Unblocking heart disease
Interview with
Heart disease and strokes are the leading cause of death worldwide. They occur 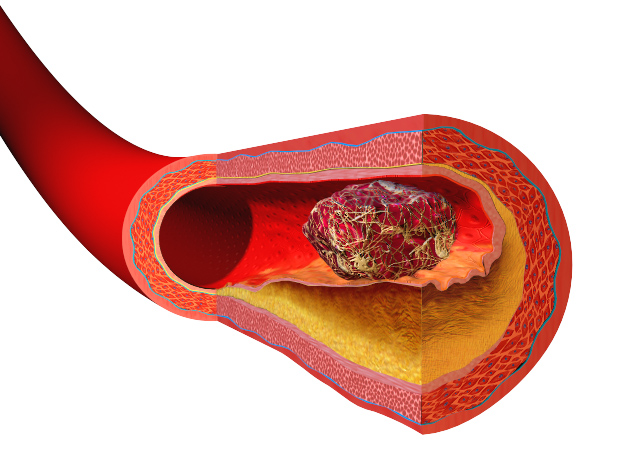 when blood vessels become furred up by fatty deposits called atheroma, which also contains dead or "apoptotic" cell debris, which the body seems to struggle to remove. Now Stanford scientist Nick Leeper thinks he knows why, and may also have got to the heart of solving the problem as he explained to Chris Smith...
when blood vessels become furred up by fatty deposits called atheroma, which also contains dead or "apoptotic" cell debris, which the body seems to struggle to remove. Now Stanford scientist Nick Leeper thinks he knows why, and may also have got to the heart of solving the problem as he explained to Chris Smith...
Nick - Our body will turn over as many as 100 billion cells per day, everyday. That is a process known as efferocytosis, and efferocytosis is Greek which means carry 'the dead to the grave,' and it essentially refers to the process by which a dying cell is recognised for clearance by a phagocytic cell like a macrophage. It's quite interesting because this process of efferocytosis is highly efficient and, actually, if you look very carefully throughout the body, it's nearly impossible to find any evidence of dying or apoptotic cells in healthy tissue.
The one exception to that is within the diseased blood vessel where there is really an amazing accumulation of these cells and other necrotic debris. And previously, we didn't quite understand why these cells were accumulating and not being cleared by the macrophages and other cells that seem to be so efficient at clearing cells elsewhere in the human body.
Chris - So if one looks around the body, every day the body is turning over, renewing, getting rid of dead cells and making new ones, and there's no evidence of the debris left behind. But for some reason, it doesn't get cleared away properly in the walls of blood vessels and this is what leads to the progressive narrowing of blood vessels and, therefore, their ultimate blockage?
Nick - That's exactly right. And previously our laboratory had decided to pursue the top GWAS locus for cardiovascular disease (GWAS stands for Genome Wide Association Studies). And this is work that was made possible by the humane genome project, where investigators could look for the first time across the full genome to identify hotspots on the chromosomes that are associated with risk for heart attack and stroke. We found that the top inherited locus for cardiovascular disease was associated with a novel pathway. People who are born with the genetic variance seem to have reduced expression of a so-called 'eat me' ligand, and this is a molecule that is expressed on the surface of a dying cell that makes it edible and appealing to these phagocytic cells like the macrophage. And we began to wonder if this was the driver of the accumulation of this debris in the growing atherosclerlotic lesion.
Chris - If that's true, why are cells in the rest of their body nonetheless cleared up efficiently then, if they're less digestible?
Nick - That's a great question. Investigators have long known that there is the presence of an inflammatory molecule called TNF-alpha that seems to accumulate and be responsible for the growth of the diseased vascular plaque. Now in the current study we've found that TNF-alpha seems to drive the expression of a don't eat me molecule. This molecule renders a cell inedible and so we think that in patients with developing aphroscotic plaque, local inflammation is driving expression of this antiphagocytic molecule that's rendering the cells inedible and unable to be cleared.
Chris - You're suggesting that blood vessels, when they're injured or damaged by disease, release inflammatory chemicals, including this chemical TNF-alpha, and that then turns on a don't eat me signal in the cells that are accumulating there, so they're not cleared and that leads to the progression and build up of cells and cell debris in the walls of arteries, narrowing them?
Nick - That's exactly right. And now we understand that this TNF-alpha promotes this don't eat me signal and this seems to make the cells inedible. It's almost like it's providing a cloaking device for these cells so that when they die they are invisible and unable to be cleared.
Chris - And if you take animals that are predestined to get furred up arteries and you block these signals in those animals, do they have lower levels of arterial clogging?
Nick - Exactly. What we found was, when we treated our mice who had very high cholesterols and were prone to developing atherosclerosis with antibodies that blocked this don't eat me signal, we found a profound improvement in cardiovascular disease.
Chris - You do think then that if you intervene in this way in a person who has established heart disease, you might be able to chemically unclog arteries?
Nick - Well that's our goal! Obviously our mouse findings are preliminary and need to be confirmed in human studies, but the extrapolation of our results would suggest that as we block the don't eat me signal, that we would be able to inhibit progression of atherosclerosis and perhaps even be able to melt away plaque by shrinking the necrotic core.










Comments
Add a comment