Good news for hayfever sufferers - by cracking the crystal structure of the histamine receptor, scientists are on the road to developing more effective antihistamine drugs to treat allergy and inflammation.
 Histamine is a molecule produced by special immune cells in response to certain foreign bodies and potentially dangerous pathogens. It has a variety of effects depending on which of the four types of histamine receptor it binds to, named H1 to H4.
Histamine is a molecule produced by special immune cells in response to certain foreign bodies and potentially dangerous pathogens. It has a variety of effects depending on which of the four types of histamine receptor it binds to, named H1 to H4.
Histamine signalling, particularly through the H1 receptor, is known to play a crucial role in a variety of allergic conditions, including hayfever, asthma, food allergies and the itchy response to insect bites.
Conventional antihistamines, such as cetirizine and acrivastine (that's Benedryl Once a Day and Benedryl Allergy Relief respectively), work by blocking the H1 receptor.
Using X-ray crystallography, Tatsuro Shimamura and colleagues unravelled the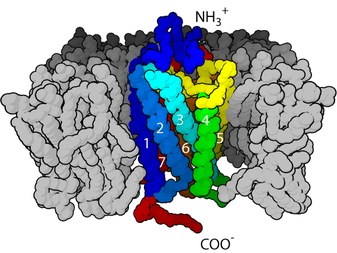 molecular structure of the H1 receptor, with a resolution of 3.1 Angstroms, or 0.00000031mm. So that's very fine detail!
molecular structure of the H1 receptor, with a resolution of 3.1 Angstroms, or 0.00000031mm. So that's very fine detail!
All four of the histamine receptors are part of a much larger family of 'G-protein coupled receptors', or GPCRs, which includes thousands of different proteins. Shimamura's group showed that the first generation antihistamine, Doxepin, interacts at a particular site in the H1 receptor that is found in most G-protein coupled receptors. This explains why doxepin was so non-selective, and hence why it had so many side-effects, including sedation, dry mouth and heart arrhythmias - because as well as blocking H1 receptors, it blocked many other GPCRs as well. In addition, doxepin could cross the blood-brain barrier and block signalling in the brain, adding to the sleepiness.
Second-generation antihistamines, like cetirizine and acrivastine, have far fewer side effects and are considered 'non-drowsy'. The X-ray crystallography suggests that this improvement is due to a second interaction with a different site on the H1 Receptor, in addition to the one that older antihistamines bind to. This second site is not found in other G-protein coupled receptors, which, combined with their reduced uptake across the blood-brain barrier, helps explain their greater selectivity and reduced side effects.
This detailed look at the H1 receptor and its interactions with antihistamines could help guide future drug discovery, so antihistamines used to treat allergies like hayfever may become more effective and with fewer side effects even than current medications.
And that work was published this week in the journal Nature.
Ben - So it's a bit like the old antihistamines where a sort of skeleton key that would fit all of these different receptors and it's only now that we really know the structure can we design exactly the right key that will only interact with the histamine receptors and that should enable us to block hay fever without all of these nasty, sleepy side effects.
Will - Absolutely. The old antihistamines just acted on so many different receptors and it went straight into the brain and so, it had a very blanketing effect on the whole of the brain.
- Previous TB Booster Better Off Alone
- Next Rocking to a Good Night's Sleep










Comments
Add a comment