For centuries, breeders have been selecting plants and animals with desirable genetic traits. So how have some of these changes come about, and where will new genetic technologies take our food in the future?
In this episode

01:09 - Jennie Pryce - Breeding a better cow
Jennie Pryce - Breeding a better cow
with Jennie Pryce, La Trobe university Australia
Kat - This month I'm continuing my reports from the Genetics Society Spring Meeting, Breeding for Bacon, Beer and Biofuels, held up at the Roslin Institute in Edinburgh - birthplace of Dolly the Sheep. As we heard in the previous episode, selective conventional breeding has wrought huge genetic changes in the plants and animals that end up on our plates today - and then there's the new frontiers of genetically modified, or GM, organisms as well as the latest precision gene editing technology. Jennie Pryce, from La Trobe University in Australia, is bridging the gap between breeders and geneticists, in order to breed better dairy cows. I started by asking her to explain what farmers are looking for in the perfect milker.
Jennie - Modern breeding objectives which are, I suppose, things that include all the traits that are important for productivity in farms, they'll include a diverse range of traits. So obviously, milk production is very important because that's what farmers are paid for. But equally, it's very important that the have cows that can get in calf - to produce milk has have to calf every year, so that's obviously a very important trait. They're also interested in breeding for disease resistance, so resistance to mastitis, lameness and so on, and also, increasingly, feed efficiency. So, it's really incorporating all the traits that contribute to profitability but also with a view on animal welfare and sustainability for the future.
Kat - You mentioned feed efficiency. I don't often think about cows as being efficient. So, what's that about?
Jennie - So actually, what we've seen over the last 50 years or so is a massive improvement in efficiency. So, a cow that was around in the 1950s would produce, say, about 1700 litres of milk and the modern dairy cow produces somewhere about 7,000 litres of milk. The weight of cows hasn't increased that much which shows us that the efficiency has improved phenomenally. So, we reckon that it's somewhere in the region of doubling efficiency just through the breeding objectives that I described earlier so, selection for production primarily.
Kat - So, these cows are better at turning the food they eat into milk coming out the other side.
Jennie - Exactly, yeah and that's what we've achieved simply through selecting for production. But there are other opportunities as well. What we haven't known in the past is how much cows actually eat and we're actually able to measure that now, but only in very small populations, so research populations.
Kat - How do you go about figuring out how much cows eat and then how to incorporate that into breeding better cows?
Jennie - So, we actually measure, we weigh, exactly how much a cow would eat in a day and that's not a trivial matter. What we have is pretty high tech equipment that we use to measure each individual meal. if you like, that a cow eats. So, a cow will have an electronic ID in her ear and when she goes into this specially designed equipment, it will record her identification as well as the amount of food that she eats. We can calculate the average that she eats over a day or over several months if we want to.
Kat - So, you can look at all these different cows, you can see how much they're eating, how much milk they're producing. How do you then tie that back into their genetics and which are genetically the best cows?
Jennie - So, this is the absolutely fantastic thing that we now have this technology called genomic selection where we can use many genetic markers on each individual cow or bull that we're interested in and we marry that with the data that we have on feed efficiency, or feed intake to put it simply. And we look for patterns in terms of associations between those genetic markers and feed efficiency. Together, that tells us how good a particular animal might be in terms of its genetic potential for feed efficiency. So, the great thing about it is that we can translate information that we have in a research population and apply it to animals that have genetic markers or genotypes but don't actually have a measure of feed intake. That's incredibly powerful because all of a sudden, you can have that information available to a very large population of cows or bulls.
Kat - So basically, what you're doing is you're measuring the cows in your research population saying, okay, they have gene markers A, B, C, and D, they're very efficient these ones, and then you can go and look at totally unrelated bulls - breeding bulls - and go, they've also got A, B, C, and D. These are going to be the ones you want if you want to turn less food into more milk.
Jennie - Exactly. The principle is right. But in fact, in dairy cattle populations, there are a lot of relationships between individuals. That actually works to our advantage. So, if you like, the problem that they have in human genetics is that they don't have those relationships between individuals which makes it harder to get those genetic predictions in dairy cattle. Because we've used a few bulls as the sires of the next generations, it means that we can make those relationships a lot more easily.
Kat - Why is it important to have efficient cattle? What benefit is that for farmers?
Jennie - It's obviously important because feed is the largest variable cost on most dairy farms. So, if they can cut their feed bill, it's got to be a good thing. If you actually ask farmers, it's one of the main priorities in terms of traits that they'd really like to breed for, feed efficiency.
Kat - At the beginning of your talk, you showed a picture of a cow from the 1950s and a cow from today. They looked very, very different and you'd think that's some kind of weird mutant cow that's gone on there. but this is all the changes that we've seen in our cattle. They've all just been done by selective breeding overtime. Have there been any traits introduced by this breeding that while they've been good for farming have been negative traits for the cattle?
Jennie - Yeah. There's a few examples actually. One of the most important is the relationship between production and fertility. Back in the 1950s, right through to the '80s, when we were selecting primarily on production, mainly because we didn't have the data on the other traits that there was actually a deterioration in fertility. It was through the '90s into the 2000s that we started to realise that fertility in modern dairy cows was deteriorating quite rapidly. We needed to put a spot to that. At that point, there was a massive effort from around the world to develop breeding values for fertility, mastitis resistance and so on, so that we could broaden breeding goals to include not just production but also those traits that we want to guard against getting worse.
Kat - There's been quite a lot of talk recently about using some of the new gene editing technology. Obviously, there's a lot of talk about that. Do you think that the future of farming as well as involving breeding might involve at some point, genetically modifying animals whether that's dairy or for food?
Jennie - From my perspective, I think that there's still a lot of scope with the tools that we have currently. So, genomic selection, just using the same sort of principles that we've used in the past, but with smarter tools to enable us to identify the best animals for certain situations. So right now, I don't see a major role of gene editing or genetic modification in most dairy systems, but who's to say what would happen in the future.
Kat - That was Jennie Price, from La Trobe University.
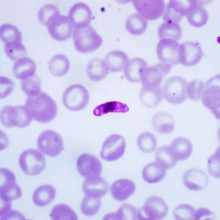
09:20 - David Baker - Viagra for malaria?
David Baker - Viagra for malaria?
with David Baker, LSHTM
Kat - Malaria kills hundreds of thousands of people every year worldwide, and the parasites that cause the disease are rapidly evolving resistance to all the treatments that we can throw at them. By studying the molecules that cause certain forms of malaria - known as gametocytes - to change shape, David Baker, from the London School of Hygiene and Tropical Medicine, may have found an unusual new approach for anti-malarial drugs - and it comes in the form of a well-known little blue pill.
David - In humans, the problems caused by malaria, all the symptoms and pathology are caused by quickly replicating farms and they invade red blood cells. As they grow over 48 hours, they rupture and burst out up to 20 or 30 new malaria parasites which invade red cells. So, you can imagine as those parasites expand, you become anaemic and that's often associated with severe malaria. So, those are one type of malarial parasite.
For reasons we don't understand very well, some of those malaria parasites when they invade red blood cells, turn into sexual forms - male and female gametocytes, we call them. We call them that because they're the precursors of gametes. Because when they get into the mosquito, the male and female fertilise to continue the cycle. So, those gametocytes, they don't divide in the human and they simply mature over about 2 weeks.
These sexual forms, these male and female malaria parasites, they get quite large and crescent-shaped and normally, if a red blood cell, it comes misshapen like that, it would be filtered out by our spleen in our body, aiming to hide in the body, stop being cleared by the immune system in the spleen. They do that when they're very young. They go into the bone marrow and hide.
It's only when their mature that they become flexible and they enter the circulation. Once they're flexible, they're safe from the spleen because they can squeeze through the capillaries in the spleen and circulate throughout the body. So, when the mosquito takes a blood meal, it will take up some of these gametocytes so that the gametes can fertilise inside the mosquito and continue the cycle.
And so, what we want to understand was exactly how it becomes flexible. So, we focused on a particular signalling molecule called cyclic AMP. We wanted to ask the question, does cyclic AMP make these sexual cells, these males and females become flexible to allow them to circulate in the bloodstream.
Kat - So, how did you go about investigating that? How does a molecule - cyclic AMP - how does that change the shape of these parasite cells?
David - That's a very good question and really, it's a next step for us to understand the mechanism how it happened. The first step was just to ask whether it happened or not. So, we used two ways, in the lab, to find out whether increased levels of cyclic AMP made these sexual cells become flexible. So, one approach we took in my lab, we knocked out a gene encoding an enzyme called a phosphodiesterase in the malaria parasite. Normally, phosphodiesterase enzymes degrade this cyclic AMP signal. So, when we deleted the gene, the levels of cyclic AMP went up as we would've predicted. So lo and behold, when we knocked out this gene, the gametocytes became less flexible.
Kat - So normally, low levels of cyclic AMP is keeping them bendy and when it rises up, they become stiff and rigid.
David - That's right. I mean, that was the problem. When we raised the cyclic AMP levels due to knocking out that gene, instead of becoming bendy, they became stiff essentially. And the other way we raised the cyclic AMP levels was to use small molecule inhibitors of phosphodiesterases. So, we used several and one of the ones we used was Viagra. Now, that's quite a well-known inhibitor phosphodiesterase that's used to treat male erectile dysfunction. And so here, we had a situation where increasing cyclic AMP levels with Viagra, we could make these sexual cells. Malaria parasites become stiff as well. In a human, if that happened, if those mature gametocytes became stiff, it will be filtered by the spleen and inactivated, and you wouldn't get any transmission.
Kat - I was there a little bit sniggering in the lab when you realised that that what was going on.
David - Yeah, absolutely. I'll always want to mention we're working with Viagra in the lab for people sort of have the odd snigger, so absolutely. Katherine came up with a good headline that it made gametocytes stiff, so yes.
Kat - That was David Baker, from the London School of Hygiene and Tropical Medicine.

14:07 - Alison Bentley - Redomesticating wheat
Alison Bentley - Redomesticating wheat
with Alison bentley, NIAB
Kat - Wheat is a hugely successful and important agricultural crop, originating from simple grass and grown in many parts of the world. But while its golden ears may seem as commonplace in the countryside as bread, pasta and other wheat products are in the kitchen, its genetics are very unusual. I spoke to Alison Bentley from NIAB - the National Institute of Agricultural Botany - in Cambridge to find out about the journey from grass to wheat, and how she's trying to recreate it.
Alison - So, the domestication and evolution of wheat started about 100,000 years ago in the Middle East when two grass species came together and created an intermediate wheat form and this later hybridised another time to produce what we know today as wheat and what we grow to produce bread and grain for animal feeds.
Kat - The wheat that we have today, that didn't just get there. it's been helped along the way by farmers going, "I like the look of that one, I like the look of that one."
Alison - Exactly. That plays a big role in it. So, these grasses are growing in fields in Turkey and areas in the Middle East and the early farmers were selecting the plants that had evolved through this coming together of multiple species and they were selected and moved forward and disseminated to different regions. That was really what drove this domestication and evolution of the wheat we know today.
Kat - Tell me a bit about the genetics of wheat. What's going on under the hood, because they are quite unusual the genetics of the wheat, the kind of wheat that are grown today in agriculture?
Alison - Yeah. I mean, when you drive past the wheat field, it just looks like a grass growing there. But underneath that is extremely complex genetics. So, it does still maintain the genomes of these three ancestral grass species which grew individually in fields in the Middle East. Those three genomes still exist and their huge genomes and they're very difficult to decode. So, we have a really big challenge in trying to understand the underlying genetics in wheat.
Kat - I heard they had about a hundred thousand genes something like that?
Alison - Something like that and there's a huge amount of repetitive DNA and all sorts of challenges in the way if you want to sequence any of that information.
Kat - In terms of the wheat crops that are grown maybe in Europe, in the US, in other parts around the world, how similar are they all?
Alison - So, they're fundamentally very similar. So, they vary just for a few adaptive characteristics that enable them to be grown across that range and that's really when wheat moved out of the Middle East. Those are the type of changes that allowed it to move out of those regions - so, into hotter, drier environments, to wetter, cooler environments. Those genes really play a big role in the spread and domestication of wheat throughout the world.
Kat - And then presumably, over the past couple of hundred years, as it became commercialised and farmed on a grander scale, fewer and fewer types got grown.
Alison - Exactly. So, a selective breeding has really narrowed that base so we're really making varieties then crossing those varieties to select new varieties. So, we're working on quite a small base of genetic diversity.
Kat - Tell me about the work that you're doing then to try and increase the genetic diversity in wheat.
Alison - So, that's exactly what we're trying to do. We're saying, this genetic basis is very low and that's a limitation to our ability to improve wheat in the future. We need to do that because the levels of yields are plateauing all throughout Europe. So, what we're doing is going back to the Middle East, collecting a wide range of accessions of the original grass species saying, "Can we recreate wheat as it was created in nature all those years ago? Can we do that?" by doing that, introduce new diversity that we can then exploit in breeding.
Kat - So, the grasses that are growing now, they've had thousands of years to accumulate new traits or maybe just combine in new and more interesting ways.
Alison - Exactly and I think the original hybridisation events of these are initial events that created wheat occurred in quite a small region whereas these grasses occur over really wide geographic range. So, you've got the Tibetan plateau, you've got inner Mongolia. So, lots of different environments. So, you can imagine in each of those regions, they've evolved to different circumstances, different nutrient availabilities, different temperature and climate. So, it's a really rich source of diversity for us to use.
Kat - One of the hot topics it's hard to avoid in science is climate change. In a lot of places where crops are grown, they may be getting drier or saltier. Do you hope that these new kind of reconstructed wheats might be better equipped to cope with these challenges?
Alison - Yeah, exactly and that's what we see whenever we look at these grass species and the material we derive from them. So, we have lines that perform, maintain their yield at lower input level so you can apply the same or lower amounts of inputs and that's a really topical thing in agriculture. You can apply less nitrogen and hold the yield. So, these are bringing in really new exciting diversity for those kind of traits which are very topical.
Kat - So, less fertiliser basically.
Alison - Exactly. So, if you could maintain the yields - so, even if you could only maintain current yields but apply half the nitrogen, that would represent a significant cost and environmental saving. So in this case, if we can do that and increase the yields then that's really - we're on a winner with that.
Kat - It's a lovely picture to think of you and your colleagues collecting these grass around the Middle East and crossing them in your lab. How do you turn that into the amount of seed you'd need to fill an entire field or whole country's fields?
Alison - Yes. So, there's definitely that issue of scaling and the time it takes to do that in wheat. So, we have what we call a pre-breeding pipeline where we move the material through from those initial crosses in the glasshouse, doing all that really fine scale work. and then we have the ability to scale that up fairly quickly to a material which we can disseminate to other people and that they can grow and assess as well.
Kat - In a recent podcast, we had a discussion about intellectual property, particularly around crops and agriculture and whether there should be patents and these kind of things on crops. Are there strains that you're coming up with the kind of things that could be patented, that companies could still control?
Alison - Not really. So, there's a treaty governing the use of wild relatives and they belong essentially to the country in which they were collected and they can't be directly commercialised on. The project that we work on funded by BBSRC, in that project, all the material we create is completely IP free. So, none of the data, none of the material is held or owned by anyone. So, we're freely distributing that material, really with the view that it can be used to improve UK wheat and wheat more widely. So, it's really what we call public good plant breeding. So, we are producing resources that are then freely available for people to use.
Kat - NIAB's Alison Bentley there.

21:27 - Helen Sang - Counting your GM chickens
Helen Sang - Counting your GM chickens
with Helen Sang, Roslin Institute
Kat - Every year roughly ten chickens are raised for every human on the planet, and these birds are a vital source of protein, not just for eggs but their meat too. Mmmm, wings... But avian flu - or bird flu - is a big problem in many regions, putting chickens, farmers, and the wider population at serious risk. Helen Sang, from the Roslin Institute in Edinburgh, has dedicated her career to developing genetically modified chickens, with the hope that they can be made resistant to avian flu.
Helen - So, we're really interested in disease resistance. So, there are all these billions of chickens and because we keep so many of them, there are a number of diseases that can wipe them out and one of those diseases is avian influenza or bird flu. I've been working for some years now with Lawrence Tiley at Cambridge University to try and generate genetically modified chickens that are resistant to bird flu.
Kat - How are you doing with that because obviously, bird flu doesn't affect just chickens? It's a serious problem in humans too, so it's very big concern.
Helen - Yes, it's a big concern and that's one of the reasons that it's our prime target - is that bird flu gives rise to human flu and causes a human pandemic. So, not only is it a real problem to the chickens who get sick and die, to the farmers who produce them because it causes huge losses, but it is also a potential source of human pandemic flu. So, if we could work out a way of genetically modifying chickens so they're resistant to bird flu, I think it would be really seriously considered as an application of GM in commercial chickens.
Kat - Tell me about how you go about that. How do you make a chicken that either can't get the flu or can't pass it on?
Helen - So, this is where Lawrence comes in because he has a lot of bright ideas because he's a virologist. He works on influenza virus. So, he's developing ways that you can express very small molecules in the chicken that will block the replication of the flu virus. So, when a chicken is infected by flu, it can't copy itself and shed more virus, which would infect more chickens or infect people. So, that's our aim - is to find neat ways where we can generate small molecules in the chicken that will be there when the chicken is infected with flu and will block the infection.
Kat - How is that work coming along so far?
Helen - Well, we've had some success. Our first work which was published a few years ago, we expressed a small molecule which seems to stop not the chickens getting infected -they do get infected - but it seems to stop them passing the flu virus on. We're now trying out sort of elaborations on that thing to see if we can make that response stronger so that the birds don't succumb to infection at all. We also want to understand more about the original experiments, why those birds don't pass on the disease.
Kat - We hear a lot about GM crops. Some people are very concerned about eating foods that may have been altered with GM technology. Do you think there's a big challenge if you say, "Okay, this is a genetically modified chicken." People might go, "Woah! No, I am not interested in eating that."
Helen - Yes, I think it's the response of some people but I give a lot of talks and we have an open day here at the Roslin Institute every year where we specifically talk about and present this. I think that if you give people a chance to understand why you're doing the experiments and what it is that you're doing, they can make up their own minds of whether they think it's of value or not. When I give talks, I quite often ask towards the end, "Would you eat a genetically modified chicken that was resistant bird flu?" if there was bird flu in this country - because you don't need it if you're not threatened by the disease. I usually get more than half of the audience - will say that they would consider this. So, I think if people have the opportunity to understand what you're doing and why you're doing it then they will make up their own minds rather than just seeing it as a blanket, "GM is bad. I won't have anything to do with it."
Kat - The techniques that you've been using to make chickens resistant to bird flu, they've involved adding genes in, adding molecules into the chickens. Tell me about some of the other approaches that you're trying.
Helen - So, there is a whole new technology coming along called genome editing where you can make very small changes to the genes in the chicken. So, you're not adding anything new and you can tweak the genes in the chicken to give them different characteristics. This is very new technology. So, we know it's likely that maybe one breed of chickens will be - not bird flu I don't think, but with some other diseases that affect chickens only, not humans as well - that they may have a copy of the gene that makes them resistant to infection with that disease. But commercial chickens don't have that copy of that gene. They have a different version and they're still susceptible to the disease. So, we're hoping that these new genome editing technologies will allow us to change the gene in the commercial chickens so that it's the resistant form that we've identified somewhere else. So, it allows us to move versions of genes around between different breeds of chickens without crossing lots of breeds together and losing the good characteristics that we want to keep.
Kat - What do you think the chicken of the future is going to be like? How have chickens changed over the past 50 years and what do you think the chicken will be like in the years to come?
Helen - Yes, the chicken has changed hugely in the last 50 years. But it's a very gradual change. The poultry breeding makes small changes and when you add them up over the years, it turns out it's a big change. The chicken of the future will maybe be more resistant to a lot of different diseases. I think that's going to be one of the big targets.
One of the things I'm interested in as well is, can we add in a new gene to the chicken that expresses enzymes that will breakup 'undigestible' feed. So, a lot of food that chickens eat are wheat and maize which are very valuable grains and that is an expense and competes with humans for those grains. But then there's a lot of waste in production of those grains which the chicken can't digest. If we could add in new enzymes into the chicken gut so they can breakdown these materials to release the energy components of those plant materials then maybe chickens will be able to eat lower quality foods and not be so expensive in terms of using high quality grains.
Kat - That was Helen Sang, from the Roslin Institute.

28:15 - Gene of the Month - Spaghetti
Gene of the Month - Spaghetti
with Kat Arney
And finally, it's our Gene of the Month, and this time it's Spaghetti, a fruit fly gene that may be the missing link between the bodyclock and neurodegenerative diseases such as Alzheimer's. The Spaghetti gene helps to control another gene known as Doubletime, which is involved in controlling the length of a fly's daily circadian rhythm, or body clock. If Spaghetti is missing, levels of Doubletime fall and the fly's natural daily cycle becomes longer. Intriguingly, this also leads to the breakdown of a molecule called Tau, which is associated with neurodegeneration and brain problems similar to dementia in humans.
Although there's a lot more work to be done to understand the complex clockwork of how circadian rhythms might be linked to dementia, Spaghetti provides an important clue to help untangle the problem.










Comments
Add a comment