The Chemical that Keeps Nerves Alive
Interview with
Ben - Also in the news this week, scientists at Cambridge's Babraham Institute have identified a factor that helps to stop nerves from degenerating. This could lead to better treatments for degenerative diseases, but also better ways to halt the degeneration of a nerve when it gets damaged as a result of an injury or stroke. Dr. Michael Coleman leads the group responsible for the discovery and he joins us now. Hi, Mike.
Michael - Hello.
Ben - So first of all, what does a nerve cell look like?
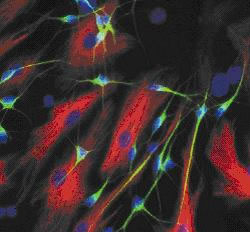 Michael - Let's start with the cell body - which is essentially the equivalent of what happens in most other cells. In this cell body, you have the nucleus which contains all the genetic material, compared to other cell types the nucleus might be slightly bigger. There's a little bit more metabolic activity and protein synthesis going on in that cell body, but by and large, the cell body is not so different from other cell types. Then we have, coming into that cell body, what we call the dendrites. Now there could be a very huge number of these - literally thousands or tens of thousands of dendrites coming into that cell body, and the job of the cell body really is to integrate the signal that comes from this enormous number of dendrites, and to place what we call an all-or-nothing response. That all-or-nothing response, the electrical activity transmitted to the next cell, then goes down to what we call an axon, and that's the bit we're interested in. There are two things really that are special about the axon. First of all, there's only one of them and this means that this is effectively the most vulnerable part of the neuron because if you lose that axon, you have totally lost the functional capability of that neuron. The second thing that's interesting about the axon is its length. This can be enormously varied between different types of neuron, but in its extreme case in humans, this can be anything up to a meter long. It can go the length of your arm or leg and it can go the length of your spinal cord.
Michael - Let's start with the cell body - which is essentially the equivalent of what happens in most other cells. In this cell body, you have the nucleus which contains all the genetic material, compared to other cell types the nucleus might be slightly bigger. There's a little bit more metabolic activity and protein synthesis going on in that cell body, but by and large, the cell body is not so different from other cell types. Then we have, coming into that cell body, what we call the dendrites. Now there could be a very huge number of these - literally thousands or tens of thousands of dendrites coming into that cell body, and the job of the cell body really is to integrate the signal that comes from this enormous number of dendrites, and to place what we call an all-or-nothing response. That all-or-nothing response, the electrical activity transmitted to the next cell, then goes down to what we call an axon, and that's the bit we're interested in. There are two things really that are special about the axon. First of all, there's only one of them and this means that this is effectively the most vulnerable part of the neuron because if you lose that axon, you have totally lost the functional capability of that neuron. The second thing that's interesting about the axon is its length. This can be enormously varied between different types of neuron, but in its extreme case in humans, this can be anything up to a meter long. It can go the length of your arm or leg and it can go the length of your spinal cord.
Ben - They certainly do sound like the fragile part, the weak link in the chain. What happens when a nerve becomes damaged?
Michael - We've already pointed out that the axon is very long and clearly, the axon has to be supplied with all sorts of material from that cell body. Most of the proteins, certainly all of the RNA and many of the organelles are made within the cell body, and they have to be shipped out. There's a very intricate system of what we call motor proteins, ATP using proteins, that are responsible for taking out and controlling the delivery of those proteins and organelles to the further parts of the axon. Clearly, like any pipeline or supply system, that's going to be vulnerable in various ways. So in various disorders, which may be inherited or neurotoxic or viral disorders for example, and protein aggregation disorders, you can have a blockage in this axon which prevents materials from getting to the far end. Sooner or later, that can result in functional impairment and ultimately, the death of that axon.
Ben - You've been able to identify a particular factor that seems to be a "stay alive" signal for the axon itself. How did you find it? Why did you know that there was something like this there?
Michael - That's right. So, what we did was effectively ask the question - among these thousands of different cargoes that are being transported down the axon, is there something which is actually a limiting factor for its survival? A nice analogy here might be a car accident on the motorway, causing a huge build up of traffic behind it. Among that traffic, among those vehicles caught upon the motorway, you will have an enormously different number of reasons why those people are trying to get from A to B. Some will be relatively trivial and not a major problem and some might be a life or death issue, in the most extreme cases. For example, the family trying to get to the beach for a day out will be very frustrated to be held up for half an hour, but it might not be a major problem. But if the ambulance is trying to get to the accident at the front is held up then you quickly have a life or death issue on your hands. What we have effectively done is to go in there and say, "What is the first protein that becomes life threatening to that axon if it cannot get through to the far end?"
Ben - And how did you identify it?
Michael - I should say that the experiments didn't happen in this order, but when we stand back now and take a sort of broader look at it, we can interpret it in this way. Effectively what we did was to cut the axon - clearly, that results in a catastrophic death of the distant parts of the axon. That's something called Wallerian degeneration which we spend most of our time studying. And then we ask - what, within there, is the first factor that's not being able to get through that kills that axon. To do that, what we have done (not always knowing at the time) is to replace that factor by something that can substitute for its action. We knew that there was something which will keep those axons alive because experiments back in the University of Oxford in the late 1980s indicated that there was a mutant strain of mouse which acquired a spontaneous and harmless mutation, and this, in experiments where those nerves were being cut, actually delayed the degeneration of those nerves by tenfold. Over the subsequent 10 years or so, we and others identified the gene that's underlying this process. In the last 10 years, this has led to us trying to understand why that, or how that protein works.
Ben - So, by identifying the gene in these mutant mice, you were able to work out which protein or at least which family of proteins it was that was responsible? Proteins always have strange names that are very hard to remember. What's this one called?
Michael - Yes. It's called Nmnat2, nicotinamide mononucleotide adenylyltransferase 2.
Ben - So, difficult to say, as well as difficult to remember.
Michael - Yes.
Ben - What does it actually do? We know that it seems to keep the nerve alive, but by doing what?
Michael - That's an interesting question. It certainly has an enzyme activity. It makes a molecule called NAD which the biochemists among you will know is heavily involved in energy metabolism inside the cell. That is, in a way, the most obvious potential consequence of this protein being missing when it can't get into the axon with enough quantity. However, sometimes the most obvious direction to take is not the correct one. We've seen this a number of times and there is some discussion in the field at the moment about whether NAD synthesis is the most important or the key function of this protein that's involved in the axon degeneration or whether it's something else. Maybe it works in reverse or maybe it catalyses a different reaction as well.
Ben - Clearly there's still some work to do, but what's the next stage for you?
Michael - So what we try to do often as scientists is to keep away from animal experiments, where we possibly can, by taking cell culture alternatives or work in other organisms such as fruit flies. The work that we've done up to this point has been in a cell culture system. At some point there will be a need to confirm this looking at a mammalian nervous system to know that what we've seen is physiologically relevant. That's a very important step because if we always stick to alternatives, then there is also a risk of diverting the science if we don't actually confirm that we are looking at the right thing. So that's one very important step to take in the near future and another one is to look at what this means in terms of disease. So, we need to actually remove this protein now and ask whether the nerves actually start to die back and whether this mimics certain disease situations.
Ben - But certainly very promising work. I do like the fact that we seem to feature all these really promising things and hopefully, we can follow up with you in the future and find out how it's doing?
Michael - Yes. That would be good.
Ben - Well thank you ever so much, Michael. That was Dr. Michael Coleman. He's based at the BBSRC's Babraham Institute. They've published this discovery in the open access journal, PLoS Biology.










Comments
Add a comment