TWiSH - The death of Daniel Nathans
Interview with
This week in science history saw, in 1999, the death of Daniel Nathans, microbiologist and co-winner of the 1978 Nobel Prize for Medicine or Physiology for his work on restriction enzymes, essential tools in the world of genetics.
Born in Wilmington, Delaware in 1928 to Jewish immigrant parents, Nathans grew up in a supportive household, and followed his sisters and brothers to the University of Delaware, where he studied chemistry, philosophy and literature. Coming to the end of his degree, he chose to study medicine, and gained his MD degree in 1954. After a time spent working in clinical medicine, Nathans knew his heart lay in medical research, rather than in treating patients.
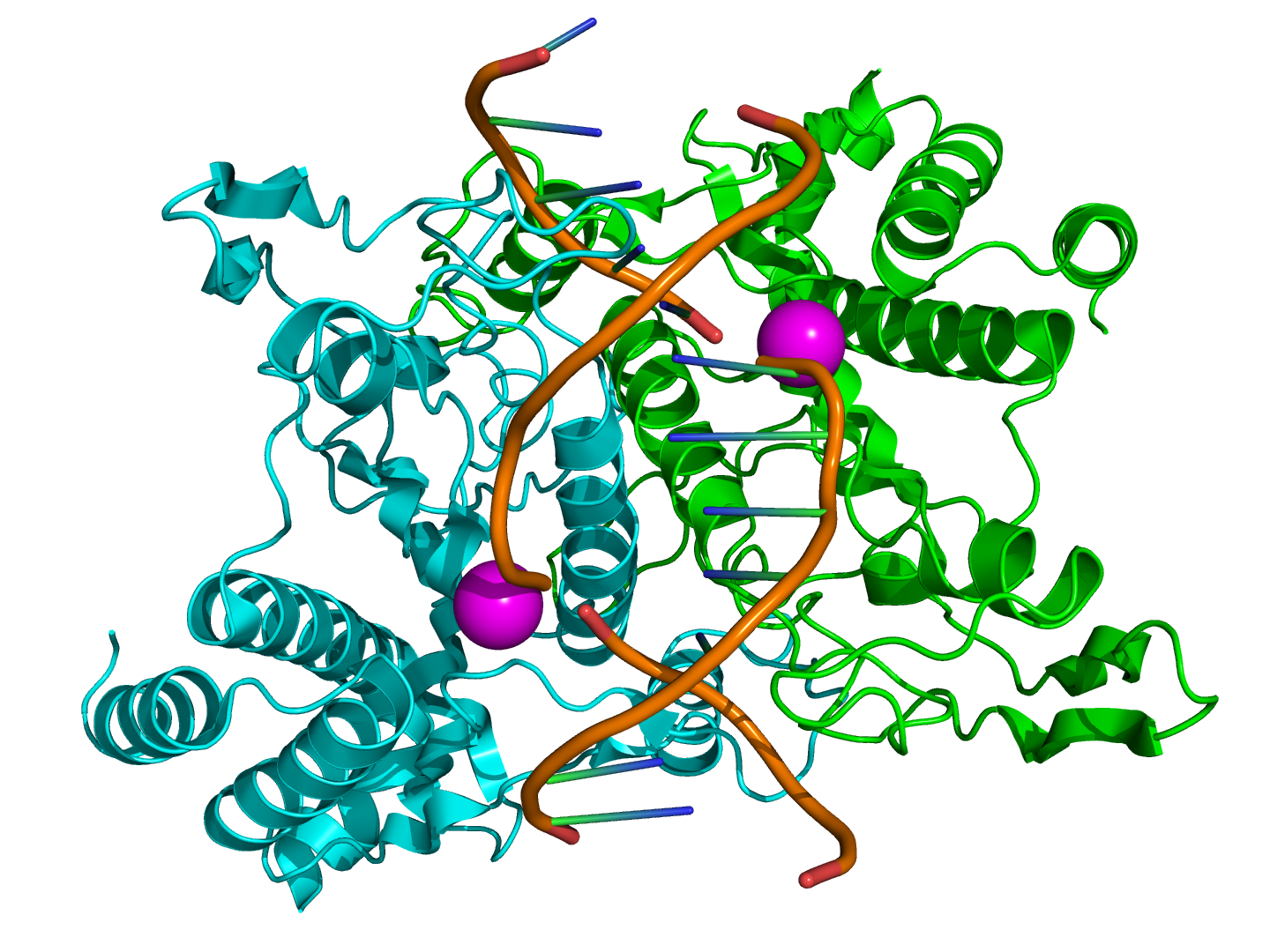 The two other winners of the Nobel Prize, Werner Arber and Hamilton Smith, discovered restriction enzymes in the 1960s, but it was
The two other winners of the Nobel Prize, Werner Arber and Hamilton Smith, discovered restriction enzymes in the 1960s, but it was
Nathans who realised their potential for use in genetics. The enzymes, also known as restriction endonucleases, were first isolated
from bacteria, where they helped to protect the bacteria from infection with viruses called phages. They did this by chopping up the
virus' DNA, while another enzyme protected the bacteria's own DNA.
So how do restriction enzymes work? Well, they scan along a DNA molecule until they reach a particular sequence of the bases A, T, G and C. For example, the enzyme EcoRI recognises GAATTC, and TaqI recognises TCGA. When it finds this sequence, the enzyme cuts the chemical bonds in the backbone of each strand of the double helix. They either cut or 'cleave' the DNA strands straight across,
known as a 'blunt end' or in places a few bases apart on each strand, leaving overhanging bases on each strand, known as 'sticky ends'. The enzymes useful to genetics are the sticky ends types, but more on that in a bit.
Nathans was working at Johns Hopkins University in Baltimore in 1969 when he received a letter from Hamilton Smith detailing his discovery of the restriction enzyme in the bacterium Hemophilus influenzae. Nathans used this enzyme to cleave the DNA of the simian virus SV40 into 11 specific fragments. This and later work, where the virus' DNA was cleaved in different places using different enzymes, showed that the base sequence of a whole genome could be determined using restriction enzymes.
Cutting DNA using restriction enzymes, known as a restriction digest is the first step in many genetic techniques DNA fingerprinting in crime and paternity cases, RFLP analysis to detect genetic diseases such as cystic fibrosis and for genetic recombination. This last technique may not ring any bells, but it will have saved your life if you suffer from diabetes, it's how scientists can produce human insulin by inserting the gene into bacteria and making them produce it in bulk.
Nathans showed that the 'Sticky ends' of the DNA left by some of the enzymes were sticky for any corresponding sticky end, whether it had come from the original DNA or not making joining human and bacterial DNA together possible. As long as the bacterial DNA was cut with the same enzyme used to cut out the human gene, the sticky ends would match and the gene could be inserted.
Nathans continued to work at Johns Hopkins until his death, becoming President of the University in 1994. He was awarded the National Medal of Science by President Clinton in 1993, and Johns Hopkins named its Institute of Genetic Medicine after him and Victor McKusick in 1999 after his death. In his autobiography on acceptance of the Nobel Prize, Nathans paid tribute to his interesting and cordial colleagues and his family, saying he felt 'struck by the good fortune that came [his] way' in his life.
Many genetic techniques that we now take for granted would not have been possible without the work of the three Nobel winners, but it was Daniel Nathans' vision that helped him realise the full and far reaching potential of restriction enzymes.










Comments
Add a comment