It was 1880 in Würtzberg, Germany, and Frau Speyer knew her daughter Maria was terribly ill. Over several days, she had watched the five-year-old become more and more lethargic. Then, while bathing her one evening, she noticed bruises on her arms and legs. The child complained of a headache and a stiff neck. Speyer summoned Herr Doctor Stern, the family physician who had delivered the child. He noted Maria was pale and weak and covered with enormous bruises. He also found an enlarged spleen that filled most of her abdomen.
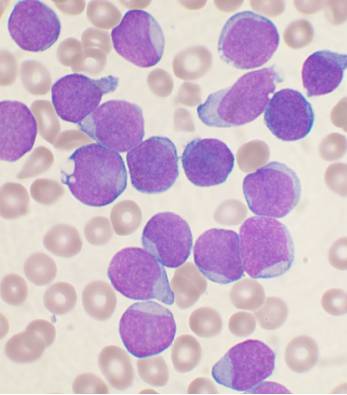 Stern, who had never seen such a case, consulted Herr Doctor Biermer, a specialist in rare diseases. To obtain a diagnosis, Biermer brought the latest medical instrument -- a microscope. Using a candle to illuminate a smear of Maria's blood, he discovered an alarming increase in the number of white cells.
Stern, who had never seen such a case, consulted Herr Doctor Biermer, a specialist in rare diseases. To obtain a diagnosis, Biermer brought the latest medical instrument -- a microscope. Using a candle to illuminate a smear of Maria's blood, he discovered an alarming increase in the number of white cells.
This confirmed Biermer's suspicions: the patient suffered from a disorder termed "Leukamie". In 1839, two French physicians had reported a forty-four-year-old Parisian woman with similar symptoms. As more reports appeared in the medical literature, doctors realized the disease was caused by a malignant overgrowth of white blood cells that killed its victims by destroying their vital tissues.
For the next eight decades, leukaemia would remain a death sentence, killing hundreds of thousands of patients, most of them children. Victory was slow in coming. The cure lurked in a realm as stark and brutal as the disease itself.
The Magic Bullet
In 1908, during a wide-ranging effort to cure the venereal disease syphilis, an assistant in Paul Ehrlich's laboratory synthesized a compound that came to be known as Salvarsan. Ehrlich had found what he called a "magic bullet," a compound that could be aimed at the patient's "foe" (in the case of syphilis, the spirochete Treponema pallidum) while missing the patient's "friend" (the organs essential to life).
Ehrlich's success inspired a wave of optimism in the medical community. During that era, once a tumour had spread to distant organs, it resisted every treatment. This was especially true of leukaemia, which scatters its cells throughout the host's organs from the moment of inception. Now there seemed to be a new hope: if Salvarsan kills millions of bacteria while leaving the patient unharmed, how hard would it be to find a drug that killed cancer cells without injuring their host?
The answer came slowly, decade by decade -- very hard indeed.
On the tree of life, the two branches that would give rise to humans and bacteria evolved distinct biological structures and metabolic pathways. Thanks to these differences, many drugs can destroy bacteria while leaving the patient's cells unharmed. Thus Salvarsan proved an effective treatment for syphilis only because Treponema pallidum bears little resemblance to Homo sapiens. Cancers are a different matter. Thanks to genetic aberrations, malignant cells grow without restraint, yet they maintain a strong biologic resemblance to the normal cells from which they arose.
This similarity between friend and foe means cancer therapies must invariably damage patients' vital tissues. The physicians who treated leukaemia victims could find no magic bullet. Instead, they had to use poisons -- drugs that injured both the tumour and the host. The horrific dimensions of this struggle were revealed by animal studies: if treatment left just one viable leukaemic cell, it could proliferate into the billions, causing a full-blown relapse. Doctors could only hope that when their drugs finally killed the last malignant cell, their patients would retain enough healthy cells to survive the gruesome struggle.
The Toxic Cloud
During the First World War, in the second Battle of Ypres, the Germans released chlorine gas against the enemy for the first time. The Allied soldiers were terrified by the clouds of mysterious vapour drifting toward their trenches. Chlorine choked and blinded its victims on contact. But among those who survived, the long-term effects proved relatively mild, and many returned to fight another day.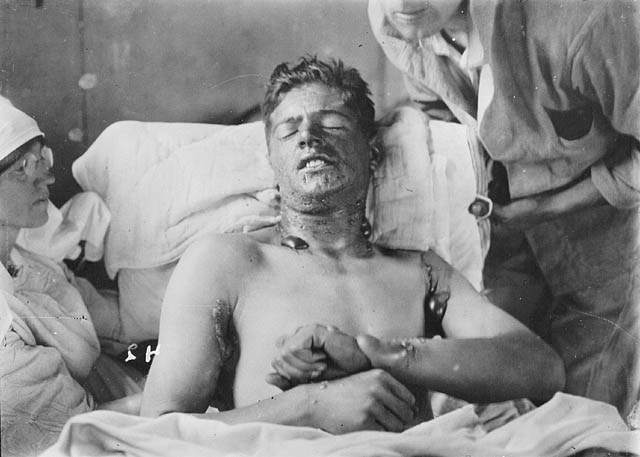
However, during the third Battle of Ypres the Germans upped the ante by releasing mustard gas (dichlorodiethyl sulphine). The victims noticed a difference in this new toxic onslaught: the immediate effects were devastating.
Mustard gas penetrates cells, destroying their vital structures. In addition, surviving cells lose their ability to divide, an effect that slowly erodes the delicate tissues of the eyes, nose, and throat. Exposed skin develops blisters that burst and become infected. Recovery takes months, leaving ugly scars. The most devastating long-term effects are on the eyes, causing permanent blindness, and on the lungs, where inflammation and the sloughing of dead tissue can drown victims in their own fluid. The gas led to over 2000 casualties among British troops at Ypres alone.
Yet redemption would come one day. Mustard gas targets rapidly dividing cells. And while this caused the maiming and lingering deaths so dreaded by its victims, in the hands of daring physicians the compound would serve an extraordinary purpose.
The Search for a Cure
The first stride toward an effective treatment for leukaemia is difficult to pin down. Perhaps credit should go to the German scientists who performed autopsies on the casualties at Ypres. Their motive was malign -- a search for yet more effective toxins -- but their findings would prove vital to medical progress. In 1942, researchers at Yale Medical School showed that certain extracts from mustard gas can shrink malignant lymphomas in mice. A lymphoma patient responded to one extract with a dramatic remission (although he soon relapsed), while at the Chester Beatty Institute in London, Doctor Alexander Haddow synthesized other mustard derivatives that would become important agents for treati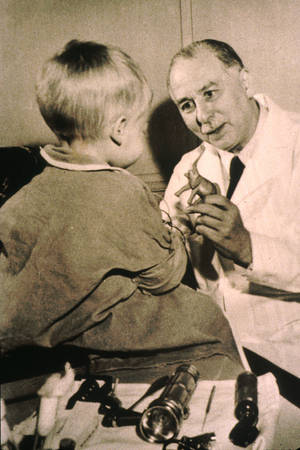 ng leukaemia.
ng leukaemia.
In the 1940s Sidney Farber began treating cancer patients at Boston's Children's Hospital. His success came largely through collaboration with Yellapragada Subbarao, a chemist at American Cyanamid, who synthesized a number of compounds known as folic acid antagonists. Farber tested these compounds on animals, selected the ones that seemed to offer the greatest promise, and then gave them to sixteen leukaemia patients.
Among these was a seven-year-old boy who had an enlarged liver and spleen. Tests showed a heavy burden of leukaemic cells in his blood. An occasional injection of aminopterin, one of the compounds synthesized by Subbarao, seemed to offer no benefit, but injections given three times a day produced a dramatic remission. However, severe mouth ulcers forced the doctors to stop the treatment, and an immediate relapse followed.
Farber and his colleagues reported their findings in a landmark paper published in The New England Journal of Medicine. Of the sixteen patients treated with aminopterin, only ten showed a definite benefit. This was the first time in history any drug had delayed the inexorable progress of leukaemia, yet there was little cause for celebration. The results showed the grim challenges that would plague physicians for decades to come:
1. No one knew how to determine the drug or dosage best suited to each patient.
2. The most effective drugs produced severe side effects that forced doctors to limit the dosage or discontinue the drug. Then, inevitably, the disease recurred, dragging the weakened patient ever closer to death.
3. No matter how dramatic the remissions, all the children died.
Rolling the Dice
By the mid-1950s, despite a concerted ten-year effort, 100 percent of children with leukaemia still died. Paediatric oncologists read journals and attended conferences to learn their colleagues' methods, but the resulting data gave little useful insight. Since physicians relied on their own judgement when selecting different drugs and dosages for each patient, no one could determine the best regime. Without a method to measure the long-term benefits, the future promised an unending string of disasters.
Three gifted scientists staffed the paediatric ward at the National Cancer Institute: Gordon Zubrod, Tom Frei, and J. Freireich. In desperation, they turned to a bold method used by Ronald Fisher, a British statistician, to measure the value of streptomycin for treating tuberculosis. Known as a randomised control trial, this would evolve into a paradigm of modern medicine, saving millions of patients across the globe.
The first trial began with an unnerving scene. Zubrod sat in an office with the parents of a desperately ill child. The mood was bleak, since the leukemia had not responded to earlier treatments, and the victim lay in a nearby room, her burden of malignant cells growing by the hour. The parents faced a daunting decision -- what should be done next? A new drug, 6-azauracil, had been synthesised and tested on laboratory animals, but no one knew whether it would help or harm the patient.
Zubrod had two goals: provide the patient with the best available treatment, and gather data to improve the treatment of future patients. His proposal must have shocked the parents. In deciding whether to administer or withhold the new drug, he would rely on random chance -- virtually, a roll of the dice.
The parents of thirty-nine patients agreed to enter their children as subjects in an experiment no different in concept from those done on laboratory animals. This study, the world's first randomised clinical trial of cancer therapy, showed that while 6-azauracil lowered the number of malignant cells in a patient's blood, in the long run it caused more harm than benefit. With or without the drug, all the children in the trial died. Zubrod, Frei, and Freireich were discouraged, but they had learned a vital lesson: the new drug did not work, and thus no more victims would suffer needlessly from its toxicity. Zubrod and his colleagues persevered, designing a series of randomised trials that continued for decades.
The Beginning of the End
With each new trial the fund of knowledge grew, leading to ever more effective treatments. A device that separated blood into its various components helped to control the anaemia, infections, and hemorrhaging that killed so many patients. Physicians also discovered the benefits of multi-drug therapy: if tumour cells became resistant to one agent, a combination of two or more administered at the same time might increase the likelihood of cure. 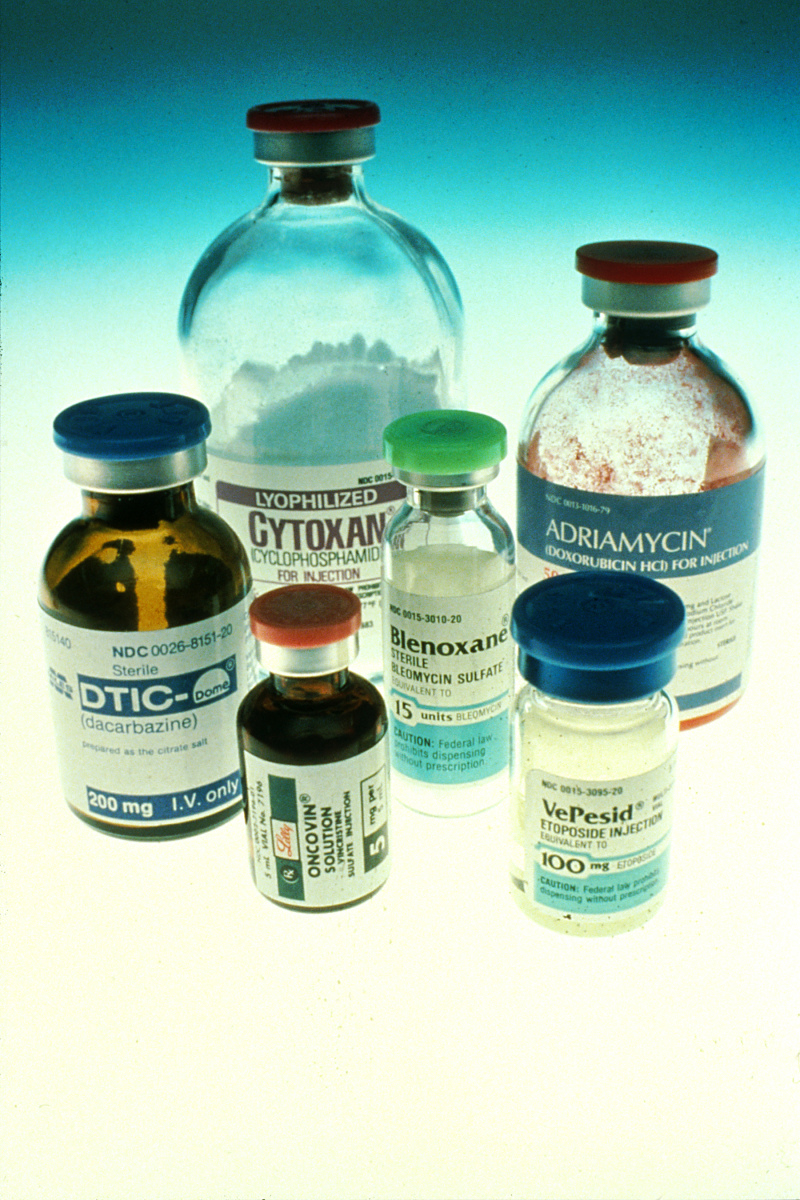
Each round of treatment destroyed a fraction of the malignant cells plus a fraction of the patient's bone marrow and gastro-intestinal tract. By monitoring the dosages and intervals between treatments, oncologists hoped to enhance the likelihood of cure while minimizing the toxic side effects.
Each new trial brought new insights, and the survival rates slowly increased. In the first trial described above, the survival curve dropped to zero after fourteen months. In the second trial, some children survived more than four years, but by five years all had died. In 1972, the third trial's survival curve reached a steady plateau: two per cent -- three of the 151 original patients -- had been cured! For the first time in history, the last leukaemic cell had died, leaving the victim to pursue a healthy, productive life. By the fifth trial, which included 265 patients, the cure rate approached twenty per cent.
Over the following decades, survival continued to inch its way upwards. Current regimens now deliver cure rates greater than eighty-five per cent. In addition, detrimental side effects have been greatly reduced, and paediatric oncologists all over the world continue their search for more effective treatments by enrolling many children with leukaemia in clinical trials.
The Risk of Victory
All therapies -- whether administered with a pill, needle, or knife -- pose some risk. Even the antibiotic penicillin, which has saved countless lives, causes a life-threatening reaction in one out of every 10,000 patients. During the early efforts to cure leukaemia, the only remedies were poisons with harrowing side effects, yet the pioneers forged ahead, risking the deaths of some while hoping to save others. They had the wisdom to search for a cure among the most toxic chemicals known to man.
There is no instance in the history of medicine when compassion has served a more noble purpose. But compassion alone was not enough. The doctors also had the clear-eyed, cold-blooded judgment to know that when benign remedies fail, a cruel disease demands an equally cruel treatment.










Comments
Add a comment