Implants to improve impaired vision
Interview with
Chris - Taking a present example, the cochlear implant has been around since the 1970s and has helped over 200,000 profoundly deaf people to regain some sense of their hearing.
Well now, the stage is set for the visual equivalent of the cochlear implant and a team based at Oxford University are developing an implant that will return sight to people who have lost their natural vision, owing to disease processes like the disease retinitis pigmentosa.
We're joined by Dr. Marcus Groppe who's from the Nuffield Laboratory of Ophthalmology. Let's first of all look at what the disease is that you're trying to remedy. So, can you just fill us in on what retinitis pigmentosa is?
Marcus - Retinitis pigmentosa is a genetic disease, so a condition which is passed from generation to generation and it's kind of a mixed bag of conditions which are all grouped under the same name.
The main problem is that patients with this condition will lose their photoreceptors. So in our eye, at the back of the eye, we have a layer of cells which make up the retina, which works a bit like a sensor in a camera, or a film in a camera. One layer of the retina, the photoreceptors, convert light energy into an electric impulse.
Patients with retinitis pigmentosa have genetic defects which don't allow these photoreceptors to work properly over a long time and within their lifetime, they will get more and more degeneration of photoreceptors. Most of these patients are initially affected by losing peripheral vision and night vision, but later on, quite a lot of these patients loose central vision and eventually end up being blind.
Chris - And their diseases manifest just within those photoreceptor cells that convert the light waves into electrical signals, but presumably, you leave the other structures in the eye, including critically the optic nerve, intact?
Marcus - Yes, and most patients, if you wait long enough, there will be some more degeneration in layers of the retina which make up cells which form the optic nerve. But by the time patients actually become blind, the ganglion cells which form the optic nerve are still intact in most of these patients.
 Chris - So if you can get signals back into them, then you can help them to experience at least some semblance of vision again. Is that your starting hypothesis?
Chris - So if you can get signals back into them, then you can help them to experience at least some semblance of vision again. Is that your starting hypothesis?
Marcus - That is exactly the idea behind the retina implant. So the retina implant we are using in our clinical trials which will start very shortly here in Oxford, is a chip which contains light sensors and an amplifier which converts the light energy into an electric impulse and then can connect to the ganglion cells so that the information of light is sent to the brain via the optic nerve.
Chris - So because you've lost the photoreceptors, the bits that see the light, your chip takes the place of those photoreceptors you've lost and then couples itself onto these ganglion cells that make the optic nerve that would transmit that signal into the brain?
Marcus - That is correct and the chip sits exactly at the same place where the photoreceptors used to be before the degeneration took place.
Chris - Wow! How big is it then?
Marcus - The actual active part of the chip is 3x3 mm and contains 1,500 light sensors, which gives you obviously a quite pixelated picture but theoretically can give enough information for patients to be able read big letters again. The main aim is to give patients who are completely blind some visual sensation back which helps them in everyday situations. For example, to find a door frame or to see a plate in front of them on a table, or to find things in their environment which they misplaced, which is very difficult for a blind patient who is only reliant on his feel and touch sensation.
Chris - How do you get the chip in, in the first place?
Marcus - It is an operation where a pocket of fluid is created via an injection underneath the retina to create some space for the chip to slide in and then a tunnel is created through the sclera which is the wide leathery bit of the eye, and the chip is then sliding towards the centre of our retina, the macula and the fovea, and is placed just underneath these structures because that allows the highest density of ganglion cells to project the best quality of picture to the brain.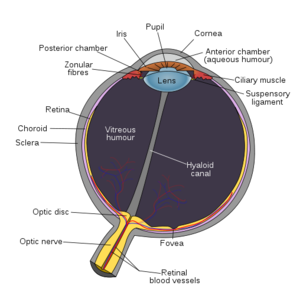
Chris - And how do you power the chip?
Marcus - With the same technology as it is for cochlear implants. So it is a magnetic plate sitting behind the ear and the chip is then powered by electromagnetic power, so everybody runs around with a small battery pack which then clicks onto the skin behind the ear via a magnet.
Chris - And what data have you got so far that this is going to be a success? The fact that you're going into humans argues that you must have satisfied enormous amounts of regulation and other preliminary data requirements to show that this is likely to work, but how soon do you expect to see results?
Marcus - At this stage, we are involved in a multicentre trial in Oxford here, so most of the work for this type of chip, and there are few other type of chips, was done in Tubingen in Germany, Currently, the state is that at this stage 1 trial, 11 patients have been implanted in Germany, and we will be starting to implant hopefully in a few months in Oxford in the first patient.
Chris - And how many people are you going to do in total? When will you know whether or not you've got a success on your hands? What's the long term follow up here?
Marcus - The trial is set to follow these patients up for one year and depending on the outcome, we are planning to do 18 patients in altogether 5 centres but that can be extended to about 32 patients.
- Previous What is cybernetics?
- Next Potentially Pandemic H5N1










Comments
Add a comment