Ingredients
| Some popping candy/pop rocks | A saucer of water | ||
| A mortar and pestle or a spoon and a plate |
Instructions
To try and work out how popping candy explodes in your mouth you could try a variety of things.
Just to get the full experience try putting it in your mouth.
It is possible it is the heat of your mouth setting them off so try putting some in cold water and see what happens.
It could be the water in your mouth weakening the sugar so, try crushing some in a pestle and mortar or under a spoon. Compare this to sugar as a control experiment
Result
You should find that all the approaches cause the rocks to explode.
| Popping candy video |
Explanation
There are a couple of ways that popping candy may work, the most obvious would be some kind of chemical reaction between and acid and a carbonate which gives off carbon-dioxide, like sherbert. Although the water experiment is consistent with this, the explosions produced by breaking the rocks indicate that it is the wrong explanation.
What is actually going on is that the popping candy is made by mixing sugars (sucrose and lactose, and some flavouring) then heating them up until they melt. This molten mixture is exposed to very high pressure carbon-dioxide at a pressure about 40 times greater than atmospheric ( 600psi ). Lots of carbon-dioxide then dissolves in the sugar. The mixture is then cooled to room temperature and the pressure is dropped.
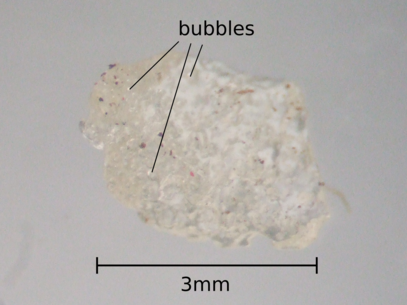
At which point all the dissolved carbon-dioxide wants to escape, and forms bubbles, just like when you open the lid of a bottle of fizzy drink. But in this case the sugar is solidifying, so the high pressure larger bubbles shatter the sugar into small lumps or rocks.
The small bubbles are preserved still at an enormous pressure, and when you break the sugar mechanically, or weaken it by dissolving the outer layers, the bubbles explode, providing that 'unique' popping experience.









Comments
Add a comment