Ingredients
 | Some salt |  | A piece of thread |
 | A bowl or mug |  | An icecube |
Instructions
- Put a piece of ice into the bowl. This experiment works best if the ice is a bit wet, just starting to melt.
- Take some cotton and lay it over the top of your ice cube. If you have a particularly big ice cube you may need to fold it over a few times to make it stronger.
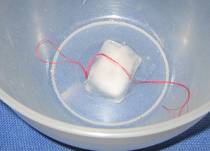
- Sprinkle a little bit of salt over the top of the ice cube, on top of the cotton.
- Leave it for about 10 seconds.
- Try to pick the cotton up!
Result
 The ice cube should stick to the thread and you should be able to pick the ice cube up using the thread lying on top of it.
The ice cube should stick to the thread and you should be able to pick the ice cube up using the thread lying on top of it.
Explanation
If you measure the temperature of a gently melting icecube, it's about zero degrees centigrade. If you then put salt on it and measure the temperature, it plummets down to about -8 or -10°C even possible to get down to around -18°C. In fact, the lowest temperature in the Fahrenheit scale is actually the lowest temperature you can get by adding salt to ice.
Water molecules behave slightly like little magnets and would like to stick together in a big solid structure. When they are cold they can do this and we call it ice. If we give the water molecules more energy by heating up the ice they can break their bonds and melt into a liquid where the molecules can move past each other and flow. Breaking these bonds takes a huge amount of energy, which means that ice can absorb a huge amount of heat as it melts so it is very good at keeping things cool.
At 0°C you can have both water and ice next to one another. At the surface of the ice there will be always some molecules that have enough energy to break their bonds and others in the water that can be captured. So there are molecules both joining and leaving the ice all the time.
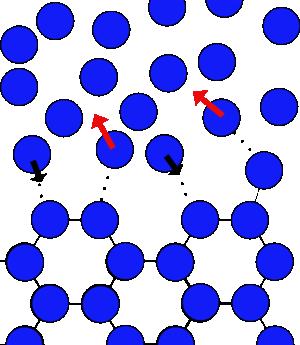
As the molecules leave they loose energy as they break their bonds, but as others join they gain the same amount, and you can achieve an equilibrium.
If you add salt to the water at the same temperature there are the same number of water molecules leaving the ice every second, but the water molecules in the water are at a lower concentration - they get lost in amongst the salt, and take longer to get back to the ice.
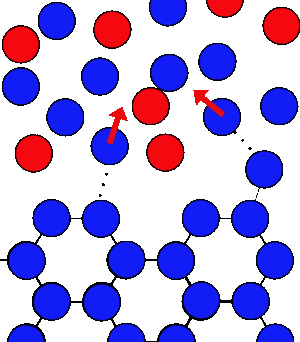
This means that the ice will melt, but this involves breaking lots of bonds which takes a lot of energy - the only place this energy can come from is from heat. So the ice and water gets colder.
What's happening with the thread then?
In the places where the salt is, the icecube is forcibly melted and also those places get very very cold. In the other places where salt didn't land, you now have melted water sitting on a much colder icecube. This causes those parts to quickly refreeze. This sticks the thread to the icecube, and you can pick it up!So why salt the roads?
The reason they do that is salt on the roads will melt the ice, making the roads less slippery. The icy water now freezes at perhaps -5°C rather than 0°C. So it has to be a colder day to make the roads ice up. If you get down to -20 or -30°C, the ice will no longer work. At these temperature you now need to add a different chemical. Sodium Acetate is the flavour in salt and vinegar crisps, and it works at lower temperatures and isn't so bad for the environment, either!
- Previous The Chemistry of Coppers
- Next Why Put Salt on the Roads?










Comments
Add a comment