Epigenetics controls the activity of genes inside cells and holds the key to new treatments for old diseases. We explore the impacts of epigenetics on embryonic development, cancer, and stem cell biology, and find out how epigenetic changes during pregnancy can even affect your grandchildren! Plus, why parenthood extends your lifespan, and the genetic recipe for the red blood cell.
In this episode
01:05 - Exploring the Exciting field of Epigenetics
Exploring the Exciting field of Epigenetics
with Stefanie Seisenberger, The Babraham Institute, Cambridge
Chris - In the 1950s, James Watson and Francis Crick, working at Cambridge University, discovered the structure of DNA. Made up of just 4 different bases or letters - A, C, T, G - which are linked together into long molecules that spell out genetic words or genes, DNA functions a bit like a recipe book which tells you to cook up the chemicals that they need to function. But different sorts of cells need to use different genes, or recipes, to do their different jobs and how this is controlled is partly down to a process called epigenetics.
Chris - Joining us to explain how this works is Stefanie Seisenberger from the Babraham Institute in Cambridge. Hello, Stefanie.
Stefanie - Hello.
Chris - So, how does all this work then?
Stefanie - As you said, different cells in your body need to take on completely different functions and this is all down to which genes are active or inactive in each individual cell. So, each gene has a molecular switch which can turn the gene on or off and it's those switches that we study in epigenetics.
Chris - When you say 'switches', how do they work? What sorts of switches are we talking about here?
Stefanie - One of the hallmarks of epigenetics is DNA methylation. They're small chemical groups that are attached directly to the DNA and they're usually associated with turning a gene off. But there are also proteins called histones that DNA is wrapped around. We know that DNA is wrapped and packed very tightly in a cell. Depending on which modifications are attached onto the histones, DNA can be wrapped more tightly or less tightly and depending on that, a gene can be kept active or inactive.
Chris - So, is this sort of 2-dimensional control here, isn't there? So we've got things that can stick onto the DNA physically and then things that will stick onto the proteins that DNAs wound around, and this affects the shape of the DNA. And therefore, whether or not, it gets read.
Stefanie - You have several layers or several ways of affecting gene activity by the DNA sequence itself or by the proteins that DNA is wrapped around.
Chris - How are these markers or these chemical groups added either to the DNA or to those proteins in the first place?
Stefanie - There are specific proteins that do their jobs. So there are enzymes called DNA methyl transferase that attach to methyl groups to the DNA directly, but there are also proteins that modify their histones. So again, you have several signalling layers that can be accessed.
Chris - So they're like miniature machines I suppose, they go along wondering along and adding these chemicals onto the DNA to open it up or close it off.
Stefanie - Exactly, yes.
Chris - When we talk about a cell having a certain  epigenetic profile, a cell that does a certain job say, a skin cell compared with a gut cell or something, if I compared the profile of those markers in the two cell types they would look different between the two types of cell.
epigenetic profile, a cell that does a certain job say, a skin cell compared with a gut cell or something, if I compared the profile of those markers in the two cell types they would look different between the two types of cell.
Stefanie - Definitely different, exactly. That's what we call cellular identity - each cell has a certain job or does a specific function and each cell has a specific epigenetic profile that mirrors that function, and that's basically how a cell knows what it is and what it has to do.
Chris - So, how does that epigenetic profile get set in my gut cells? How does it know to put those markers on the right places in that cell and a skin cell to do the equivalent?
Stefanie - So, that would probably start very early on already in embryonic development. So you start off with one cell basically...
Chris - That's a fertilised egg.
 Stefanie - Exactly and then that develops further into a structure called the blastocyst. And here, you have embryonic stem cells, and they can turn into any cell type in the body. These develop further and get pushed into what we call a certain cellular lineage or cellular identity. So you get certain methylation marks or other marks that contribute to cells taking on a certain function, and that can later on in life then be further modified.
Stefanie - Exactly and then that develops further into a structure called the blastocyst. And here, you have embryonic stem cells, and they can turn into any cell type in the body. These develop further and get pushed into what we call a certain cellular lineage or cellular identity. So you get certain methylation marks or other marks that contribute to cells taking on a certain function, and that can later on in life then be further modified.
Chris - So, as the embryo subdivides into these different lineages or cells that are going to produce different tissues, the cells then add these markers as they go through. So, as they become more and more specialist.
Stefanie - Exactly, so that cell fate is already decided in the embryo and then you just add onto that and get more and more specialised cells at the end.
Chris - So, looking at one very particular and important tissue which is the tissue that makes our cells that are going to enable to reproduce - in a case of a man, that's the testes, and in the case of a woman, the developing ovary to make all of her future eggs. They've got to produce a cell, sperm or ova, in which these epigenetic tags have been wiped away.
Stefanie - Yes, so it happens even earlier than that.  So, quite early in the developing embryo, you already set aside the cells that will later on become sperm or egg. And in these particular cells, what happens is that epigenetic information gets reset completely on a global scale. So they sort of wipe out all methylation marks and also the histone marks become reset.
So, quite early in the developing embryo, you already set aside the cells that will later on become sperm or egg. And in these particular cells, what happens is that epigenetic information gets reset completely on a global scale. So they sort of wipe out all methylation marks and also the histone marks become reset.
So you know how environmental influences can affect not only the mother, but also the developing child within her, but as we now know, this can also affect the cells within the developing child that will later on become the grandchildren or contribute to their grandchildren. So, should there be any environmental influence that have put on epigenetic marks in a wrong way, that can be wiped out and you provide a clean slate for the third generation.
Chris - But conversely, when a woman is pregnant with say, a daughter, a developing daughter inside her, then that daughter is going to have her own eggs being incorporated into her ovary. So, at one point, there is three generations there. There's the woman herself who's pregnant then the baby inside her, and that baby's babies.
Stefanie - Yeah, exactly. It's three generations. So, it's not only environment influences of the pregnant mother not only affect the child, but also, the grandchildren.
Chris - Do we know whether that wiping out is genuinely comprehensive at that stage or is it possible that a growing baby is being exposed to various factors from the mother's environment and that they can set genes for the life of that baby and potentially the life of the baby's offspring?
Stefanie - We don't understand exactly how, but we know it does happen. So there are multiple examples, also in history where things like that happened, but we're just starting to understand how this whole process works and how things can escape to solve the reprogramming process.
Chris - I've got a question here from Emily Seward who has just sent this in and said, "Epigenetic changes and preferences..." she's asking about why you like things and why you don't. "could it be that when you like something when you're older, but don't like it when you're younger, could some of these preferences be - and that's obviously a slightly simplified thing, but could it be that part of our changing behaviour or the way we age could be down to epigenetic programming?"
Stefanie - Yeah, so epigenetics is a very important factor in ageing and that's something that's become more and more clear in recent years. Specific traits or likes or dislikes, I don't know how much that is down to epigenetics, but I wouldn't be surprised if that is the case.
Chris - And what about reversing this because Teo Gibson has got in touch and said, "Is there any research into undoing some of the stuff that's been done?" and you were mentioning about how a baby inside a mother who's pregnant has the future babies inside that. He says here, "If our parent's lifestyles before we were conceived can determine how much of our DNA expresses itself, is there any research to potentially undoing things like predisposition to obesity, diabetes, and addiction?"
Stefanie - Undoing things, I am not sure about, but simply by having a healthy lifestyle, you can take preventative measures. And simply, by being aware that what you do, what you eat, what you expose yourself to, will affect future generations, can maybe raise awareness and help preventing damage.
Chris - Stefanie, thank you very much. Stefanie Seisenberger from the Babraham Institute.
Does epigenetics dictate our preferences?
Stefanie - Yeah, so epigenetics is a very important factor in ageing and that's something that's become more and more clear in recent years. Specific traits or likes or dislikes, I don't know how much that is down to epigenetics, but I wouldn't be surprised if that is the case.
Can we alter our epigenome?
Stefanie - Undoing things, I am not sure about, but simply by having a healthy lifestyle, you can take preventative measures. Simply by being aware that what you do, what you eat, what you expose yourself to, will affect future generations, can maybe raise awareness and help preventing damage.
09:02 - Epigenetics and Cancer
Epigenetics and Cancer
with Mark Dawson, Cambridge University
Apart from enabling us to understand how cells function and develop normally, epigenetics can also give us some new treatments for a range of diseases including cancers and Haematologist Dr. Mark Dawson from Cambridge University works on this very question...
Chris - I presume your case Mark, it's chiefly going to be diseases or cancers of the blood, leukaemia, is that you're interested in?
Mark: - That's right. I spent part of my time at Addenbrooke's Hospital caring for patients with various types of blood cancers including leukaemia as you just said.
Chris: - Which type? You've done some pretty instrumental studies looking at the role of epigenetics in these leukaemias. Which ones were you looking at in particular because they're quite a big family of diseases?
Mark: - Broadly speaking, my research interest is about  the acute leukaemias. So, these leukaemias are cancers of white blood cells, and somewhere between 5 to 8-10 people per 100,000 of the population develop an acute leukaemia.
the acute leukaemias. So, these leukaemias are cancers of white blood cells, and somewhere between 5 to 8-10 people per 100,000 of the population develop an acute leukaemia.
What's sad about this disease is that despite progress in modern medicine and supportive care, we've made very little progress in trying to cure the vast majority of these patients. In our current therapy, only less than 30% of these patients actually get cured.
Chris: - When we look back over the history of treating leukaemias though, once upon a time, we just knew people had some kind of disease that made them have enormous numbers of the wrong sort of white blood cells in their blood, and we knew that they got sick and we knew that giving them drugs that were horrendous but killed off those cells since to make them live longer. Now, we understand quite a bit about what's going on genetically about these disorders.
Mark: - That's right. So now, if you take acute leukaemia for instance, we know in over half of these patients what the initiating event is. So, this is a genetic abnormality that is called a chromosomal translocation. And what happens here is that part of a chromosome breaks off and fuses abnormally to a completely different chromosome. What this results in is the fusion of genes from two different chromosomes together.
Chris: - So if I had say, chromosome number 1 and a chunk of fell off there, it could take itself to chromosome number 2 and stick itself on instead of the equivalent chunk of chromosome number 2. Where the two joined, I've now got two sections of genetic material linked together, and I've effectively made a new gene by linking that chunk of chromosome to the other chromosome.
Mark: - That's exactly right. These are called fusion genes and they're only really present in the cancerous cells. So, none of our normal cells whilst they have two original copies of what these genes are. They don't have the fusion together.
Chris: - And what do those fusion genes do?
Mark: - The vast majority of them serve to initiate or drive the process of leukaemia and the fusions I've been studying more recently are called the MLL fusions. So here, one part of this fusion is a gene is called the MLL gene and this produces a protein related to the proteins that you and Stefanie have just been talking about, an epigenetic regulator. And what it does is it binds very specifically to a small set of genes and it modifies them by laying down a chemical group on the histone protein surrounding these genes. And what this modification does is really prepare this gene to be turned on. So the MLL gene is involved in the initiation of gene expression.
Chris: - So, there's a normal gene there whose job it is to go to a certain part of the DNA of a cell and say, "Right, you are going to turn on and your gene is going to be expressed." So when we then take that gene and fuse it to another gene, making this funny fusion, it retains the ability to address itself to different bits of the genome, does it?
Mark: - Exactly. Its targeting potential is still preserved.
Chris: - What else happens beside that?
Mark: - So, what happens is what we've realised more recently is many of the fusions that the MLL genes stuck towards - the other genes - are also genes that code for proteins that are involved in the process of gene expression. But these proteins really continue the process of gene expression and are involved in the completion of gene expression. So, what these fusions eventually do is they form this sort of turbo charged driver of gene expression. It increases the expression of several of the MLL target genes, but also increases the expression of genes that should normally be turned off at this stage.
Chris: - So, you end up with this fusion protein, taking a very potent turn on signal for genes to lots of different places in the genome that wouldn't normally get turned on like that.
Mark: - Correct.
Chris: - And that starts the cells dividing and growing in this abnormal cancerous way.
Mark: - That's right. Many of these genes that are turned on in this way actually confer upon these cancerous cells a survival and growth advantage, and that's eventually what leads to the process of leukaemia.
Chris: - Begging the question, now you know that, could you intervene and interrupt the ability of that funny protein to address itself to all these different places in the genome and turn it off?
Mark: - Yes, so that's exactly what we've been studying. We've been trying to understand. What's clear is that these fusion proteins don't work in isolation. They work with a number of collaborators. Other proteins, they perform as what we call a protein complex and each component of this protein complex plays an important role in the regulation of this gene expression.
We looked very deeply to understand what are the components of the MLL fusion protein complex and we found that there are part of this complex that contain a group of proteins whose job it is to anchor the MLL fusions at these specific genes. They do so by using a special binding module that recognises a chemical group on the histone proteins. So, binds this and locks on there and keeps the whole protein complex there.
Chris: - So, you've understood what the molecular Velcro is that sticks this strong expresser of genes to the wrong bits of the genome. So have you got some way of interrupting that process?
Mark - That's right. So in collaboration with GSK, a pharmaceutical company, we've developed a molecular decoy so to speak. This small molecule largely mimics the chemical modification that we see on the histones. And so, this works as a decoy to draw away the protein complex from being locked on to the genes to away from the genes, and then now, the genes can actually be turned off.
Chris - Was this in mice or people?
Mark - So, we use this in a number of different studies, a number of laboratory models of leukaemia, including studying cells and how they grow in a dish, what genes they turn on and turn off, and how this small molecule turns these genes off. We also were able to show very successfully in models of leukaemia, mouse models of leukaemia, that this small molecule really confers an impressive therapeutic advantage for these mice.
Chris - Are you doing this in humans now?
Mark - So, based on our study and other studies from around the world, phase I clinical trials with this compound in patients with cancer have already started in the US, and we're hoping that this will follow on and be rolled out to the UK, where some of our patients can be involved.
Chris - I suppose it's relevant with David Cameron saying he wants everyone to be in a human DNA database across the country and marry that data to our medical records. But this, I suppose we should emphasise is one particular kind of leukaemia, and you've had to do this sort of DNA detective story to work out how this disease occurs. We couldn't just assume that the same compound is going to work in a range of other diseases because they're going to have a different process, aren't they?
Mark - That's exactly right and we know that is true for this compound. It works in a very specific subset of cancers, leukaemia being one of those cancers, but it's not a panacea for cancer. So, it's not likely that this will be what we've been looking for to cure all cancers because different cancers are driven by very different genetic events.
Chris - Mark, thank you. Mark Dawson from Cambridge University.
Can we reduce cancer risk with epigenetics?
Mark - If I understand the question correctly, is there ways and means by which we can modulate whether a gene turns on or off at a particular time? In theory, this is possible because the epigenome or these various modifications that control whether a gene gets turned on or off as you've been talking about are reversible. These are not static entities, so in theory, we may be able to fine tune in the future. We're not there yet, but we may be able to do this in the future.

18:08 - Is having children good for you?
Is having children good for you?
This is a difficult area to research, because you can't conduct a randomised trial like you normally would to test a hypothesis. But a new study gets to the bottom of this tricky question. The verdict? Having children seems to lower your chance of having a long life!.
What scientists would like to do is take a group of people, and randomly assign  some of them to have children, and others not to, but this isn't possible, or ethical.
some of them to have children, and others not to, but this isn't possible, or ethical.
Prof Agerbo and colleagues from Aarhus University Denmark attempted to get around this problem by using a natural experiment. They followed couples undergoing IVF treatment, and examined the differences between those for whom the treatment was successful, and those for whom it wasn't.
Why is this an improvement on previous studies? Previously, couples who are childless out of choice and those who are unable to have children have been put in the same category- this study avoided this, as all childless couples had been through the IVF process, so were not childless by choice.
They also looked at those parents who adopted children, and compared them to childless couples, and those who had a biological child.
Criticisms of previous studies have suggested that the differences found may be due to the fact that not being able to have children is an indicator of poor health.
In this study, none of the participants, even those who ended up with children, had been able to conceive naturally, reducing the risk of this being an important factor in the findings.
So what did they find? They followed the parents for between 3 and 14 years after starting IVF, and found that childless women had a rate of death 4 times higher than those with a child, whether it was biological or adopted. Men with children also benefited, being half as likely to die as their childless counterparts.
The team also looked at instances of Psychiatric illness, and found that the rate amongst those with biological children did not differ to that of the childless couples (apart from in the case of substance abuse, which was lower).
This goes against previous, less well controlled studies, which found higher rates amongst childless couples, but this can be explained if having an illness makes people less likely to have children.
Interestingly, parents who adopted children had a lower rate of psychiatric illness that either of the other groups. This may be because the rigorous selection criteria rules out any prospective adoptive parents who have an underlying, undiagnosed, psychiatric illness.
This study does have its limitations- the authors admit that income, education and age may have confounding roles, however it provides an intriguing glimpse into the benefits of child-rearing.. you may think they are driving you into an early grave, but your kids might actually be helping you to live a long and healthy life!
24:45 - Touchscreen technology used to help understand brain evolution
Touchscreen technology used to help understand brain evolution
A study using touch screen technology has shed light on how we evolved to be so intelligent, and how this development may have made us susceptible to mental illness.
Humans have been successful because of our ability to solve problems, and think flexibly about the world around us. It seems, however, that some of the genes that allow us to do so are much older than our species- they date back to when we shared a common ancestor with mice.
A multi-disciplinary study by researchers from 8 different institutes, led by Bussey and Grant, compared human and mouse intelligence, using touch screen tasks which can be adapted to work for both humans and mice.
 They compared mice with mutations to different genes within the same family, which are involved in creating signalling molecules found in the brain. Invertebrates, which (with a few exceptions) are thought to be less intelligent than vertebrates, have only one of these genes. 550 million years ago, however, the vertebrate genome duplicated itself not once, but twice, and most vertebrates have retained several versions of this gene.
They compared mice with mutations to different genes within the same family, which are involved in creating signalling molecules found in the brain. Invertebrates, which (with a few exceptions) are thought to be less intelligent than vertebrates, have only one of these genes. 550 million years ago, however, the vertebrate genome duplicated itself not once, but twice, and most vertebrates have retained several versions of this gene.
They found that mice missing one of these genes could not complete even simple learning tasks. 2 of the others had a, interacting pattern, affecting different, more complicated learning and attention tasks. It seems that these 2 genes work in opposition to each other, to control higher level cognitive abilities.
Humans and mice diverged from their common ancestor 100 million years ago, yet it seems that some of the genes which allow us to learn about the world are the same as those found in mice. Humans with the equivalent of one of the mutations exist, and when tested on similar tasks, showed a corresponding pattern of ability. These individuals also seem to be at higher risk of developing schizophrenia, and the pattern of cognitive deficits seen in the mice matches that seen in sufferers of the illness.
The other mutation is interesting, as it seems that the mice without this gene performed better than normal mice, with very high visual discrimination and attention abilities. It seems odd that a gene would have evolved to make us worse at these tasks. However, autistic people are also very good at these types of task, so it may be that this gene keeps these abilities under control to enhance other abilities.
The whole genome duplication that permitted the development of these genes may have allowed us to think more flexibly, and to survive better in a complicated and changing world. However, it seems that susceptibility to mental illness may be the price we have to pay for this intelligence.

28:31 - eLife: A New Open Access Science Journal
eLife: A New Open Access Science Journal
with Mark Patterson, eLife
A new scientific journal is being launched this week in Cambridge. It aims to be distinct and different from existing journals, so we invited the executive Managing Editor, Dr Mark Patterson, to tell us how...
Robert Tjian, Howard Hughes Medical Institute - We do need an alternative to the pre-existing process of publication that every scientists has to undergo and allowing the process to occur in a way that's much more efficient and serves a purpose of getting the most up-to-date high quality scientific information in the hands of the scientific community is really the goal we should go for.
Herbert Jackle, Max Planck Institute - We did a new journal because the journals which are on the market don't have the mechanisms to select the best possible science.
Mark Wolpert, Wellcome Trust - This will be a journal for scientists edited by scientists. So scientists will be at the heart of the decision making process. They'll be the best peer reviewers and then scientific editors will make the decision. So, it will be a journal for peers by peers.
Robert Tjian - I certainly hope that a defining feature of this journal would be rapid, transparent, scientifically based editorial decisions, one in which we don't at all sacrifice the quality of science, but we make the process much, much more efficient.
Ginny - The journal is called eLife. The Managing Editor is Mark Patterson. So, rapid, efficient, transparent publication uncompromising on quality. It sounds great what we've just heard on that soundtrack, but how are you going to do this better than a normal journal?
Mark P. - One of the initial things that we're going to do, you heard a lot of emphasis on quality. So, one of the things that eLife is going to do is to be a great journal for publishing very influential and important science across all of biology and medicine, and make that work openly available.
Anyone with an interest in that work can read it and they can do whatever they want with it, which is really important because most of the science that you hear about in programmes like this or read about in the media, maybe 90% of that, you have to pay to read.
The first step really is to make research openly available. So that's the first thing that eLife is going to do differently, but you also heard other themes in those talks there, and they were from the people behind the project, the funders behind the project.
One of the other themes was that the journal should be run by scientists for scientists and so, another thing that's different about eLife is that we have a group of 200 scientists who are committed to a different way of reviewing the work, taking work through peer review, so it's more efficient and more rapid, and more constructive than you see in a conventional journal.
With just one example of something that we're doing differently is that when the reviews, the review comments, are sent to the authors, what happens is the authors don't actually see the full reports. Instead, what happens is the editor who's handling that manuscript assimilates and consolidates the comments of the reviewers, after a discussion amongst the reviewers, so that the authors just receive a single set of instructions, and they know exactly what they need to do in order to get the work revised and then published. And that makes the whole thing happen much more quickly. So, those were a couple of ways for which eLife will be different.
Ginny - That sounds great, but if it's so good, why hasn't it been done already?
Mark P. - Yeah, that's a great question. There are some pretty powerful forces which keep the system of journals and the way the articles are published in journals, operating in the way that it has done for decades or even centuries, and I think there are probably two main forces at the moment.
One is the fact that the publishers that publish subscription base journals make an awful lot of money out of it. So, there's the strong commercial incentive to keep things as they are.
On the other hand, the scientists who publish their work, they have to publish in journals with an established reputation. And if most of those journals are subscription based journals, then you've got a kind of a cycle that reinforces itself.
Now having said that, there are similarly powerful forces of change as well, beginning to operate. eLife will provide some real momentum towards open access by providing highly prestigious journal home for really great science, and make that science openly available to everybody.
But there are many other publishers now, new publishers now like the (Public Library of Science) that is showing how this kind of way of publishing can work successfully. So, we've come so far, we've got to about 10 to 15% of the literature is now available to everyone. There's a very powerful momentum that will take us further, but there's still quite a lot of work to do.
Ginny - So, most journals, they make their money through subscriptions. People pay to read them. If you're not making money via that way, how are you doing this? Who's funding it?
Mark P. - The three voices that you heard at the beginning in that segment there were from three funders. And so, eLife is supported by three of the most prestigious funders in the world of research, the Wellcome-Trust in the UK, the Howard Hughes Medical Institute in the United States, and the Max Planck Society in Germany.
In our case, we're funded and our job is to respond to the kind of vision of the funders behind the project, and launch the best possible journal we can.
Other publishers are showing through other business models, and other approaches, how this kind of approach to open access publishing can operate in a sustainable way through other business models.
Ginny - And just one final question, how are you going to make this appealing to the scientists, who at the moment want to get published in Nature, or one of the really prestigious journals? How are you going to make sure that your journal is just as appealing as those?
Mark P. - Well, it's another very important question. I think the things that we have going for us are, that we have - as I mentioned, these three very prestigious funders who are behind the project, so I think that lends a huge amount of credibility to the scientific community who are considering submitting to the journal. We also have a terrific group of 200 scientists who are responsible for running the journal.
I think those two things will really help us to attract great work and we have already started publishing work now, and we are in fact, receiving some really terrific science.
I think the other thing that we have to offer that's very important is just the speed of the process. Especially for people we are early in their careers; they cannot afford to wait around for months and months to get their work published. And it's not uncommon actually that people can wait - you know, spend more than a year to get a great piece of science published. And so, you know, that's another reason why I think eLife can offer something very special to scientists.

35:19 - Urban Flooding - Planet Earth Online
Urban Flooding - Planet Earth Online
with Rachel Dearden, British Geological Survey
Britain's continuing wet weather has highlighted a serious problem facing towns and cities - urban flooding. Rather than soaking through the soil, water in built up areas is blocked by concrete, tarmac and tile and can overwhelm the drains and flood.
But there is an alternative, at least in some areas - SUDS or Sustainable Drainage Systems, with some neat ideas for urban planners.
Planet Earth Podcast presenter Richard Hollingham has been speaking to hydrogeologist Rachel Dearden at the British Geological Survey in Keyworth near Nottingham...
Rachel - SUDS try to mimic the natural conditions that you would get in a hydrological system, so we're trying to store that water in the catchment instead of allowing it to flow quickly downhill. So there are a few ways we can do this. The most natural scenario is that water infiltrates straight into the ground. Where this can't happen, because the ground is not permeable enough, naturally that water would collect in ponds, in depressions and slowly that would then either infiltrate into the ground or it would flow in water courses through the catchments, but importantly we don't get these really intense flows usually in natural environment - this is really only an urban phenomena.
Richard - And one of the ideas is the idea of permeable pavements, of having a hard surface but that the water can flow through.
 Rachel - Exactly. Trying to emulate what would happen naturally. So if we have an area where we want to have a hard surface then we can just make sure there are avenues for the water to dissipate through the surface into the ground. So, importantly, we need to think about what the properties of the ground are and what sort of systems we can design to be compatible with those properties. So, for example, just to the west of Nottinghamshire we have a sandstone bedrock, it's very permeable, and we can quite happily concentrate our water flow into a relatively small area, for example, into a soak away which is just a pit in the ground. Then that water can dissipate quite happily into the aquifer. Conversely to the east of Nottingham we have the Mercia Mudstone Group which we're actually standing on now and this comprises of clay - it's really quite impermeable and as we can see now and if we step around-
Rachel - Exactly. Trying to emulate what would happen naturally. So if we have an area where we want to have a hard surface then we can just make sure there are avenues for the water to dissipate through the surface into the ground. So, importantly, we need to think about what the properties of the ground are and what sort of systems we can design to be compatible with those properties. So, for example, just to the west of Nottinghamshire we have a sandstone bedrock, it's very permeable, and we can quite happily concentrate our water flow into a relatively small area, for example, into a soak away which is just a pit in the ground. Then that water can dissipate quite happily into the aquifer. Conversely to the east of Nottingham we have the Mercia Mudstone Group which we're actually standing on now and this comprises of clay - it's really quite impermeable and as we can see now and if we step around-
Richard - We're on a bit of grass here and it's just muddy and horrible.
Rachel - It's quite muddy and horrible and in here you would try pretty hard to focus any recharge, any rainwater, into the ground and in a place like this maybe you can install an infiltration basin that actually provides storage on the surface and therefore allowing that water to infiltrate very slowly, but actually what we choose here when we designed our new buildings - behind us is a rainwater harvesting system. So rainwater is harvested on our roofs and it is used to flush our toilets and this is a good example of a sustainable drainage system which doesn't involve the ground so we can install sustainable drainage systems absolutely anywhere. It's just that in some places we can infiltrate to the ground and in other places we really should focus on either storing water on the surface or re-using it.
Richard - Could you retrofit these sorts of systems?
Rachel - Certainly retrofitting is a big area of interest. In cities, when we regenerate areas, can we possibly put in sustainable drainage systems and there's a real space issue here. So trying to find space for an infiltration basin is really quite difficult in urban areas. But, still, we can do things like put in permeable pavements, for example, instead of hard road surfaces, rainwater harvesting, there's always options.
Richard - And you've actually put together a map of where this would work in the UK and where it wouldn't work in the UK?
Rachel - Not exactly, but almost. We've created what we call an infiltration SUDS map and this map shows you what the properties of the ground are. So we cannot say "here you can install a soak away" or "here you can install an infiltration basin" because it very much depends on the design of the actual system. So, what is the surface area of the system, what is its volume. But we can tell you have permeable the ground is, whether you're on a flood plain and whether the ground water is likely to be very shallow, whether if you put water in the ground it is going to cause the ground stability problem or you could impact ground water quality. And so the map gives you data that tells you all about these considerations so that you can then go away and make a decision about what sort of system might be appropriate.
Richard - But are there imperatives for people to do this, for builders, for planners, for architects, engineers to take these sorts of things into account?
Rachel - So the case with retrofitting is less clear but certainly for new builds there's a new legislation called 'The Floods and Water Management Act' and this requires that developers must consider using sustainable drainage instead of connecting to the drainage network. This legislation hasn't been implemented yet but when it has been it will mean that developers must prioritise the use of infiltration to the ground, so they must consider the properties of the ground and they must consider using - infiltration is the most natural and sustainable drainage system. If that's not possible because of the properties of the ground they then must consider storing water on the surface in infiltration basins, for example, and if that's not possible then they may consider putting water into the drainage network. But the key thing is the right for them to connect to the drainage network is not necessarily going to be there in the future and they are going to have to think about other ways to solve this problem.
Can a baby be born with leukaemia?
Mark D. - Yes, so what we now know is that many of the infantile leukaemias actually had their origins in utero. So, when we go and have a look at whenever any baby is born - and you would've seen this with your own kids - you have a heel prick test and this is taken away to be tested for a number of common genetic diseases. When you go back and have a look at these samples [from a leukaemia patient], for instance these chromosomal translocations, these abnormal fusion genes that drive leukaemia, are they present when the baby is actually born? In a number of cases, the answer is yes. So, this abnormal genetic event is not inherited, but for some reason, happens while the baby is in utero.
41:54 - Examining Epigenetics in Induced Stem Cells
Examining Epigenetics in Induced Stem Cells
with Ryan Lister, University of Western Australia (UWA)
Epigenetics is one of the ways in which genes can be turned on or off in different cell types. And if a cell divides, so it's split into two daughter cells, this also inherits the same epigenetic programme. So, what happens when we used specialised mature adult cells like a skin cell to make adult stem cells that we can then for instance turn into brain cells or heart cells to treat diseases? Are the epigenetic markers alreset correctly in the process? Well, it's looking like the answer could be no. From the University of Western Australia, Ryan Lister.
Ryan - When people started making these adult stem cells which are able to turn into many different types of cells, the essential question was, do these adult stem cells look like embryonic stem cells which you can then turn into many different types of cells?
This is an important question because we want to know when you start with these adult stem cells, do they start from this clean slate where you're then able to then turn them into any differentiated cell type that you wish? Or they start with any residual marks of a hangover of originally being a different cell in the body?
So, what we actually did was to profile the genomes of these induced pluripotent stem cells, they're called adult stem cells and look at exactly where the methylation marks, it's chemical tags were throughout the 3 billion bases of the human genome and compared this to embryonic stem cells to see if there were any differences between the adult stem cells and the embryonic stem cells.
It turns out that there are hundreds of differences in these chemical tags, its epigenetic tags, between the adult stem cells and the embryonic stem cells. What happens is that you have some memory of the adult cell type, the adult stem cell that you've derived from say, a skin cell in a couple of hundred places in the genome has these chemical tags which still look like the tags as you would find them in the skin cell, and look different, the pattern of these tags that you would find in these stem cells.
Chris - What do you think the consequence of that could 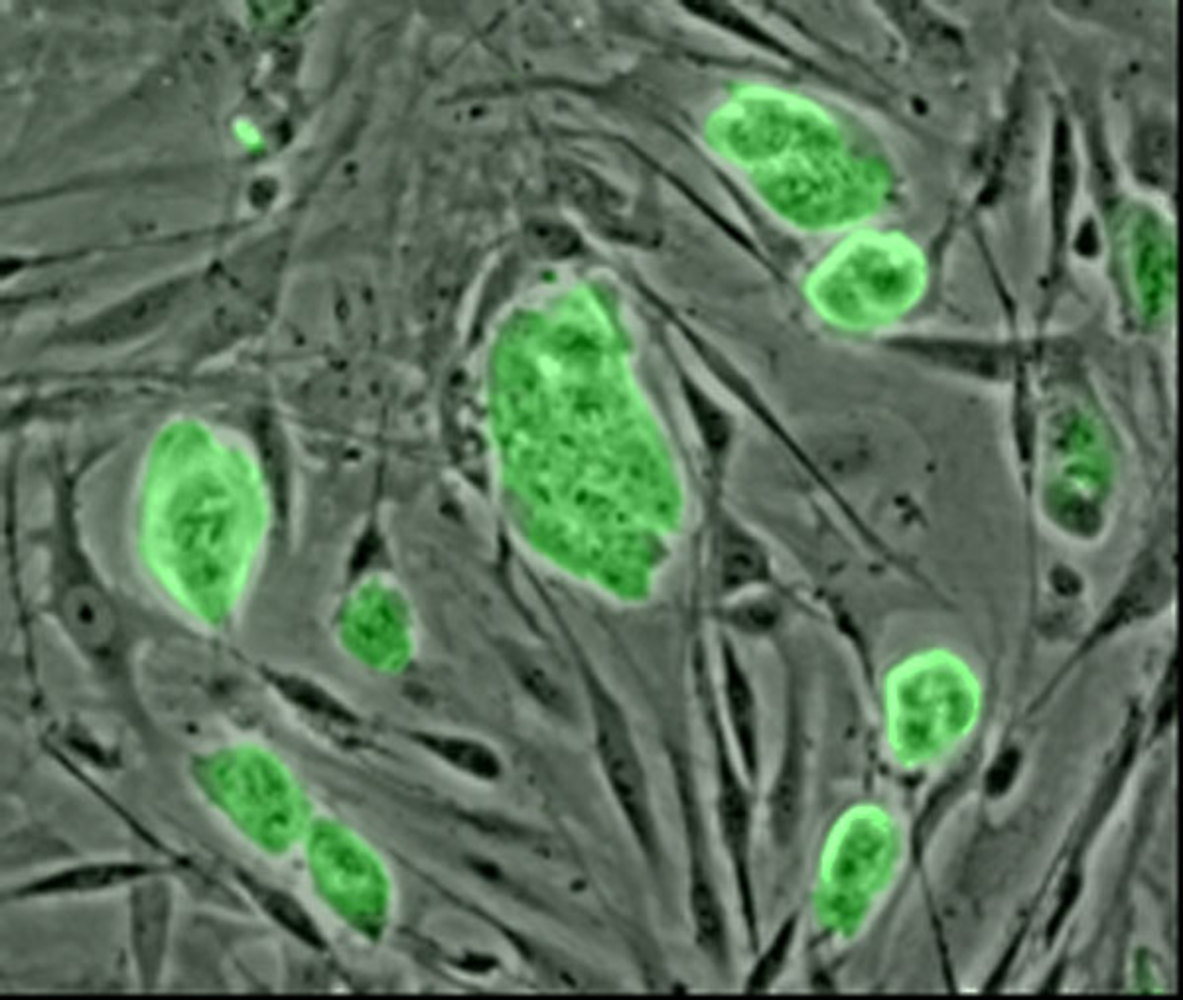 be?
be?
Ryan - Well, the potential consequence of that is that, ideally, what we want to do is to take these adult stem cells and turn them into different cell types that we require for therapeutic purposes in our body for repair's sake. You may take a skin cell and turn it into a cell required for repair of your heart. Now, if the adult stem cells that you derive from the skin cell has these epigenetic tags which prevent certain genes being turned on which may need to be turned on when the cell was turned into a heart cell, then it potentially could cause problems for deriving fully functional heart cells from the adult stem cells.
Chris - Do we know why they don't set those tags right? Is it that the reprograming that we're doing is incomplete in some way?
Ryan - That's right. So, it appears that there may be certain regions of the genome which may be difficult to reprogram. So, this reprogramming process where you create an adult stem cell typically involves taking a very small number of genes, for example, four genes and turn them back on in the skin cell, and these genes are able to bind in many places in the genome, and sort of kick start it into a different pattern of activities, so that it becomes like a stem cell.
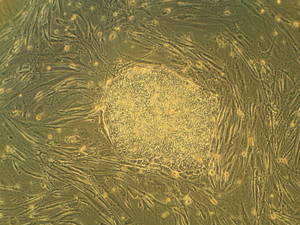 Some very recent work showed that certain regions of the genome, when this reprograming process is taking place, are quite resistant to being bound by these reprograming factors. And interestingly, those regions are where we found many of these differences in these epigenetic tags between the adult stem cells and the embryonic stem cells.
Some very recent work showed that certain regions of the genome, when this reprograming process is taking place, are quite resistant to being bound by these reprograming factors. And interestingly, those regions are where we found many of these differences in these epigenetic tags between the adult stem cells and the embryonic stem cells.
Chris - Scientists have got a number of different ways of making these so-called IPS or induced pluripotent stem cells when you take a skin cell and turn it back into this stem cell-like state. Some involve using viruses to put these four genes in, others involve just putting the four genes in via, what we call transfection - you just put the DNA into the cell. Does it make a difference, how you make the cells, whether or not you get this washing clean of the genetic slate or not? Do some methods work better than others?
Ryan - Well, from what we've seen so far, you find this memory no matter what methodology you use. That's not to say there aren't approaches that will produce perfectly reprogramed adult stem cells, but so far, from the different methodologies that we've looked at, IPS cells created with these different methodologies, we find these consistent differences between IPS cells and embryonic stem cells in all of them.
Chris - Is it just that we need to put more of these resetting factors in? Is it just a shortage of supply or is there something fundamentally missing that we need to get on top of?
Ryan - I'm not sure that it's a shortage of supply. It could be that we need to pre-treat the adult cells, the differentiated cells in order to make these certain resistant parts of the genome more amenable to binding these reprogramming factors. This is something we don't know at this stage, but they're potentially ways that we can poke the cell beforehand to try to put it into a state which is more amenable to reprogramming, but certainly an area which a lot of research is going into at this stage.
Chris - So, if in the future we wanted to use these cells, would we make a huge batch of them and then go through, using the techniques you use, and find the ones that have got the best epigenetic profile, and say, "those are the pool we'll use and we'll just discard the ones that have got a less attractive profile."?
Ryan - Yeah, I think potentially - there may be say, three different approaches that we could use in the future, and we may be able to identify reprogramming conditions which create IPS cells which look - to all intents and purposes - just like the embryonic stem cells. We may alternately be able to create many IPS cells from a skin cell for example and it might be that a small percentage of them look perfectly like an embryonic stem cell, but it's just that we need to check say, a large panel of them to find the one which looks very good. Or maybe that we are able in the future to develop methods to specifically reprogram or correct epigenetic patterns precisely where we want. And so, we could take an adult stem cell, an IPS cell, and identify through this epigenome sequencing methodologies where differences exist between the IPS cell and the embryonic stem cell, and then go in and correct them, and subsequently use them.
Chris - That's Ryan Lister from the University of Western Australia.
Does radiation aid evolution?
Mark D. - An interesting question and a philosophical question. I'm not sure we've been made to evolve that quickly. Radiation plays a very important role in treatment and especially in the treatment of some cancers, but I'm not sure that would be a very effective way for us to try and evolve.
Chris - It's reassuring. I'll avoid Chernobyl in the future then!
Can nanotechnology change DNA?
Stefanie - Well, if you think about proteins working in the cell, they are in some way nano robots that do their job. There is still a very long way to go, but yes, maybe.
Chris - So, you're saying that the mini machines in the forms of enzymes that we already have in our cells doing these jobs, they're like robots anyway. Stefanie - Yes, so if you could use them and make them do what you plan to do then, yes.
Are behaviours genetic?
Stefanie: - There are some human behaviours that are definitely hard-wired in the genetic code. Like the fear of heights, from living in trees and having to hold on, or fear of darkness coming from the fear of being eaten by a predator. I think there are some genes that we know are involved in this, but I think it's still a question scientists are working on today.
How do cancer cells survive?
Mark D. - The truth is that the process of cancer is actually a very rare event.
Many of our cells are developing genetic abnormalities all the time and the immune system does a very good job [of clearing them out]. Also some of these genetic events actually are deleterious for the cell itself, so they end up dying of their own mutations.
Very, very rarely, you get this perfect storm really where you have a cell that is able to survive and grow uncontrollably and also evade the immune system and tumour-surveillance.
The study of cancer immunology is a huge field that is trying to understand this problem...
Genetically tweaking offspring?
Stefanie - Wow, interesting question. One could imagine that this could eventually lead to something like this, but I think we're still in the process of understanding the basic mechanisms behind everything. Mark D. - I'll have to agree with Stefanie. I think it's possible. Our field of epigenetics is still very much in its infancy and understanding how genes are controlled, we're just starting to get a grip on this. So, it may be, in the future, we might be able to bespoke this process a bit better.
In-the-dish vs in-the-body?
Stefanie - This is a classic problem in experimental genetics. You first have to be sure that your in vitro system is a very good one and you have various controls for that. Eventually, you have to see if the same thing is happening in vivo, but it's a good way to start off. Chris - So, start there and then build up. Because it's generally cheaper as well. Stefanie - Exactly, yes.
53:15 - Do foetuses get cancer?
Do foetuses get cancer?
Hannah - Cancer is the unregulated growth of cells and during pregnancy, cells divide very rapidly. There are mechanisms in place to prevent cancer developing, but as Professor Graham Burton from Cambridge University points out.
Graham - About 1 in 10,000 babies when they're born will have some sort of tumour, a swelling in the body associated with abnormal growth. Thankfully, these are usually what we would call benign in that the cells do not invade into other parts of the body, and so, can be treated usually at the time of birth. In terms of true cancer, in terms of uncontrolled proliferation, the other way that a foetus could develop that is if it is transmitted from the mother. Fortunately again, this is very rare. Most maternal tumours will not cross the placenta. The placenta forms a pretty effective barrier to agents that would cause cancer in an adult so that the embryo is in a very protected environment, but will also stop cells from the mother crossing into the foetus. But occasionally, we know that there is mixing of the two circulations, the two blood systems, and if the mother has leukaemia or a similar condition, it has been reported that the foetus can develop the same problem.
Finally of course, the placenta itself can undergo a cancerous change. This again is very rare. It's associated with a very interesting, but curious condition known as hydatidiform mole. Normally, you would derive half of your chromosomes from the mother and half from the father, but in these cases, all of the chromosomes comes from the father, and this causes very rapid proliferation of the placental tissues, but interestingly, you get very, very small growth of the foetus itself. And so, often in these cases, there's no baby to be found at all, just a big mass of placenta. And because of this rapid proliferation, some of those placental cells can undergo a cancerous change.









Comments
Add a comment