In this week's podcast, we're taking on your questions! From how we make decisions to why do we go temporarily deaf when we yawn and if light wears out, these are some of the many conundrums you asked and we answered with the help of an expert panel. Plus, the top headlines in the world of science, including the four new elements discovered, why you can blame your neanderthal heritage for bad allergies and how Harry Potteresque screens could be the next big thing...
In this episode
Why do we have pubic hair?
Kat Arney put this steamy question to Ginny Smith...Ginny - That's a very interesting question, and it's one that's perplexed people a bit. There are a few different theories. The first is that it's simply there to prevent rubbing or chaffing - if you imagine you weren't wearing clothes, as we weren't when we evolved, you might find those kind of areas were quite rubby as you were walking along, so the hair could simply be to stop that. But that's not the best explanation. The best explanation I've come across is that it's all down to mating. It's all down to finding yourself the perfect partner...Kat - So many things in life...Ginny - So we produce two types of sweat around our bodies. We have eccrine glands which are found all over and they produce that watery sweat that cools us down when we get to hot. But we also have apocrine sweat glands and they produce a stickier and slightly smellier sweat, and these glands are mainly found in your armpits and your pubic region. Now the theory is, this apocrine sweat contains chemical messengers that can send out information to prospective mates.Kat - These are like pheromones aren't they? The kind of the...Ginny - Yes. A lot of animals communicate using pheromones. We're not sure if humans do it - it's still one of the big unanswered questions. What we do know is that humans are attracted to people based on smell. There have been some hilarious studies where they've got women to smell sweaty shirts...Kat - Oh yes, yes - the BO T-shirts studies...Ginny - I'm sure we've talked about these before. Well what they've show is you can choose a partner whose compatible via their smell, and it's usually looking at something called a major histocompatibility complex, which is basically how different their immune systems are to your immune system. And you want someone with quite a different immune system so that your offspring have the best chance of survival. So the theory is that the hair in those areas is to kind of wick away these smelly signals and send them out into the world so that we can find a partner.Kat - So basically armpit hair is there to help you get laid?Ginny - Yes. So armpit hair should be sexy.

Why do embers glow red?
Kat Arney put this burning problem to physicist Stuart Higgins...
Stuart - If you think about what's happening there - so you've got a lot of heat, and you've got everything that's warm gives off thermal radiation and this is caused by all the electrons whizzing around in the materials and the molecules of the wood and they're giving off this thermal radiation. Now humans do this all the time.
We do this at room temperature but we can't see it, it's in the infrared. So if you ever see one of those infrared cameras, and you look at someone and they wave and you can see their hands, you can see the hot parts of them giving off this radiation.
Kat - But we don't see that, so why do we see it coming off fire embers?
Stuart - So, in this case, the fire is a lot warmer. It's giving off a lot more energy and, in that case the photons, the radiation that's been given off is of a higher energy and it shifts from the infrared, the bellow red, into the red region of the spectrum, so we can start to see if with our own eyes.
Kat - So if humans were as hot as a fire, we would glow red?
Stuart - Yes, yes we would!
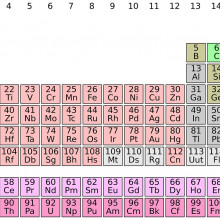
04:33 - Four new elements discovered
Four new elements discovered
with Dr Ben Pilgrim, University of Cambridge
Most school science labs have a periodic table on the wall but until now, there 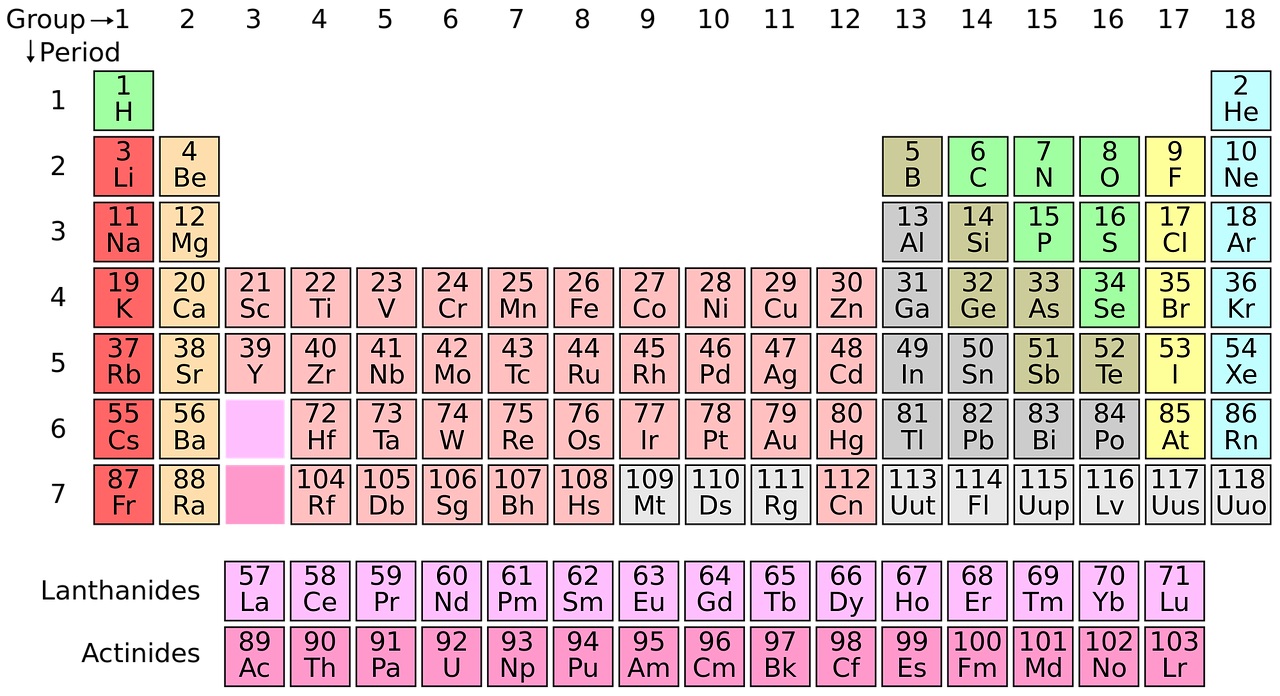 have been a few missing elements towards the bottom. Ben Pilgrim explained to Kat Arney why everyone is suddenly very excited about this historic table...
have been a few missing elements towards the bottom. Ben Pilgrim explained to Kat Arney why everyone is suddenly very excited about this historic table...
Ben - Yes, there's been a very exciting week for chemistry. So IUPAC (The International Union of Pure and Applied Chemistry), announced that four elements (elements number 113, 115, 117 and 118) have been discovered. Now these haven't all been discovered in the last few days - that would be quite a coincidence. It's actually been about 10 or 15 years since scientists first started gathering evidence to suggest that these elements did exist. So there have been gaps in the periodic table; they've been predicted to be there for a long time, but it's all about the scientists getting enough data to be able to be sure that these elements are actually there.
Kat - The periodic table - a lot of people have heard of it, a lot of people probably recognise it. What exactly is it as a way of categorising elements? How did they know that there were these gaps in there?
Ben - So, elements are placed in a periodic table depending on the number of protons, which are a particle found in the nucleus of every atom, and there's basically, you know, the first element hydrogen has one proton, the second element helium has two, and so on. The number of protons defines what element you have and, it so happens these numbers I said earlier, they are the number of protons that we hadn't found yet but, you know, they should be there because it should be possible to have one with that particular number.
Kat - So how do you go about discovering a new element - I assume you don't find it down the back of the sofa?
Ben - Yes, I mean the problem is that there's about 90 elements on the earth, sort of naturally occurring, we find them around. They might be bound up with other things but they will always be there, they're stable. An oxygen will always be an oxygen, a gold will always be a gold. The trouble with these elements is they're radioactive, they're unstable. They fall apart after a very short amount to time - sometimes fractions of a second, and so the scientists actually have to make them and they do this by firing two lighter elements at each other. So, for example, they fire a calcium atom at an atom of americium. Calcium has a number of 20 and americium has a number of 95 and that adds together to make 115. Part of the difficulty is that these new ones we make, they are unstable, they fall apart, so how do we know we've made them?
Chris - They hand around for something in the region of about a microsecond through to a couple of seconds, don't they. I mean someone was saying that one of these new elements, they'd only ever made 90 atoms ever.
Ben - Yes. One of the ones I found out that 117 apparently 15 atoms have been observed, so...
Chris - As many as that.
Ben - You don't want to...
Kat - Don't spend them all at once.
Ben - You don't want to go to the toilet and miss it or something, do you? But, imagine if you had a video, for example, of a game of snooker and someone had, by some computer wizardry, removed the cue ball from the piece of videotape that you might be able to think about where the cue ball was based on what the other balls were moving and how they were moving around the table,and that's a bit like what they have to do here. They have to kind of use what these heavy atoms decay into, and then kind of reconstruct and then assume what they had before.
Chris - Can I just ask you the really simple question though which is - why are we doing this?
Ben - Well I think it's very exciting because there's something that's referred to as the 'island of stability'. So, as we get heavier and heavier, the atoms are becoming less stable but, it's been hypothesised by a number of people that, once we get a little bit higher up we might actually get back into some stable elements again, some stable atoms and these may have new properties that, you know, haven't been seen before. So that's very exciting.
Kat - And, of course, the big question is - what are we going to call them, because numbers aren't cool? We need like brilliant names. What's the...
Chris - Ununtrium and ununpentium and ununseptium. Is that not sexy enough?
Kat - Come on, no!
Ben - Those names are a little dull, so they can be called after a number of things. A number are called after countries or places or after famous scientists.
Chris - There was a petition this week to call one after Lemmy from Motorhead.
Ben - Yes, this would be one of the heaviest of the heavy metals. Unfortunately, I think it has to be a scientist, according to the rules. There are certainly some British scientists - Humphry Davy, Michael Faraday that don't have elements named after them. Even perhaps one of our most famous scientist overall, Isaac Newton, doesn't have an element. But because the research groups - 113 was discovered by a Japanese group, the others by a collaboration between Russian scientists and American scientists, I think we might maybe see a reference to Japan in one of them, one based on the name of Moscow I've heard, and so I think that's perhaps slightly more likely.
Kat - Well, I think we shall have to see.

Why don't some people get hangovers?
Kat Arney put this question to Chris Smith...
Chris - Well I did a little bit of practical experimentation myself over Christmas and I must admit, I did have one or two.
Kat - I've got a terrible one today.
Chris - And Kat is confessing that she is currently suffering.
John - You see I've got two sisters. Myself and one sister never, ever get hangovers and my other sister does.
Chris - I suppose in answering this question John, one has to consider - well what is a hangover? A hangover is a combination of dehydration because alcohol is a diuretic and it encourages you body to lose water, and this means that the salt balance of your body goes off kilter, which contributes to you feeling not very well. The other thing that alcohol is, it's ethyl alcohol, and this is a metabolic precursor for acetaldehyde, which is about one carbon atom and a few hydrogen atoms different from the same stuff we use to embalm bodies in a mortuary - that's formaldehyde. And when you break down alcohol, one of the intermediates in the breakdown process is acetaldehyde and that then goes round your body fixing your tissues and also contributes to some of the nasty symptoms. Now, why is it that some people get these symptoms and others don't? Well there was a big study done in Australia, actually. They looked at 4,000 people on the twin registry. They found about 40-45% of the hangover effect is down to genes, the other half probably down to behaviour, and body size, and so on. In other words - what will genes be doing? Well, they'll be affecting your ability to handle the levels of acetaldehyde; they'll be affecting those levels of those enzymes in your body that break down alcohol and, therefore, eliminate those toxic intermediates from you body. And, therefore, how your metabolism handles alcohol is going to be at least half of the situation. So, if you are prone to hangovers, you're probably not going to improve through training very much. A little bit of training can improve things by increasing the levels of the enzymes your liver makes, but not very much so, it's probably better as they say to steer clear. Kat - So basically I've pickled myself today?
Chris - Yes basically - sorry.

How do snowflakes maintain symmetry?
Kat Arney put this question to physicist Stuart Higgins...
Stuart - Well, you need a big cloud of gas and water vapour and you need the right conditions - it needs to be nice and cold. And so, at the start of a snowflake, you need a speck, you need a nucleus, so typically this might be something like the dust in a cloud and the water will start to solidify and crystallise around that nucleus. And it just so happens that the way it packs is in a hexagonal structure - a regular hexagon has a symmetry of six and so that's what gives us this kind of growing six pronged structure of the snowflake.
Kat - And are all snowflakes different? Is this true or is this a myth?
Stuart - It is true to a certain extent, so they have similar structure. So snowflakes will share this common six pronged structure due to the fact that it's the packing of the water structure underneath. But what happens after that, when these arms spread out and they start to interlink and branch like tree branches, that's more dependent on the conditions, that could be the temperature and the exact pressure and all the different factors that might affect it. So, the chance of getting those two sets of conditions, to grow the same crystal in the same snowflake twice is very rare.

Does religion have an evolutionary role?
Kat Arney put this question to Ginny Smith...
Ginny - It's a difficult question to answer because humans are the only species we know of that has religion. So the question is - how, and or why, did we evolve religion? And we don't really know, but there are two ways that it could have happened. One is that religion evolved due to natural selection because it has a selective advantage. Those of our ancestors who became religious did better than those who didn't and so it was selected for. Another thing is is that it could be an evolutionary byproduct. It may be that we evolved something that was really useful, and then religion came along for the ride as a kind of byproduct of that thing that was usefull.
Kat - I mean I think there's an argument about it having some sort of social cohesion and a lot of religions have rules around hygiene, and eat this, don't do that. let's do this, let's go here.
Ginny - Yes. So that's one of the ideas that, if it did have a selective advantage, that could have been one. It seems like religions came along around the same time our ancestors were starting to live in bigger groups. When social cohesion would have been really important. They often involved watchful ancestor spirits - that kind of thing. People keeping an eye on what you're doing and, if you have that ever present person keeping an eye on you, there's this really big impetus to do the right thing, and if you're living in a big group that's really important.
Chris - Where do you think the observation from South Africa, last year, comes in Ginny - the Homo naledi findings where these primitive people (they may be 2 million years old), appeared to be burying their dead. To all intents and purposes these animals had a brain the size of an orange and we know that equivalently brained animals are not known to be Einstein. So, is it that there's some sort of primitive thing that evolves that you want to have respect for the dead, or whatever? David what do you think?
David - I mean religion in itself you can look at it from two perspectives. As the lady said earlier on, as human beings started to live in bigger communities and, obviously, there had to be some rules of control, let's just say. But I think also if we look at religion I think the concept - I mean this is maybe controversial - but once a certain intellect starts to be perceived, and the consciousness of death starts to appear, I think that religion has a role in that play as to ease the concept of death, I suppose you could say.
Ginny - Yes. That would kind of fit with the - it's a byproduct thing. That we became more intelligent because that was useful for making tools. We gained this ability to see cause and effect but then that meant we were looking for bigger causes and effects. We were looking for something more and perhaps put together things that maybe didn't go together and we saw a divine power and then, as you say, once you start to understand your own mortality, it's very comforting to have an idea of an afterlife and it does seem to be something that really pervasive all across human cultures, human religions. They all have this idea of the afterlife and what to do with your dead and I thinks that's probably right, it's got an element involved there.

Why are some elements unreactive?
Kat Arney put this question to chemist Ben Pilgrim...
Ben - Thinking back to the periodic table again, these metals are all in the same region of the periodic table, so they're at the bottom right of what we would call the D metals. And, if we think about why something's not reactive, well there's two reasons. One is that it's not favourable for the reaction starting materials to go to products. For example, you might think of a metal reacting with oxygen and forming an oxide and tarnishing and whether that's energetically favourable for that reaction to happen. So that might be one of the reasons. The other one is that it might be favourable for the reaction to happen but it just happens so slowly, there's a sort of barrier for it to start and, with some of these metals, it's a bit of both really.
Kat - So basically, the metals that don't react. It's just really difficult - you need to put too much energy in to get anything out of them?
Ben - Yes. That's one way of thinking about it. Gold does react with some things though. You can dissolve it in aqua regia. This was done in the Second World War.
Kat - That's acid isn't it, basically?
Ben - Yes. It's a concentrated nitrogen hydrochloric acid and it was done in the Second World War to save the gold Nobel Prizes of a couple of German physicists from the hand of the Nazi's. So they were dissolved in aqua regia, hidden in a bottle of acid in a lab, and then after the war, the gold was still there and it was turned back into gold and recast and the medals were re-given to the scientists.

What's the fastest thing in the universe?
Chris Smith put this question to physicist Stuart Higgins...
Chris - Well, I would say a photon is the fastest object in space as it's going at the speed of light. Would you concur Stuart?
Stuart - I would indeed. A photon, the speed of light is the fundamental speed limit in the universe.

19:45 - Neanderthals responsible for allergies
Neanderthals responsible for allergies
with Ginny Smith, The Naked Scientists
Neanderthal genes don't just result in heavy brows. Now, scientists have identified genes involved in your immune system and allergies. Ginny Smith explained to Kat Arney how these genes were passed to us and whether they actually serve a purpose...
Ginny - Well I have a story that you may be able to to blame the neanderthals for your hayfever and other allergies. Scientists have known for a while that when our ancient ancestors moved into Europe, they mated with the neanderthals and their close relatives, the denisovans. This means that those of us who don't have purely African heritage, we all have some neanderthal DNA in us and it's estimated to be around 1-6%. But this week, really interestingly, two separate papers have come out and they've shown that some of the most common neanderthal and denisovan genes that we have are actually involved in boosting our innate immune systems, and that's the first response our body mounts when it encounters a pathogen. So one of these studies looked at whole genome sequences and they analysed variability basically, and they found that there were these two sets of genes that were involved in innate immunity that were more similar to neanderthal genes than the rest of our genome was. And they also found evidence that these sets of genes had been positively selected for...
Kat - So there must be an advantage to having them?
Ginny - Exactly. And the second paper found something very similar looking at one particular set of these genes. So, this is really strong evidence that we got these sets of genes from these ancient ancestors and that they were then selected for. So, the most likely scenario was as our ancestors left Africa, travelled across the world, they came across these other ancient humans and they were already well adapted to their environments. So, when our ancestors bred with them, they kind of stole bits of their immune... well they shared their immune systems and the ones that got these genes from the ancestors who'd been there for longer, the neanderthals, they survived better because their immune systems were more reactive to these kind of local pathogens. But it isn't all good news because these genes also seem to encourage overly sensitive immune systems so, those that carry them are more at risk of things like asthma, hay fever because, of course, these are also linked to your immune system. So, you might have the neanderthal to blame when you get the sneezes.
Kat - It's something we're hearing a lot about in the media at the moment. Sort of boosting your immune system with detoxing after Christmas and you have to remember that boosting your immune system does give you allergies.
Ginny - Not always good news.

Why is ice on my car harder to scrape off?
Kat Arney put this troublesome question to phycist Stuart Higgins and chemist Ben Pilgrim...
Ben - So I've had a couple of initial thoughts about this. One thing might be how quickly the water freezes to ice. We know that things that freeze quickly tend to form crystals that are very, very small - think about snow, something like that. Whereas something that freezes over a long time, forms much larger pieces of ice and, I imagine, the larger bits of ice would be more difficult to get rid of than smaller bits. That's one of my first thoughts. And the other one might be whether the car was wet before the night, so if the droplets that are already on the car then they might be quite well adhered to the surface...
Kat - Or like if it's been misty or something like that?
Ben - Yes. Or just rained or something like that. Sort of above freezing during the day but then these droplets already on the car, they just freeze at night. Or whether it's just moisture that comes from the atmosphere during the night. My feeling is that if the car was wet already, that would lead to sort of ice that was a bit more stuck on.
Ginny - But would the easy answer not just be to boil the kettle and pour it over? Cause that would work on either wouldn't it?
Chris - Or deicer?
Ben - Yes, that would work, but you've got to be a bit careful with very hot water because you might lead to an expansion of your windscreen and crack your windscreen. But yes, certainly deicers and things like that would be good.
Kat - Stuart?
Stuart - Yes, one of thing you could do would be the surface chemistry of the windscreen itself. If you've got a layer of material on it that the ice doesn't want to stick to well to the glass, that might well help. So, for example, a thin layer of washing up liquid, something like surfactant that forms basically a layer in between the glass and the water would also be another way of doing it.
Chris - That's what's known as a tarp isn't it?
Stuart - Yes a tarp. Yes you could put a tarp over it.Kat - Does that answer your question Martin?
Martin - Yes, that's great. Thanks a lot.
Kat - Well, happy scrapping.
Where do decisions originate in the brain?
Kat Arney put this question to Ginny Smith...
Ginny - Well, as with many brain questions, the answer is we don't fully know yet. There's a very famous experiment done by a guy called Libet, where he asked people while they were in a brain scanner to move their finger whenever they felt like it but remember the time, and they were looking at a clock face, that they decided to move. And he found there was a 200 millisecond delay between them having the urge to move and the movement. But, 550 milliseconds before the urge, he could see a pattern of brainwaves that she called the 'readiness potential'. So, effectively, these people's brains knew that they were going to move before they consciously made the decision, which has been used for a long time to sort of argue against the idea of free will. Our brains are making these decisions and we, our kind of conscious selves, come afterwards. But more recent experiments have shown that actually, this readiness potential is active whether you're deciding to do something or whether you're deciding not to do it. And there was a new study out recently where they were scanning people's brains and when they saw this readiness potential, they then sent them a signal saying stop yourself from moving and, actually, they were able to stop themselves for quite a long time after this readiness potential was there. So that pattern of brain waves itself isn't enough to actually make you make the movement, but it is sort of involved.
Kat - I mean, I guess, if you had to think about every single thing you're doing all the time, you'd just be freaking out. You wouldn't be able to survive...
Ginny - Yes. A lot of what we do is effectively on auto-pilot. It's being controlled by our brains without any conscious intervention.

Why does the first tick take so long?
Chris Smith and Ginny Smith tried their hand at this question...
Chris - And it's absolutely true isn't it. You must have all noticed this and I think it comes down to what you're saying basically which is, that the processing delay through your visual system is at least 150-250 milliseconds. In other words, a pretty high fraction - a fifth of a second goes by between the time that actually something is presented to you and you actually being able to process that information through your visual areas of your brain. And it may may take up to a third of a second before your consciousness then integrates that and puts that visual stimulus into your conscious experience. So when you're looking at the clock, there's going to be a delay when you first look at the clock while your visual system compares what is the clock looking like, what did it look like - oh look it's now moved, and then it becomes a streamline process.
Ginny - The other time where you see that delay is if you've ever done that thing where you've seen something out of the corner of your eye and thought that it was a spider or a snake and had that moment of panic, and then noticed that actually it's the garden hose or it's some, you know, fluff on the side. You get that moment of panic because you're unconscious parts, the subconscious parts of your brain register the threat and then the conscious parts get the information from your visual system and go - oh no, hang on, it's just a garden hose, calm down. So then you can really see that difference between how fast your conscious and unconscious processing is.

Does light wear out?
Kat Arney put this question to physicist Stuart Higgins...
Stuart - The short answer is - no it doesn't wear out. It never wears out, it keeps travelling for ever and that's because light actually is a wave of energy, it doesn't have mass, and so things that have mass, normally decay into smaller things and break down. In this case light doesn't have it. It will just keep going for ever. However, that doesn't really match our everyday experience. If I shine a torch in your eyes and it's blindingly bright, and then I stand the other side of a field and shine it towards you, it looks dimmer, it doesn't seem as bright, so what's going on there? There's different things that actually that could make light wear out as it were, and that's where the light might be absorbed by the particles of the air, or the atmosphere, or scattered off the dust. Or it might be the lights just not reaching you, it's shooting at a slightly different angle.
Kat - So, if there was a perfect vacuum in the field between you with the torch and me, then it would seem as bright as if I was standing right next to you.
Stuart - Not quite. So if it were maybe a laser, and the laser was shining and the laser travelling in a straight line, that would be fine but, actually, the torch, if you think where the lights being made by the filament, it passes through a lens, that scatters some of the light, it passes through. There's usually a bit of metallic foil in their as well that kind of pushes it toward the front of the torch, but even still the light is spreading out and so, if you imagine the number of photons that are reaching your eye, it's actually decreasing the further away you are as your eye is only a fixed area that can receive them.

Why are my rubber bands losing their spring?
Kat Arney put this question to chemist Ben Pilgrim...
Ben - Actually, the thing that usually causes rubber to go off rather than being slightly too warm is exposure to a chemical that degrades the rubber. So there's some types of polymer, things like ozone in the atmosphere can react with but, even without that actually light is one of the big problems. Light can split oxygen into these radical species, these peroxides and, over time, these degrade the structure of the rubber. So, rather than keeping it cold, keeping it in the dark would certainly help. Now, it is interesting about the cold because, actually, if you cool rubber down too much, you actually cause it to decompose much faster and this is due to the rubber falling below something which we call 'the glass transformation' or 'glass transition' temperature, when it sort of has these rubbery like properties. If you go below that temperature, it becomes brittle. Now one of the most famous consequences of this was actually the cause of the Challenger shuttle disaster back in 1986. So the 'O' rings, which were made of rubber in the kind of engines and boosters for the rocket were very cold on the launch pad that day, because they'd had very freezing, very low temperature conditions and, therefore, they weren't flexible enough to seal properly and this then led to sort of hot gases coming out and eventually the very sad disaster. And this was demonstrated quite famously by Richard Feynman in sort of the inquest when he took one of these bits or rubber and put it in a glass of iced water in front of the panel and showed how brittle it became. So, I'd be careful about sticking it in the freezer - that might not be the best thing to do.
Chris - But when it warms up again, wouldn't it go back to normal behaviour?
Ben - Yes. But I say one of the main things is keeping it out of light and if you could keep it in a sort of airtight container so it doesn't have, for example, oxygen getting to it, that might help as well.
Kat - So. Keep them in a sealed, dark box.
Ben - Yes.

31:55 - Harry Potter style newspapers created
Harry Potter style newspapers created
with Dr Stuart Higgins, University of Cambridge
If you're a Harry Potter fan, you may remember the newspapers they read in  the film. They're not too dissimilar from when you read an article on your tablet - there are moving images and graphics. But one key difference is that your tablet is a hard, inflexible surface whereas the newspapers in Harry Potter are bendy, much like paper. Stuart explains to Kat Arney how this felixible style newspapers may not be too far off...
the film. They're not too dissimilar from when you read an article on your tablet - there are moving images and graphics. But one key difference is that your tablet is a hard, inflexible surface whereas the newspapers in Harry Potter are bendy, much like paper. Stuart explains to Kat Arney how this felixible style newspapers may not be too far off...
Stuart - This news story really caught my eye because this is right up my street - this is my area of research. There is the Consumer Electronics Show in Las Vegas that's this big trade fair that happens every year. It's just finished this year and it's basically for the tech companies to show off their latest gadgets and inventions. And the one that really my eye was from LG Display, and they've made this flexible screen that's about 18 inches in diameter and you can roll up and bend it. It looks like a TV screen but on a piece of plastic that's as thin as a piece of paper.
Chris - Is this for people with sort of non-ideally shaped living rooms then? You can sort of bend it into the corner, is that the point?
Kat - Or slap it on the coffee table?
Chris - Exactly. Quite literally.
Stuart - You could put it anywhere. One of the things they say is you can actually roll it down or so, you know, once you've finished watching TV you roll it up like a poster and put it in the cupboard. It has all kinds of other applications. This sort of technology could also be used in things like intelligent packaging, incorporating screens and circuits into packaging.
Kat - So how does this actually work and, the big question, is it actually any good?
Stuart - Right. So, it's a mixture of things. We've had this technology for a while now because we've had OLED TVs - we've might have these nice big flat screens. And part of the problem is, the way these TVs work is that you have to have a big white light behind them that shines through, red, green, blue filters - he colours that make up all the colours of the rainbow that we see - and you basically work the screen by turning off which bit of the light is shining through each filter. So instead here, they're using OLEDs, which means they have little tiny lights in there that are directly generating the red, green, and blue that you need to see, and that means they can get rid of the backlight behind it, the big white light, and that makes the whole thing thinner and a lot easier to put onto a piece of plastic.
Kat - But is it actually any good? If you keep rolling it up, shove it in your pocket, get it out again, is it just going to get knackered?
Stuart - Well this is the problem because, I don't know, did you have an unbreakable ruler at school, which it claimed to be unbreakable. But, I tell you, if you bend it enough times it will break. And this is the same thing, plastics fatigue, they wear, they have mechanical strain, and if you look very closely at these screen, what you're looking for occasionally, especially the flexible ones, is a thing called dead pixels. The little ones the lights aren't turning off or aren't turning on.
Chris - I thought I had those but it turns out it was insects.
Stuart - In your screen?
Chris - Yes, because they put in front of the LCD, they put a fixed layer because the LCDs obviously much more delicate, but these little tiny thunder buggy thrip things obviously discovered it's a nice bright light source, it's nice and warm, they can get in between the two. And I thought I had a dead pixel or two and then I discovered it was moving across the screen, and the next day it's in a different place. The real bummer is they die in-situ and then you have this permanently dead pixel but it's a dead insect. I tried shaking the screen, but it's kind of lodged in there.
Kat - Given that this does sound like stuff of the future, how close is it to being a consumer reality?
Stuart - So the screens themselves are getting there. We might be maybe five years away from having that into a product. But the challenge is not the screen itself, it's really the circuits and the technology around it that power the display. So how are we going to make a flexible battery, how are we going to make the processor that actually puts the display on the picture? Those are the limiting factors at the moment.

Why do I go deaf when I yawn?
Kat Arney put this question to Dr Chris Smith...
Chris - The reason is that the way that you hear stuff is that the ear canal, which is the bit that you can put your finger into in your ear, ends in a structure called the eardrum, and the eardrum is a sheet of tissue which, when vibrations in air, which is what sound is - its mechanical vibrations, those vibrations impact on the eardrum, they make it vibrate and those vibrations are then conducted through three tiny bones into your inner ear, which is also known as your cochlea, and that is where soundwaves effectively become brainwaves and your brain then decodes what those vibrations meant, and that's how we interpret the sound world that we live in. Now the problem is that the middle ear, which is where those three tiny bones are, is connected to the back of your throat through a structure called the eustachian tube. And you need that because when the outside pressure changes, if there was no way for the pressure on the inside to be equalised with the pressure on the outside, then, if say you went up a mountain, you'd have lots of pressure inside your your head and no pressure outside and your eardrum would bulge painfully outwards. If you go diving and you had no way to equalise, the pressure of the water would push painfully in on your eardrums. And some people who go diving and do find they get pressure in their ears, that's exactly what's happening. But when you yawn, you're applying a lot of pressure inside which is conducted down your eustachian tube and pushed outward on your eardrum and it changes the ability mechanically of the eardrum to respond to those vibrations in the air. It affects its stiffness, which means that the hearing is shifted out of the regime of most of the frequencies that we interpret as sort of speech and meaningful sounds. Your ear becomes much less capable of responding to those sounds, those frequencies when you've got that high pressure in your ear, and that's why you experience this momentary deafness while you're in the middle of a yawn.
How did male and female first evolve?
Kat Arney put this question to Ginny Smith...
Ginny - Yes, well. If we're going to talk about the origins of male and female, we do actually have to start by talking about the origins of sex because, of course, when at first it was all single celled organisms, they mainly reproduced through asexual reproduction. Bacteria, yeast, things like that still do it.
Kat - They split in two, don't they? One cell becomes two, becomes four...
Ginny - It's very, very quick. It's very, very easy. You don't have to bother finding someone else to do it with...
Kat - And buying them dinner and all that kind of stuff...
Ginny - So why did we stop doing it? Well the problem is, bad mutations can build up because you're just producing a clone of yourself. And it also means, if something new comes along - a new pathogen or a new environmental influence, it's going to wipe out the whole line. So it turned out that mixing your genes with another animal's genes is a much better way of producing offspring that are likely to survive. So sex was one of the best ways of doing this, and the first sexual encounters were likely to have been between two organisms that were effectively the same, not male or female, both somewhere inbetween. They just started swapping some information.
Kat - Because bacteria do this. They swap little things called plasmids. Tiny little bits of DNA they just sort of go - do you want some of that, here you go. And this is usually how antibiotic resistance spreads, doesn't it? It's carried on plasmids.
Ginny - Exactly, exactly. So that's kind of the first precursor to real sex. But, over time, many species then evolved to having two mating types - call them type A and type B. Now, we aren't quite sure why this happens, but it might be something to do with keeping our mitochondria in check because only one of the two parents now passes down mitochondria. We get all our mitochondria from our mother, so it may be that A and B evolved because one of them passed down the mitochondria and the other one didn't. Now once this had happened, it was an advantage for each of the mating types to specialise. So one type started making more smaller gametes and that meant that they could have more offspring because they could mate more easily with more of them, and the other one started putting all it's resources into ensuring the health of the offspring and the survival of the offspring they produced.
Kat - All it's basket in one egg!
Ginny - Exactly, yes. So that then over lots of time as they became more and more specialised...
Kat - Give it a billion years, and here we are...
Ginny - Became male and female and that, of course, leads to the choosy female - ardent male system that we see in most animals now. And, interestingly, there's some evidence that there's a real advantage to species that do that because it ensures that only the fittest males get to pass on their DNA and it improves the fitness of the next generation as a whole.
Kat - And certainly, as a woman, it means I get taken out for nice dinners!

Does stress cause grey hair?
Kat Arney put this question to Chris Smith...
Chris - People have looked at this in some detail actually, and there's not really a consistent story here.
There's a reason, a clear reason, why hair goes grey, or white, because the natural colour of hair - it's a protein called keratin. It's the same protein that makes your fingernails and toenails, and the natural colour of that protein is white.
And the reason hair is coloured is, when hair grows out of the hair follicle, which is a little ring of stem cells embedded in your skin that makes the hair, there's another population of cells in the follicle, called melanocytes, and melanocytes, as you probably know - the clue is in the name - make melanin.
There are different "flavours" of melanin, the chemical. There's what we call eumelanin, which is a very black colour, and there's phaeomelanin, which is a more yellow colour; and the different ratio of these (the black to the yellow) affects the colour of the hair. These melanin pigments are added to the hair as it forms, and that gives it it's colour.
For some reason, as we age, the hair follicle soldiers on but the melanocytes peg out and die; they stop making a colour-contribution to the hair and it reverts to its natural state, which is a white colour.
Whether or not this can be the consequence of stress, actually, we just don't know. One way that could happen is because, for instance, the follicles become biochemically stressed. Perhaps when you are under periods of extreme stress it could biochemically stress the hair follicles - but there's not really any good evidence for that.
Another reason, people have speculated - and it's certainly been shown in animals - that there's a build-up of hydrogen peroxide at high levels in hair follicles, and that hydrogen peroxide can damage the melanocytes. So, perhaps, for some reason when you're stressed you have less ability to defend yourself against hydrogen peroxide attack?
On the whole, we're pretty unconvinced and it's probably genetic because some people keep their hair colour for much longer than others and that tends to run in families.
So, it doesn't actually look like Barack Obama's grey hairs are down to increased stress in the job, it's probably just a lack of "Grecian 2000"!

Why does water go off?
Kat Arney put this question to chemist Ben Pilgrim...
Ben - It's almost always because there's something in it. I mean water is an inherently stable molecule. In fact, they've found water that's 2.6 billion years old, sort of buried deep under the ground in Canada and nothing's happened to that. The problem is that if there are small contaminants within the water molecules, sort of microbes, things like that. Then, over time, if they have the right conditions these microbes can sort of grow and proliferate, and make toxins, and so on.
Kat - So it's not the water - it's the stuff in it!

Could we send all the plastic to the Sun?
Kat Arney put this question to physicist Stuart Higgins...
Stuart - Well we can. Occasionally old spacecraft and things, maybe not towards the sun, but are bashed into planets all the time to get rid of them. But in terms of plastic - the problem is the sheer quantity of plastic that we have. So last year, the Earth produces about three hundred million tons of plastic. A million tons is about the weight of of 750 Empire State Buildings - it's a massive quantity.
Kat - So okay. In terms of like the jet fuel that we'd need to get a rocket made of that much plastic up there would - it's not economic is it?
Stuart - It's not. So I did a quick back of the envelope calculation, I must stress.
Chris - It wasn't a plastic envelope?
Stuart - It wasn't a plastic envelope.
Kat - A little plastic window in it though.
Stuart - So the heaviest rocket - the heaviest lifting rocket that we've had was Saturn 5. It was the rocket that took astronauts to the moon and that had the capability of lifting 50 tons-ish to the moon. So if we think, actually, it would take 6 million rocket launches to get all the plastic from just one year up towards the moon, not even to the sun. And if we think there are only about 85 rocket launches a year - that's about 70,000 years it would take just to shift last year's plastic.
Kat - And Enzamul does goes on to say that this use of plastics is clearly bad and that we should stop using them - are they all plastics bad? Maybe Ben as well, you'd like to come in on this. Are all plastics bad and there any really viable alternatives to get us off our fossil fuel plastics?
Ben - So plastic's a material called polymer, sort of formed from long chains, and part of the problem is that these chains are quite chemically unreactive. They persist in the environment because the microorganisms can't attack them and start to break them down, but there has been a lot of work recently on more biodegradable polymers. So these are ones that still have the sort of mechanical properties to be a useful plastic, but can sort of be attacked on over time by the microbes and might rot away in the ground after a year or two. And, certainly, they wouldn't be as bad because eventually they would go from a landfill site, unlike the ones at the moment.
Kat - Stuart - what do you think?
Stuart - Well I think this is it. So one of the things in our field - we were talking about plastic electronics earlier. And one of the challenges we have to consider as we make things is what we're going to do with all the plastic we're using and generating. So, I think further research into biodegradable plastics and how we process them if we can do recycling on them, is really important.
Kat - Yes. I guess what you were saying about the flexible screens. You know if you kind of throw it up, put it in your pocket and eventually it dies - just chuck it away! Is there any progress in trying to recycle these kinds of electronics?
Stuart - Yes. So, in general, you can recycle some electronics. You kind try and recover the metals and things out of them, but this is very energy intensive. It takes a lot of energy to do and so there's always a trade off there, so maybe if we can look at ways of developing new techniques and new materials and biodegradable polymers - that would be a better approach.
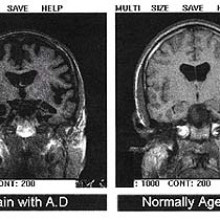
What's the latest in Alzheimer's research?
Kat Arney put this question to Ginny Smith...
Ginny - Well. It's quite a difficult question because, actually, we still don't fully understand what causes Alzheimer's. Now Alzheimer's is the most common form of dementia but it is only one form of dementia - there are other types. These are brain diseases which cause problems with thinking, memory, and often all sorts of other areas, including language. One of the long running theories is that what's causing Alzheimer's is a buildup of protein in the brain, called beta amyloid, and you can actually see sort of lumps of this in the brain of people with Alzheimer's
Kat - They're kind of tangles and plaques and things like this aren't they?
Ginny - Exactly, yes. And so a lot of the research has been looking into whether you can break those down. The theory being, if they're what causes it then breaking it down should improve people, and some mouse studies have shown improvements when they've broken down these plaques and tangles. But, interestingly, research that has been coming out recently has shown that in humans, breaking these down doesn't always improve people's symptoms, and you can also get some people who don't have any symptoms, but when you look at their brains, they do have these buildups of these proteins in them. So now people are starting to think, well actually, may it's not a direct 'cause and effects' thing with these proteins and perhaps we should be looking in kind of other directions for potential treatments. So, at the moment, there are a few drugs which can help with the symptoms but we don't have anything that stops the progress of the disease but there's a huge amount of work being poured into trying.
Kat - Because I know from the genetics perspective, lots of people are hunting for the genes involved in Alzheimer's and the problem being, you know, there's lots of different types of dementia and, even if you can find a gene or a gene variation that increases the risk, we don't know if there's anything we can do about it to mitigate it. That, I guess, is the biggest problem, isn't it?
Ginny - There is a little bit of research that's coming out that's quite interesting about what you can do throughout your life to keep you brain healthy and, hopefully, stave off Alzheimer's. And that's things like keeping active, lots of exercise, keeping social interactions going, keeping you brain active...
Kat - Doing things like Sudoku and puzzles and, I guess, listening to The Naked Scientists can keep you brain active...
Ginny - Yes, exactly. And also social interactions, so seeing people and talking to people seems to be protective. And what you eat is also important as well, so lots of green veg, berries, nuts, olive oil - all of these things have been shown to help keep your brain healthy.

Why do you suddenly get desperate for a wee?
Kat Arney put this question to Chris Smith...
Chris - I think this is a case of 'mind over matter' or maybe, more accurately, 'mind over bladder' in this case. When you are stressed about something, and I would put it to you that being stuck in a car on a motorway with an overactive bladder, thinking crikey where is the toilet, this is a stressful situation and this will initiate your 'fight or flight' reaction, which is a part of your autonomic nervous system, which is out of your control. It's under sub-conscious control and when you are under stress, then what happens is that you send very powerful supplies of nerve impulses to your guts to turn off your intestines, and to your bladder to make your bladder relax, because the last thing you want to do when you're trying to fight or run away is think, I need a wee, and this deters the bladder from activity. Now when you get home though, you now know - ahh respite is within my grasp, I am nearly home. And then what happens is those nerve impulses that were strongly inhibiting the contraction of your bladder and were making your bladder relax, and relax, those nerve impulses are now turned off. And so now the bladder begins to gain activity, and begins to compress the urine and you become much more exquisitely sensitised to the stretch in your bladder - thinking I really, really, really need to go to the loo, and so I'm going to run to the loo. And that's the reason why. I think it's because it's a psychological thing. You now know that helps at hand, you know you can make it, you now feel much more relieved before you relieve yourself and so you begin to then think - now I really do need to go to the loo.
Kat - But, of course, you should have gone before you left. That's always the thing, isn't it?
Ginny - I think there's a pavlovian element as well in that you associate seeing a toilet with going to the toilet, so seeing the toilet makes you need it more. I know that for me, if someone mentions the word 'toilet', it makes me want to go.
Kat - Like turning the taps on and things like that?
Ginny - Yes, all of that. I'm terrible.
- Previous Naked Genetics 47
- Next Surgery in Space










Comments
Add a comment