With the precision design of superalloy materials
(as written about here by David Collins and Bryce Conduit), we are able to manufacture materials capable of withstanding the extreme conditions inside a jet engine where temperatures routinely exceed 1500°C and the loads on the materials have more momentum than a family car flung three miles into the air. But making materials strong and temperature-resistant isn't the whole story, because making them chemically durable is also key.
In this pair of articles, materials scientists Bill Clegg and Cathie Rae explain how special coatings can be applied to turbine blades to render them resilient to harsh, highly-oxidising environment in order to prolong engine life and boost efficiency.
Dr Cathie Rae, from the Department of Materials Science & Metallurgy at Cambridge University, uses a range of techniques to study the way that coatings interact with the underlying alloy, and how this "interaction zone" may alter the overall performance or lifetime of the material...
Cathie Rae:
 |
| Figure 1 - The interaction layer between a nickel superalloy turbine blade at the bottom and a nickel-aluminide coating. |
To survive in the engine, blades need coatings. While the inside of the blade is designed to carry the enormous load from the rotation of the turbine, the outside has to withstand the hot gases which, over time, turn the metal to oxide powders. A more mundane example is the problem of dealing with rust - uncoated iron will quite quickly oxidise to form iron oxides, which are far weaker than the original, unoxidised, iron. However, a coating of paint keeps the metal separated from the oxidising atmosphere, prevents the rust from occurring, and extends the useful life of the material.
In a jet engine, coatings are made of aluminium and nickel and often include precious metals such as platinum. They are deposited on the surface by passing a gas containing aluminium over the superalloy blade.
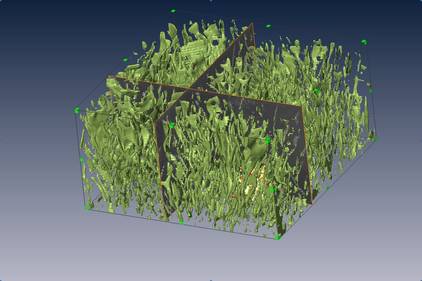
Figure 1 shows part of one of these coatings: At the bottom you can see the characteristic structure of the blade - the little cubes of the superalloy. At the top is the base of the coating layer. In the middle is the layer where the two different materials overlap and mix. Holes, in this case the darker structure that looks like a little dinosaur - are one of the results of this mixing.
The "dinosaur" is 20 microns from nose to toes (50 would fit in a millimetre) and sits in a layer where the coating has grown into the blade and altered the structure. This can be a problem as the strong structure of the cubes has gone and the striped structure that replaces it, although made of the same elements, is much weaker. If this layer grows too much it can seriously weaken the blades.
To better understand how coatings affect the structure of the blade and how to overcome these problems, we use a scanning microscope - an essential tool in materials science. With some additions a scanning microscope can look inside the structure using software that was initially developed to allow medical scanners to see inside the body. The second picture, figure 2, is a 3D reconstruction of the white streaks shown in the middle of the first picture. This image was created by collating the scanning micrograph images of a series of slices, which were taken by eroding a slithers of the surface away between each picture. This reveals the extraordinary shapes these make as they grow into the blade, beautiful but very damaging. By understanding the way these structures grow we can design coatings that protect the surface but do not damage the superalloy structures underneath.
|
| Figure 2 - 3D reconstruction of the interaction layer (or Secondary Reaction Zone) between a coating and the superalloy blade. |
Professor Bill Clegg, from the Ceramics & Inorganic Materials Group at Cambridge University, researches the way that the crystal structure of a material alters how it behaves at high temperatures...
Bill Clegg:
The thrust from a jet engine comes from the air that is forced through it. In order to do work on the gas (in the compressor) and to extract work from it (in the turbine) the moving air must hit the blades on a rotating turbine disc. Air that escapes around the edges of the turbine blade and the shroud is useless and the engine efficiency falls. However, a design that completely stops this would cause friction, much like a bicycle wheel rubbing on a mudguard, and this could cause the engine to seize.
So, to surmount this problem, engineers have come up with many complicated designs. Some involve developing tortuous gas paths to prevent the gas escaping. In others, materials are inserted that are strong enough to withstand the passage of any gas, but sufficiently weak that they present no obstacle to the turbine and, even more importantly, do not wear away the turbine blade which would otherwise produce a gap. To do this, materials such as felts and honeycombs, made of thin strips or fibres, are commonly used.
But the main challenge is posed by the hottest parts of the turbine. The conventional approach has been to use a shroud - a large piece of metal that sits on the tip of the turbine blade, protecting the tip. It has the further advantage that it keeps the blades more firmly fixed in their place on the turbine disc.
But this also applies a huge centrifugal force to the blade as it spins, reducing the lifetime of the blade in the turbine disc - a problem that gets worse as the temperature in the high pressure turbine increases. To get the increased turbine efficiency we need, future engines will need to operate at even higher temperatures, so there is a real need to think of alternatives to using the shrouded blade to minimise gas escaping over the turbine blade tip.
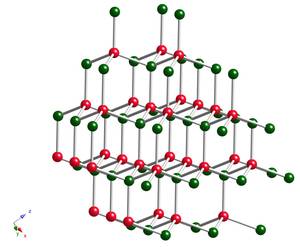
Figure 3: The crystal structure of the cubic form of boron nitride, c-BN © Bill Clegg One solution has been using the turbine blade itself to cut a track in a very porous ceramic material-an abradable. By rotating in the track the amount of air that can escape is reduced. However there can be substantial wear on the tip of the turbine blade. The blade rubbing against the abradable is just like any two surfaces rubbing together - the friction between them gives rise to local heating. And because of the rotation speeds and forces involved the blade tip can get so hot it melts. The surface can be protected by applying a film of hard material that can cut through the abradable, but this needs to be non-continuous so as to avoid distributing heat around the blade. In other words, the tip of the turbine blade is coated with a hardened abrasive.
At present the cubic form of boron nitride is used (see figure 3). This is bonded to the turbine blade using an alloy of nickel, cobolt and aluminium (Ni,Co)Al, which is suspended in a matrix of a nickel and cobolt solid solution. To provide extra resistence against oxidation (the non-iron equivalent of rusting), this then has small quantities of yttrium and chromium added in a process known as "doping". There are a whole range of these materials, known as MCrAlYs, where M is Nickel or Cobolt.
Cubic boron nitride is used as an abrasive generally and although it is cheaper than diamond, it is still sold by the carat, making it an expensive option. While this has worked well, boron nitride oxidises rather easily in air to form boric oxide. This melts at a mere 450 °C, evaporates rapidly above 1,000 °C and boils at 1500 °C. To meet conditions inside a jet engine we need to find alternatives that will be hard enough at high temperature to act as abrasives. Just to add to the coplications, the MCrAlY bonding material is also reaching the limit of its capability.
So are abrasives reaching the end of their use? Here at Cambridge University, we're working to understand how this abrasive system - the abrasive and the material used to attach it to the blade - behaves, so that we can determine what properties we need if such abrasive systems are to be of use in the future.
- Previous Processing Nickel-Base Superalloys
- Next Steeling the Show










Comments
Add a comment