In this NewsFlash, we find out how a diet of glycerol makes yeast live longer, how microbes in mosquitoes can block malaria, and how planting trees could reduce your electricity bills. We hear about the European Space Agency's Planck and Herschel missions, due to launch this week to study the formation of galaxies and the fate of the universe. Plus, Sarah Castor-Perry takes us back to this week in Science History.
In this episode
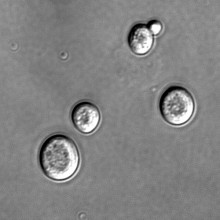
Glycerol diet for long life?
Researchers in the US have discovered that living on a diet made up solely of glycerol could double your lifespan - if you're a yeast, that is.
 Previous studies have found that severely restricting calories can also double the lifespan of yeast, but in this new research, published in the journal PloS Genetics, Valter Longo and his team have found a more filling alternative.
Previous studies have found that severely restricting calories can also double the lifespan of yeast, but in this new research, published in the journal PloS Genetics, Valter Longo and his team have found a more filling alternative.
The scientists tried feeding glycerol to yeast after they discovered that yeast that had been genetically engineered to have a much longer lifespan also showed increased activity of genes that produce glycerol. The yeast also have low activity levels of a molecular pathway known as TOR1/SCH9, which is thought to be important for extending lifespan in many different animals from worms to mice.
At the moment this work only applies to yeast but it is certainly intriguing. Longo suggests that it may be possible to extend even the lifespan of human by changing the makeup of our diet. We know from previous studies that extreme calorie cutting can extend human lifespan, although - who would want to live a life without cake anyway? Perhaps changing the energy sources in the human diet may also have an effect on lifespan.

Giant shark mystery solved
Basking sharks, the second largest sharks in the world, have been tracked on epic, thousand-mile migrations into the deep waters of the West Atlantic, solving a long-standing mystery of where they spend the winter. Gregory Skomal from the Massachusetts Division of Marine Fisheries in the US led a team of scientists who tagged 25 basking sharks off the coast of New England.

As the satellites began beaming information back to the team they were amazed when the sharks kept on swimming south, into the Caribbean Sea and beyond. One shark even crossed the equator ending up at the mouth of the Amazon River on the Brazilian coast where it hung around for a month.
Their discovery has revolutionised our understanding of these mysterious basking sharks which until now were thought only to live in temperate waters. They have never been seen in this part of the world before, probably because they swim very deep down between 200 and 1000 metres.
The obvious explanation for their migrations is that these cold-blooded sharks need to find warmer water with lots of plankton, their favourite food. But what puzzles Skomal and his team is why the sharks bother going so far south. If food and warmer water were all they were after they could stay in northern Florida. Why bother going all the way to Brazil? One idea they came up with is that the sharks are moving to as yet undiscovered birthing and nursery grounds.
Amazingly, scientists have never seen a young or embryonic basking shark, so we know virtually nothing about how they breed. Previously it was thought that basking sharks form many separate sub-populations but now it seems they are well connected and could form a single ocean-wide population. That raises important issues of how we protect them, since any impacts on basking sharks in one area could have affects on the population as a whole. It could be nations around the world will need to get together and create a global basking shark conservation programme.
And this study just goes to show just how much more we still have to learn about some of the biggest creatures that roam the oceans today.
“Good” bacteria block malaria
Mosquitoes are a major problem around the world, not just because they're annoying, but because they spread deadly malaria which kills over a million people worldwide every year, mostly children in Africa.
This week, researchers at Johns Hopkins Bloomberg School of Public Health in the States have made an important discovery that could pave the way for new ways to tackle these pesky insects. Their work is published in the latest edition of PloS Pathogens.
The researchers focused on bacteria found in the gut of Anopheles gambiae mosquitoes, and discovered that the bacteria help to prevent the mosquitoes from becoming infected with the parasite that causes malaria. When mosquitoes were treated with antibiotics to kill their gut bugs, they became much more susceptible to infection with the malaria parasite. The team also found that infection with the bacteria shortened the mosquito's lifespan.
This is good news because it takes about two weeks for the malaria parasite to complete its life cycle within a mosquito host, so if the mossies are dying earlier, as well as being more resistant to infection with the parasite, it means they're less likely to pass on malaria.
The researchers think that the stimulation of the mosquito's immune system caused by the bacteria also helps to block infection by Plasmodium falciparum, the malaria parasite.
Lead author George Dimopoulos suggests that deliberately introducing the bacteria to wild populations of mosquitoes could be a potential way to control malaria infections. He and his team are currently trying to identify which strains of bacteria trigger the strongest mosquito immune defense against the malaria parasite.

Plant trees. Save electricity.
With all the talk of climate change and trying to cut down our carbon footprint, scientists this week have come up with evidence of a new way to help cut down on electricity bills - the solution may be as simple as planting some trees.
 Two researchers from the States, David Butry of the National Institute of Standards and Technology and Jeffrey Donovan of the U.S. Department of Agriculture, have conducted a study looking at how planting trees around your house can help shade it in summer and reduce the need to switch on air conditioners. We all know that trees provide shade and a nice spot to sit under in the summer but this is the first wide-scale study to investigate whether this can translate into significant energy savings in the home. Butry and Donovan studied 460 homes in Sacramento during the summer of 2007 - they looked at what trees were growing around the house and then linked this to the energy bills of each household.
Two researchers from the States, David Butry of the National Institute of Standards and Technology and Jeffrey Donovan of the U.S. Department of Agriculture, have conducted a study looking at how planting trees around your house can help shade it in summer and reduce the need to switch on air conditioners. We all know that trees provide shade and a nice spot to sit under in the summer but this is the first wide-scale study to investigate whether this can translate into significant energy savings in the home. Butry and Donovan studied 460 homes in Sacramento during the summer of 2007 - they looked at what trees were growing around the house and then linked this to the energy bills of each household.
They found that planting trees on the west and south sides of the house decreased summer electricity use, by an average of 5% a year, while trees on the north side of houses actually increased the use of electricity and those on the east had no effect. Fast-growing trees work better than smaller, slower growing species. They worked out that a London Plane tree planted on the west side of a house could reduce carbon emissions from air conditioners by an average of 30% over 100 years.
And of course trees have the added benefit that they not only shade houses but they absorb and lock away carbon dioxide from the atmosphere too. This study sounds very promising and the authors are keen for researchers in other regions of the world to see if they find a similar pattern.

09:46 - Studying Space - The Launch of Planck and Herschel
Studying Space - The Launch of Planck and Herschel
Dr Anthony Challinor, Institute of Astronomy, Cambridge University
Kat - Thursday 14th May, all being well, we are going to see the launch of the European Space Agency's Herschel and Planck missions which are studying the formation of stars and galaxies and background radiation, all sorts of exciting things.
On the show today we are joined by Dr. Anthony Challinor who is here to tell us about the mission and how he is going to be looking at some of the data from it. So thanks coming on the show Anthony.
Anthony - That's quite alright.
Kat - So tell me what is the mission that you are involved in and what is it doing?
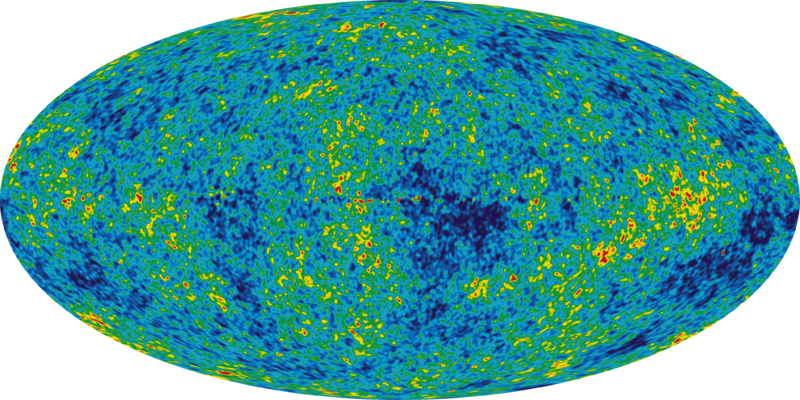
 Anthony - So we are involved in the Planck mission and what Planck is trying to do is study what's called the cosmic microwave background radiation. So this is really the oldest light in the universe. It was essentially created in the big bang itself and what we are going to try and do is study very sensitively the tiny variations in temperature in this radiation when you look in different directions and from that we have to learn a lot about both the early universe and also something about what the universe is composed of, what its geometry is and perhaps even the ultimate fate of the universe.
Anthony - So we are involved in the Planck mission and what Planck is trying to do is study what's called the cosmic microwave background radiation. So this is really the oldest light in the universe. It was essentially created in the big bang itself and what we are going to try and do is study very sensitively the tiny variations in temperature in this radiation when you look in different directions and from that we have to learn a lot about both the early universe and also something about what the universe is composed of, what its geometry is and perhaps even the ultimate fate of the universe.
Kat - Crikey. What's the actual satellite going to be like? Where is it shooting off to?
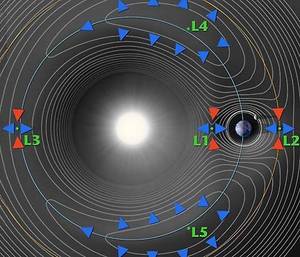
Anthony - Planck, its ultimate home is going to be what's called the second Langrange point which is a very special point about one and half million kilometres from the Earth and it is peculiar in that it rotates at exactly the same angular speed about the Sun as the Earth does. So thermally it's a very stable environment which is exactly what you need when you are looking for these tiny temperature variations - just about a millionth of a degree fluctuations we are looking for.
Kat - And so you are looking for these tiny fluctuations in temperature, how far back in time are you hoping to be able to look, you know you are hoping that this data will shed light on?
Anthony - So the cosmic microwave background radiation was produced very, very early in the history of the universe and the early universe is very, very opaque but eventually it became essentially transparent about 400,000 years after when we think the big bang occurred and at that time the microwave background effectively decoupled from all the matter in the universe.
 So when we look at it today, we are effectively seeing a snapshot of conditions in the universe 400,000 years after the big bang or about 13 billion years, 14 billion years back in time from now.
So when we look at it today, we are effectively seeing a snapshot of conditions in the universe 400,000 years after the big bang or about 13 billion years, 14 billion years back in time from now.
Kat - And how is the Planck mission special or different from the sort of previous microwave measuring experiments that have been there before?
Anthony - Well so Planck is Europe's first satellite mission to try and measure the microwave background. There have been two other NASA missions before, the first called KOBE, the second called WMAP which is actually still observing.
Planck is an improvement in that it is much more sensitive. It will observe very, very much wider range of wavelengths and it has better angular resolution as well.
Kat - And if it is so far away from the Earth how is it sending the signals all the way back for you to analyse back in the lab? How long does it take that data to get to you?
Anthony - Well the data is transmitted, it is not transmitted continuously but it's sort of buffered on board and then there's an hour or two-hours' slot everyday when it is all transmitted back.
Kat - And how long does it take to get back to you?
Anthony - What from the...
Kat - Yeah, from the satellite.
Anthony - It takes, it is 1.5 million kilometres so however long light takes to travel that distance.
Kat - I don't know, any of our listeners would like to do that calculation and tell us, that will be great. Tell us a little bit about Herschel, the other satellite as well, what's that up to?
 Anthony - Okay, so we are not directly involved in Herschel although there are groups in the U.K. that are and what Herschel is basically trying to do is it is looking at dust within the universe.
Anthony - Okay, so we are not directly involved in Herschel although there are groups in the U.K. that are and what Herschel is basically trying to do is it is looking at dust within the universe.
So it is an infrared satellite and it is basically looking at galaxies that otherwise we can not see in optical light because that the star light is sort of shrouded and absorbed by dust. But that dust is then heated by the star light and re-radiates in the infrared. So Herschel will be able to see those sorts of environments directly.
Kat - I love the idea of a satellite going out looking for dust out to the universe and doing the dusting, "How Clean is Your Galaxy?"
Anthony - Some people are very interested in dust, yeah.
Kat - Some people are. So where is the mission launching from this Thursday?
Anthony - So it's going to launch from Kourou in French Guiana.
Kat - And you didn't manage to get a ticket out there?
Anthony - Unfortunately no.
Kat - And when are the first, the first bits of information going to be coming back? How long is it going to take to get into position?
Anthony - So it takes about three months for Planck to reach L-2 where it will start observing from and the plan then, it takes a couple of months further to sort of settle down and properly be commissioned but after that Planck will do basically two complete surveys of the sky, which will take about 15 months.
Kat - And then you get all the data back and come back on the show and tell us all about it.
Anthony - That's right, I mean we get the data back essentially as soon as Planck starts observing but it will be probably about three years before there is any real public data release of the cosmological data.
Kat - Brilliant, well we will really look forward to hearing that. Thank you very much. That's Dr. Anthony Challinor from the Institute of Astronomy here in Cambridge. So if you are a space-excited person then watch out for the launch of the satellite this Thursday.

15:16 - This Week in Science History - Valence Theory
This Week in Science History - Valence Theory
Sarah Castor-Perry
This week in science history saw, in 1852, the publication of a paper by Edward Frankland describing the valence theory of why chemical elements bond with only a certain number of other elements. Although he did not know the precise mechanism behind it, his idea has shown to be correct and is still taught in schools today as one of the basic principles of chemistry.
 Born in Lancaster, England in 1825, Frankland began his career in chemistry straight from school. After several apprenticeships, he was a student at the University of Marburg in Germany for a time before taking up a position as professor of Chemistry back in London. He later went on to become a professor of chemistry at the Royal Institution and a member of the Royal Society of Chemistry.
Born in Lancaster, England in 1825, Frankland began his career in chemistry straight from school. After several apprenticeships, he was a student at the University of Marburg in Germany for a time before taking up a position as professor of Chemistry back in London. He later went on to become a professor of chemistry at the Royal Institution and a member of the Royal Society of Chemistry.
During his work soon after returning to England, Frankland noted that some chemical elements seem to form compounds involving always the same number of other elements, either in groups of three or five - for example nitrogen combining with three hydrogen atoms to form ammonia, NH3. In the paper of 1852, he uses the phrase 'combining power' to describe what we would now understand as valency - how many chemical bonds will be formed by an atom of a given element.
All atoms are made up of a positive nucleus surrounded by negatively charged electrons, organised into 'shells'. Chemical bonds are the result of the most energetically favourable arrangement of the outer shell electrons of the atoms between their nuclei. Bonds either occur as a result of the atoms sharing the electrons between them, or by one atom donating electrons to another (in this case the atoms are known as ions, because they have a positive or negative charge).
 Take hydrogen gas for example. In the gas, the hydrogen atoms pair up to form H2 molecules that make up the gas. In the H2 molecule, each hydrogen atom has one electron in the outer shell, and when they pair up, the electrons are equally attracted to the other hydrogen's positive nucleus.
Take hydrogen gas for example. In the gas, the hydrogen atoms pair up to form H2 molecules that make up the gas. In the H2 molecule, each hydrogen atom has one electron in the outer shell, and when they pair up, the electrons are equally attracted to the other hydrogen's positive nucleus.
The number of bonds an atom will form is determined by the number of electrons present in its outer shell. The outer shell of an atom can contain a maximum of 8 electrons, depending on its position in the periodic table (or 2 in the case of a few elements like lithium and hydrogen). All atoms 'want' to have a full outer shell of electrons. In the case of a metal like Lithium, its outer shell, which could contain up to 8 electrons, only has one, which means it 'wants' to lose that electron so that the next shell down, which is full, will be the outer one. Oxygen has six electrons in its outer shell - 2 short of a possible 8, so it will want to grab two electrons from another atom or two to fill those spaces. This means that lithium has a valency of one - it will form a single bond using the one electron in its outer shell. Oxygen has a valency of two - forming two bonds, such as in H2O, water.
 As with many discoveries, we now know the situation is more complex than Frankland realised and that although some elements such as carbon and fluorine always form the same number of bonds (4 and 1 respectively in this case, such as in CH4, methane and HF hydrofluoric acid), some will vary, like iron, symbol Fe, which will form compounds as iron II and iron III - so you can get FeO, iron II oxide, and Fe2O3, iron III oxide, which we know as rust.
As with many discoveries, we now know the situation is more complex than Frankland realised and that although some elements such as carbon and fluorine always form the same number of bonds (4 and 1 respectively in this case, such as in CH4, methane and HF hydrofluoric acid), some will vary, like iron, symbol Fe, which will form compounds as iron II and iron III - so you can get FeO, iron II oxide, and Fe2O3, iron III oxide, which we know as rust.
Despite the huge advances in chemistry over the past 150 years, and without the understanding we now have of the structure of atoms and the physics behind chemical reactions, Frankland's analytical mind drove him to discuss the idea of valence before anyone else, still one of the key ideas in chemistry today.
Related Content
- Previous Jumbo Aerobatics
- Next Clean Water and Alien Invasions










Comments
Add a comment