Flexible friends - protein chaperones
Interview with
Historically, a chaperone was someone who accompanied you when you went out and they made sure - in the words of University of Michigan scientist, James Bardwell - that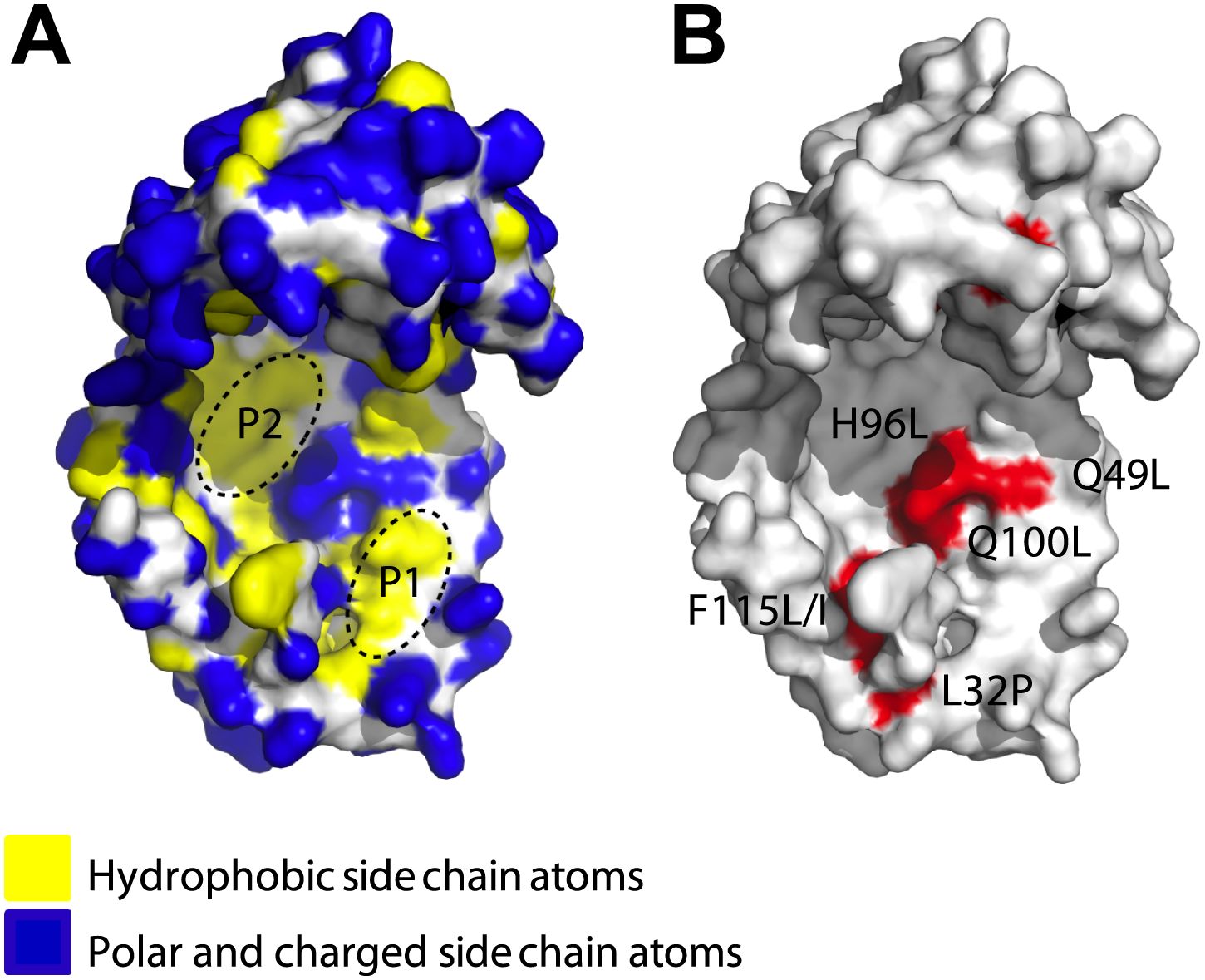 inappropriate interactions didn't happen. Well proteins also have chaperones which are other proteins that help them to stay in shape and in the correct company, but how do they work?
inappropriate interactions didn't happen. Well proteins also have chaperones which are other proteins that help them to stay in shape and in the correct company, but how do they work?
James - Chaperones have been discovered back in the '60s and it's been known for a long time that they help proteins get their proper shape. But the real mystery is, how they could do that just not for one protein, but for a whole bunch of proteins because of course, every protein has a different shape and how can you have one particularl protein help a whole bunch of different shapes take place. There's been an idea for some time that these proteins that help other proteins fold have to be very flexible because they would have to of course, bind to proteins that have all different kinds of shapes to help them gain their proper shape. So, we just started out by taking this chaperone that had this, I think, lovely name, Spy. And we decided to try to make a better Spy.
Chris - So, how did you seek to explore what Spy was doing and then adjust it?
James - Well, we had developed a way to force organisms to fold proteins better. We actually linked the proper folding of a protein to antibiotic-resistance that an organism encoded. There's a gene that encodes resistance to ampicillin. So, we could set things up so that an organism was in sort of a fold or die situation. They'd have to either improve their folding or they would be killed.
Chris - So, how did you try to persuade the cells to optimise or improve this by?
James - Well, we just simply took Spy and we mutated it and then we demanded a higher level of antibiotic resistance than it had before. Nearly all of the bugs died, but those few lucky ones that had improved Spy survived. We looked to see what changes there were in those new Spies that work better than the Spy that nature had created.
Chris - So, if you were able to improve on Spy, why hadn't nature done that already?
James - That's a brilliant question actually and we wondered that for quite a while. So, what we did actually was, we went back and we looked to see what nature had done to Spy in other organisms. The organism we worked in was E. coli. Interestingly, in other organisms, nature has in many cases come up with the same solutions we did. So then the question becomes, well why isn't E. coli the best? I think it boils down to the fact that evolution optimises rather than maximises. For instance, I workout sometimes with a trainer and he's so muscle-bound that sometimes all his muscles just seize up and he can't even walk. So, he's incredibly strong, but he's not very agile.
Chris - But on the other hand, if you can see how it's improved and why it's improved under certain circumstances, you can then begin to infer how it's doing, what's it's doing, how it's achieving that improvement.
James - Exactly and that was the major motivation of our work, was to make something better and then maybe we'd understand how it worked in the first place.
Chris - So how did you do that? Did you have to then look at crystals of Spy to see what the structure was in its wild type, the original form, and then the mutant, the better form, to see what have changed?
James - Yes, so we had a structure so we could see at high resolution what the original Spy looked like and we knew what changes we had made. The changes we'd made that made Spy better were very, very specific.
Chris - If you look at those tweaks, do they give you some insights into what the function of Spy is in stabilising those proteins, how it's doing its job normally?
James - Yes, so if you look at them, a good number of them, in fact a majority of them, work by making an area in the centre of Spy. Now, Spy is a sort of very thin cradle-shaped structure. It looks kind of like a cupped hand. A lot of the changes were right in the middle of the palm and they made one part of the palm that we already knew was hydrophobic, in other words, sticky to oily substances, it made that part even oilier, even more hydrophobic. But it clearly wasn't just that, because it also seemed to make the protein much more flexible. So nearly, all the mutations we got made the protein more able to wrap around these other proteins and sort of cradle them and protect them.
Chris - And now, you've found this, what do you think are the key questions that you need to answer next?
James - Well, I think the real key question that we launched into in the paper was exactly what chaperones do to proteins and I think that's really the key question. So far, we're starting to get glimpses of that. I mean, we, meaning the whole chaperone community and it's a long-standing problem about exactly what chaperones do to proteins to help them fold. And so, I think that that's really the key question that this work opens up, that at least for this particular chaperone that we might be able to understand at anatomic resolution, what it looks like, a protein bound to a chaperone for instance.
- Previous Twisting and turning: bacterial flagella
- Next And so to sleep









Comments
Add a comment