Ingredients
 | An item of acidic fruit, citrus fruits, apples or pears work well. |  | Something made out of copper, eg a coin; we used copper nails |
 | Something made out of zinc, eg a galvanised nail. |  | A multimeter to measure currents and voltages |
 | Objects made of other metals eg. an ungalvanised nail |
If you actually want to charge your iPod or iPhone, you will also need a spare USB extension cable and a LOT more fruit and nails...
Instructions
If you just want to make a battery (or technically a cell - a battery has more than one cell):
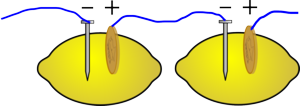
Push the zinc object into the fruit
Push the copper object into the fruit close to the zinc one (but make sure they don't touch or they will short out), if you are using a citrus fruit, try to get it in the same segment
Measure the voltage between the zinc and copper objects.
Now measure the current it will produce.
Try using different metals, see what happens.
If you actually want to charge your MP3 player:
Beware this may damage your mp3 player!
String at least 12 of these cells together and - ideally - use multiple nails in each fruit.
Cut your USB cable open, and work out which wires should carry current by plugging the male end into a computer and measuring voltages.
Connect the battery to the exposed wires in the USB cable that you have cut open.
Plug in your MP3 player.
Hope you are not going to get into trouble for ruining all the fruit in the house!
Result
 You should find that there is about 1 volt between the copper and zinc objects in the fruit, which sounds good, but it will support only a tiny current. Ours would only produce about 0.1mA at the most.
You should find that there is about 1 volt between the copper and zinc objects in the fruit, which sounds good, but it will support only a tiny current. Ours would only produce about 0.1mA at the most.
You should find that different metals will produce different voltages, for example iron to zinc or copper will produce about 0.5V.
If you use a whole bowl of fruit, and have enough metal objects, you can just about get enough current to cause an mp3 player to begin charging.

Explanation
When you insert the two different metals into the fruit, a chemical reaction will occur. Metallic zinc dissolves to form zinc ions, releasing energy; it also loses electrons. If the zinc is connected to the copper via an electric circuit, these electrons can flow around the circuit and reduce copper ions in the lemon. The energy released is what charges your mp3 player.
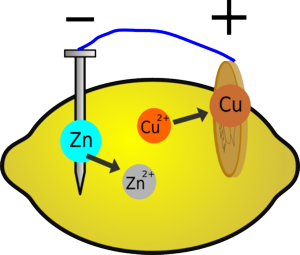
You have built a battery (or technically a cell), which works on the same principle as the cells you buy in the shops, although these more often use different metals, a more optimised design, and less fruit; but they will all involve a similar reaction that requires electricity to flow for the reaction to occur.
Why are there copper ions in the lemon?
There will be a few there naturally, but most of them will be created by the tarnish on the copper dissolving in the acid (see the cleaning copper coins experiment) releasing the copper ions in the copper oxide tarnish).
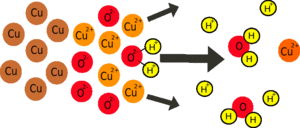
Why doesn't the reaction happen without the circuit?
If a zinc atom is to dissolve, it must form Zn2+ ions; this involves losing 2 negatively-charged electrons. If these electrons can't go anywhere, the zinc object will become so negatively charged it will attract as many positively charged zinc ions back onto itself as are dissolving, so the reaction will stop.
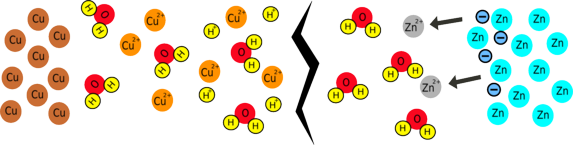
What happens when you complete the circuit?
Electrons can flow around the circuit to the copper electrode; here, copper ions are attracted to the negative electrons and, when they meet, they are reduced, forming copper metal. Because zinc is more reactive than copper, this whole process releases about 1 joule of energy for every coulomb of charge that is moved, which is the same as saying the battery produces about 1 volt. This voltage is related to the difference in reactivity between the two metals you are using, so if you change the metals you will change the voltage.
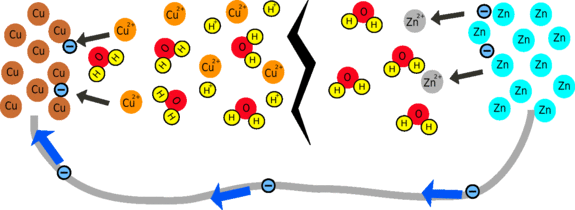
Why does the battery produce such a small current?
To produce a large current, both chemical reactions have to be able to take place quickly, so the larger the surface area and the more reactants there are in the solution, the faster the reaction will occur. There is a very low concentration of Cu2+ ions so the reaction is likely to be quite slow, limiting the current that can be produced.
The other limiting factor is that it is difficult for ions to move around in the fruit. This is because, after a while, the region around the piece of zinc becomes positively-charged and the region around the copper becomes negative. This means that there is less difference in voltage between the zinc and copper nails, so instead of giving out 1V, the cell may only produce 0.5V. But, if you wait for a while, ions will flow through the fruit to cancel out this effect.
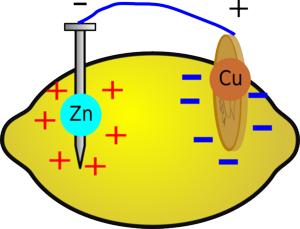 | 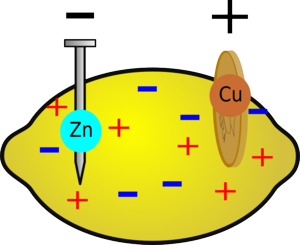 |
| After a while the region around the zinc will become positive due to all the extra zinc ions and the region around the copper will become negative, reducing the voltage of the battery. | But if you wait for a while, without drawing a current, the ions in the fruit will redistribute themselves and the voltage will build up again. |
This is why we needed to use 12 x 1V cells to produce the 5V to charge the mp3 player: each cell was actually producing less than half a volt. This is also why commercial batteries, when they are almost flat, can still produce a good voltage but this drops as soon as you draw a current.
- Previous Squashing Bottles
- Next Blood Circulation










Comments
Wheres the i pod part?
Wheres the i pod part?
I think something might be
I think something might be wrong with Cu2+ . According to the https://en.wikipedia.org/wiki/Lemon_battery , it is the two H+ receive the 2 electrons and form H2 gas. Cu is only a media act as a cathod, like Carbon does in some batteries.
It is true. Carbon or
It is true. Carbon or Graphite does not react at all. Reactive takes place at Zinc electrode.
This was very helpful for my
This was very helpful for my science fair research!
Add a comment