This week, we catch up with the latest from the front line of cancer research. Kat Arney reports from the National Cancer Research Institute's annual conference, we find out how proton therapy is promising for targeting tumours and look at the hormones and stem cells involved in breast cancer. Also, the role of aspirin in the 1918 Spanish flu epidemic, how recession could be healthy and tuning in to the Earth's vibrations. Plus, in Kitchen Science, we show you how to see using sound!
In this episode

Aspirin and flu
Little white pills of aspirin have been popped by millions of people since the drug came to market in 1899. And today around 40,000 tonnes of the drug are sold every year around the world. But a new paper published in the journal Clinical Infectious Diseases suggests that misuse of aspirin to relieve the symptoms of flu could do more harm than good - at least in the case of the 1918 flu pandemic.
 This is work from Karen Starko in California. She thinks that very high doses of aspirin given to some patients during the flu pandemic in 1918 and 1919 - up to 30 grams per day - might have caused dangerous side effects, such as a buildup of fluid in the lungs.
This is work from Karen Starko in California. She thinks that very high doses of aspirin given to some patients during the flu pandemic in 1918 and 1919 - up to 30 grams per day - might have caused dangerous side effects, such as a buildup of fluid in the lungs.
In turn, this would contribute to the deadly effects of the flu, and increase the chances of lung infections. We know that doses this high can cause such side effects, as seen in people with aspirin poisoning. Adding to the evidence, the US Surgeon General recommended the use of aspirin for flu just before a massive spike in flu deaths back in 1918.
Based on what we know about the percentage of people who get fluid in their lungs when they take high doses of aspirin, this could have affected up to one in thirty people treated with the drug. And when you add up how many thousands of people died from the flu at that time, quite a significant number of them could have been due to aspirin. What's more, Starko thinks that the unusually high number of deaths in younger people - who usually fight off the infection - might have been down to aspirin use, as well as the flu.
Back in the early 20th century, aspirin was a relatively new drug, and doctors weren't sure exactly how to use it, and how much for people to take. It was also pushed hard by the pharmaceutical companies at the time, and doctors would prescribe it in order to just be able to do something for patients - back then there wasn't any Tamiflu!
Today we know much more about aspirin, and the complex ways it can affect the body. But we can certainly take this as a warning from history not to get carried away with unnecessarily high doses of drugs for relieving the symptoms of flu.

Depressions are good for you
The shadow health minister Andrew Lansley was criticised earlier this year when he pointed out that, fiscally painful as they are, recessions are nonetheless good for a nation's health.
 Now there's more scientific evidence to support that claim thanks to a paper in the current edition of PNAS by University of Michigan researchers Jose Tapia Granados and Ana Diez Roux. The duo have compared the state of the US economy over the period straddling the Great Depression of the 1930s with the population mortality rates over the same period, with striking results.
Now there's more scientific evidence to support that claim thanks to a paper in the current edition of PNAS by University of Michigan researchers Jose Tapia Granados and Ana Diez Roux. The duo have compared the state of the US economy over the period straddling the Great Depression of the 1930s with the population mortality rates over the same period, with striking results.
The years 1923, 1926, 1929 and 1936-37, they report, were all economic booms and on each of these years the population mortality peaked. But in 1921, 1930-33 and 1938, which were all recessions or depressions, the death rate fell to its lowest levels. Initially this seems surprising because one would assume that during recessions people would be financially stretched, have little money for healthcare and healthy living and would be generally more stressed, all of which would translate into a high risk of dying. But instead the reverse appears to be true.
Some have suggested that this relationship reflects a lag effect, whereby people become ill during a recession but by the time they die the economy is booming again. The researchers discount this theory on the grounds that the timing just doesn't work because the periods between boom and bust are not constant each time yet the mortality rates change directly in step with the state of the economy.
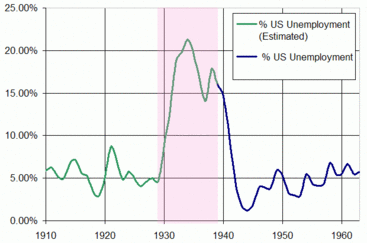 Instead, they point out that although a fast-growing economy might be good for your bank balance it is potentially very bad for the health of the populace because people tend to work longer hours during a boom, they have more disposable income to spend on alcohol and cigarettes, consumption of which is known to increase at times of prosperity, there is also more traffic, more pollution and industrial accidents are more frequent. People often migrate in search of lucrative high-paid work, leading to social isolation. All of these factors add up to more ill-health during the so-called good times. So, rather than rue the recession, welcome it with open arms...and see how long your bank manager will swallow the story that your empty account and maxed-out credit card are all part of a healthier lifestyle...!
Instead, they point out that although a fast-growing economy might be good for your bank balance it is potentially very bad for the health of the populace because people tend to work longer hours during a boom, they have more disposable income to spend on alcohol and cigarettes, consumption of which is known to increase at times of prosperity, there is also more traffic, more pollution and industrial accidents are more frequent. People often migrate in search of lucrative high-paid work, leading to social isolation. All of these factors add up to more ill-health during the so-called good times. So, rather than rue the recession, welcome it with open arms...and see how long your bank manager will swallow the story that your empty account and maxed-out credit card are all part of a healthier lifestyle...!
Gene controls brain cell count
Wouldn't it be fantastic if we could flick a genetic switch and increase the number of brain cells we have? But it would be bad news if this production line ran out of control, because then you'd end up with a brain tumour.
Now researchers in the US have tracked down the gene responsible for maintaining this tricky balance, making sure we grow enough new neurons, but not so many that things get out of hand.
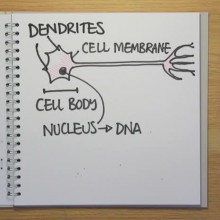
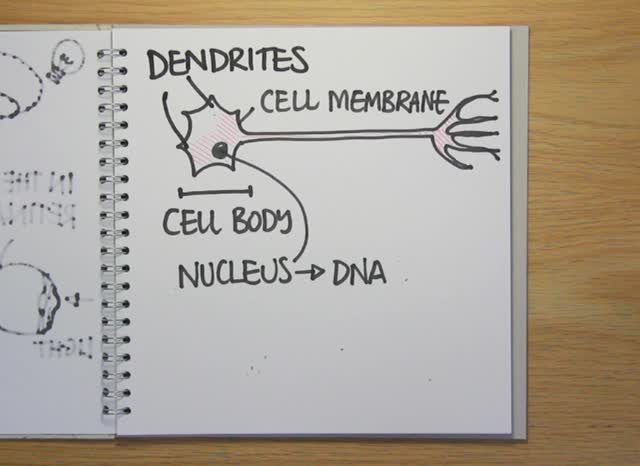 Led by William Snider at the University of North Carolina, the scientists discovered that a gene called GSK-3 controls the signals that determine how many nerve cells we have in our brains. GSK3 is a kinase - an enzyme that sticks little molecular tags on other proteins, switching them on or off to send signals in the cell. They've just published their results in the journal Nature Neuroscience.
Led by William Snider at the University of North Carolina, the scientists discovered that a gene called GSK-3 controls the signals that determine how many nerve cells we have in our brains. GSK3 is a kinase - an enzyme that sticks little molecular tags on other proteins, switching them on or off to send signals in the cell. They've just published their results in the journal Nature Neuroscience.
The researchers used genetic engineering to create mice whose GSK3 could be removed at a very specific time during the development of the mouse embryo - at a time when a type of brain cell called radial progenitors have just been made. These stem cells produce the bulk of the nerve cells in the brain.
The researchers found that removing GSK3 at this crucial time meant that the progenitor cells were locked into a pattern of constant proliferation, churning out endless new stem cells, rather than mature neurons.
As a next step, the researchers want to find out if adding GSK3 back into the brain after this massive burst of proliferation will make the stem cells mature. They think that they could make mice with three to four times as many neurons as normal mice.
So one day, by manipulating GSK3 levels, we could perhaps increase our brain capacity - Snider describes it as "dialling up and down the number of neurons that are generated in the brain."
The other potentially important thing is that GSK3 has also recently been fingered for a role in a number of psychiatric illnesses, including schizophrenia, depression and bipolar disorder. And lithium,a common treatment for bipolar disorder, works by shutting down GSK3.
The researchers suggest that perhaps doctors should avoid giving drugs like lithium to younger children whose brains might still be growing, in case it causes problems like an overgrowth of cells, which could potentially lead to cancer.

Tuning in to see inside planet Earth
Scientists have discovered how to use the natural hum inherent to the Earth to see deep within the planet's interior.
 Various processes, including ocean swells and atmospheric disturbances set up very low frequency vibrations - less than 0.01Hz - that propagate through the planet. Because the character of these waves is highly random it's possible to identify and follow specific signature sounds as they travel around the planet. The sounds change as they pass through different geological structures, so by tuning into them from many different places on the planet's surface you can build up a picture of what must lie beneath.
Various processes, including ocean swells and atmospheric disturbances set up very low frequency vibrations - less than 0.01Hz - that propagate through the planet. Because the character of these waves is highly random it's possible to identify and follow specific signature sounds as they travel around the planet. The sounds change as they pass through different geological structures, so by tuning into them from many different places on the planet's surface you can build up a picture of what must lie beneath.
The work has been published in the current edition of the journal Science by Kiwamu Nishida at the University of Tokyo and the studies are based on recordings made between 1986 to 2003 by 54 seismic stations dotted around the world.
This approach is very similar to another method that scientists use to see inside the Earth, which is by looking at how vibrations triggered by Earthquakes spread around the planet. The data generated by the two techniques agrees very well, proving that it works, but the new approach offers the additional advantages of continuous sampling, the ability to see very deep inside the Earth - down as far as 500km say the scientists - and could even be used to reveal the internal structures of other planets.
According to the team atmospheric disturbances would set up a similar hum on Mars, allowing scientists to map the planet's interior.

13:51 - The National Cancer Research Institute Annual Conference
The National Cancer Research Institute Annual Conference
with Dr Kat Arney
Kat - Well this is a conference that's organized by the National Cancer Research Institute, the NCRI, which is kind of a virtual institute. They're an umbrella that brings together all the funders of cancer research in the UK. So organizations like Cancer Research UK, Leukaemia Research, some pharmaceutical companies, basically to make sure that everyone is doing cancer research in a good way, not missing any areas and not duplicating too much work. So it's really - it was setup a few years ago to address the fact that people didn't really know what was going on in other labs. So basically, this is a conference where cancer researchers from all over the UK, from all over the world, get together to talk about the latest results to discuss collaborations. And it's not only scientists here, but there's doctors, nurses and also patient groups here as well. So, it's a really diverse range of people.
 Chris - Now every year, when you go to the NCRI conference, you come back with some new hot kid on the biological block. So, what are the hot topics in cancer research this year?
Chris - Now every year, when you go to the NCRI conference, you come back with some new hot kid on the biological block. So, what are the hot topics in cancer research this year?
Kat - Well, there's just been a talk this afternoon and by a chap called Larry Norton who's from the Memorial Sloan-Kettering Cancer Centre in New York and it was really, really interesting. When you think about cancer that starts to spread, there's this idea that there's a starting tumour and cancer cells go off around the body and find new places to go, such as the lungs, the liver, and they start new tumours. His idea is while that cancer is growing, stem cells; spreading cells, they go off, they travel around the body and then they come back to the original tumour, and they start growing there, so this idea of self-seeding. And what he's proposing is that, say, you treat this original tumour, you get rid of it with surgery, with radiotherapy, with chemotherapy. There are still these cells, out travelling in the body like the prodigal son, and they try to come back, but there's no original tumour there and they think, "Well, I should go somewhere else" and then they go and start growing in the lungs, in the liver and in the brain.
Chris - So by chopping out the cancer paradoxically, the primary tumour, we could be encouraging the process to spread?
 Kat - Well, that's basically the idea and his ideas are really very new and at the moment, he's only got research in mouse models that might support this. But it's certainly an intriguing idea and he's got all sorts of ideas of how he might use this to treat cancer for example. So basically, the idea that the original tumour is acting like a kind of a sponge absorbing back in these cancer cells. So, it could be, we make some kind of fake sponge that would then mop up the cells that have started spreading? Can we find out the signals that they're giving out and try and mimic them or block them that might stop cancer cells spreading? And he's designing some cancer intuitive regimes of different drugs you might give that would actually help to stop this process and treat cancer more effectively. So, there's some really exciting work to be done in the future there, I think.
Kat - Well, that's basically the idea and his ideas are really very new and at the moment, he's only got research in mouse models that might support this. But it's certainly an intriguing idea and he's got all sorts of ideas of how he might use this to treat cancer for example. So basically, the idea that the original tumour is acting like a kind of a sponge absorbing back in these cancer cells. So, it could be, we make some kind of fake sponge that would then mop up the cells that have started spreading? Can we find out the signals that they're giving out and try and mimic them or block them that might stop cancer cells spreading? And he's designing some cancer intuitive regimes of different drugs you might give that would actually help to stop this process and treat cancer more effectively. So, there's some really exciting work to be done in the future there, I think.
Chris - Okay. Well Kat, thank you very much.
You can keep up with the latest news and videos from the NCRI conference
on the Cancer Research UK Blog.
17:39 - Proton Therapy
Proton Therapy
with Professor Karen Kirkby, University of Surrey
Chris - Professor Karen Kirkby is at the University of Surrey and she's developing a way of treating cancer, using proton therapy. Now, this is a targeted beam of protons, they're positive charges, and you can aim these at individual cancer cells to get rid of them. Hello, Karen.
Karen - Hello.
Chris - Great to have with us on The Naked Scientists. So, tell us a bit first of all about the problem with cancer that you're trying to tackle. I mean, everyone's heard of cancer but it's a generic term, what do we actually mean by it?
Karen - I think cancer covers a whole range of diseases and Kat covered it quite nicely earlier on when she said about the brain cells. Obviously, we all like more brain cells, but we don't want them to go mad and form brain tumours. And that's effectively what cancer is. It's where the cells go mad and starts to form tumours in parts of the body where you don't want them.
Chris - And so, if you could just summarize, what are the current strategies that we use to get rid of cancer, before we start talking about your technique.
 Karen - Okay. Well the current strategies, there's surgery which is very effective and you're looking at about 50% of cancer cures using surgery. Then we've got radiotherapy which comes in at about 40% and then chemotherapy, which combined with the other two modalities comes in at about 11%.
Karen - Okay. Well the current strategies, there's surgery which is very effective and you're looking at about 50% of cancer cures using surgery. Then we've got radiotherapy which comes in at about 40% and then chemotherapy, which combined with the other two modalities comes in at about 11%.
Chris - And your technique?
Karen - Our technique is one that's used rarely in the UK. There is a centre in Clatterbridge which uses it to treat eye cancers, but it's becoming very, very widely used in the states and Europe, largely because of advances in medical imaging. You've got to see the tumour before you can use this technique. So, it was thought about in the late '40s, but because at that time you couldn't really see the tumour, it wasn't very good to use. It's a very targeted technique - whereas with x-rays, if you irradiate a tumour with radiotherapy, the damage that's induced by the x-rays is induced around the tumours, so in front of and behind it. Whereas if you use protons and heavier ions, you use something called the Bragg peak - this is the way the ions actually stop. And if you change the energy so that most of the energy is deposited in the tumour and very little in the surrounding tissue, then you can imagine you put most of the damage into the tumour, very little into the tissue in front of it, and practically none into the tissue behind it.
Chris - So this is a way basically of minimizing side effects because radiotherapy is very effective - you're basically giving a beam of radio waves, x-rays, microwaves, whatever people are using, ionizing radiation into the cells. This damages the DNA of the cells and they die, but the problem is, that as you say, it's unfocused and takes down adjacent tissues which are healthier and this make side effects. How do you manage to target your therapy so appropriately just into the tumour itself then?
 Karen - Well I think it's largely the physics. Physics works as for us beautifully because of this Bragg peak. You get a very, very sharp peak. So, if you can imagine going in through the tissue, you put a tiny bit of damage into the tissue in front of the tumour then there's this big peak as the protons deposit their energy, and then beyond the tumour, there's practically none.
Karen - Well I think it's largely the physics. Physics works as for us beautifully because of this Bragg peak. You get a very, very sharp peak. So, if you can imagine going in through the tissue, you put a tiny bit of damage into the tissue in front of the tumour then there's this big peak as the protons deposit their energy, and then beyond the tumour, there's practically none.
Chris - First of all, can you just explain the proton bit of it. Why is that novel and how does that work? And where do you get these protons from?
Karen - Well, protons as I say, they've been thought of for cancer treatments since the work was done or back in the - I think they've first proposed in 1946 and some work was done in Berkley in the states. But the results were a bit unequivocal largely because they would irradiate a large amount of the body simply because they didn't know exactly where the tumour was because they haven't got the imaging techniques. So, the results weren't particularly good. But now, we can find the tumours, we can target the protons at them. If we use MRI imaging for example, we can target the protons exactly at the tumour and it's very useful if you've got a tumour very close to a particular structure such as the spine because there's not an exit dose. And therefore, you can put all the damage into the tumour and run into the vulnerable tissue beyond it.
Chris - When the tumour gets impacted by the beam of protons, what do the protons do to the tumour. Why do they destroy it?
Karen - Well protons work in a very similar ways to x-rays, but of course, protons being bigger, they're particles coming in, rather than electromagnetic radiation. They basically induce double-strand breaks in the DNA. Now we know those double-strand breaks can be repaired, but they're much more difficult to repair. And therefore, you can start to destroy the tumour much more easily than you could with x-rays. It's sort of - it's a bit like throwing cannon balls, rather than ping-pong balls at the tumour.
Chris - Which is a good thing. The question is though, that as you've said yourself, now we have the ability to image tumours really well and we can see where they are and you can target your therapy. That's fine. But what's the resolution of the scanning? In other words, one of the reasons people die with malignancies is not because of the primary tumour usually. It's because it's spread to elsewhere in the body. So, are you able to use this kind of therapy to pluck off, not just the primary tumour, but those spreads, those metastases as well?
 Karen - It's being done a lot in Japan. There are stories there in the literature of it being treated for small lung tumours and tumours in the liver, and it's been termed, the form of Atomic scalpels. In Japan, they're tending to use protons and carbon ions. So the Japanese are further ahead then we would be in the UK at the moment. But they're using combined modalities of treatment and there are stories of fishermen being taken off fishing boats, taken to hospital, given something like 10 to 15 fractions, being flown back to the fishing boat and being completely cured. As I said, these are some of the stories in the literature and I've seen pictures of liver tumours where the tumour has completely been removed. And for example also, tumours in kidney which would be difficult to treat with conventional radio therapy, simply because of the collateral damage on things like the bowel.
Karen - It's being done a lot in Japan. There are stories there in the literature of it being treated for small lung tumours and tumours in the liver, and it's been termed, the form of Atomic scalpels. In Japan, they're tending to use protons and carbon ions. So the Japanese are further ahead then we would be in the UK at the moment. But they're using combined modalities of treatment and there are stories of fishermen being taken off fishing boats, taken to hospital, given something like 10 to 15 fractions, being flown back to the fishing boat and being completely cured. As I said, these are some of the stories in the literature and I've seen pictures of liver tumours where the tumour has completely been removed. And for example also, tumours in kidney which would be difficult to treat with conventional radio therapy, simply because of the collateral damage on things like the bowel.
Chris - And is it pretty much any kind of tumour or the specific kinds of tumour for which this is more appropriate?
Karen - In the UK, the feeling is, first of all, it's for paediatric tumours because of - for example, if you're treating tumours of the spine in children, if you use conventional radiotherapy, obviously, you'll bathe the rest of the body in radiation. You bathe things like the lungs and the heart. And obviously in children, you want to minimize the chance of secondary cancers later on in life. So that is one of the particular ones. It's also very useful if you're close to critical structures. It's part of the armoury. It's not going to replace conventional radiotherapy, but it might be useful for cancers that we can't use radiotherapy for at the moment.
Chris - Thank you , Karen. We'll leave it there, but do stay with us. That's Professor Karen Kirkby. She's from the University of Surrey and she's working on charged particle beams for cancer treatment. They use protons. So needless to say, she's very positive about her research.

24:58 - Breast Cancer and Stem Cells
Breast Cancer and Stem Cells
with Dr John Stingl, Cancer Research UK Cambridge Research Institute
Kat - And now, it's time to join Meera Senthilingam who's gone along to the Cancer Research UK Cambridge Research Institute this week, to find out how John Stingl and his team are investigating the role of stem cells in breast cancer development. Now, stem cells are known for their ability to regenerate and differentiate to form lots of the cells in our bodies. But as well as this crucial role in our growth and development, it seems that rogue stem cells might be at the heart of cancer formation in many cases including breast cancer. So, Meera spoke to John about how he's studying this kind of change in the human breast.
 John - The breast has traditionally been considered to be composed of two types of cells. We have luminal cells which are cells that make milk and an underlying layer of myoepithelial cells, just the name implies, they're muscle-like cells, and they're literally a cell that contracts and squeeze the milk out of the mammary gland. So our hypothesis is that the mammary gland is probably more complex than just milk-producing and muscle-like cells and it's our hypothesis that there's also stem cells present - mother cells present in the mammary gland that can generate both these luminal cells and myoepithelial cells. It's been basically our research for the last number of years now, where we basically try to identify mammary gland stem cells, trying to figure out what all the different types of differentiated cells in the breast and as well, other cells in between stem cells and the differentiated daughter cells. Cells we would call actually, progenitor cells.
John - The breast has traditionally been considered to be composed of two types of cells. We have luminal cells which are cells that make milk and an underlying layer of myoepithelial cells, just the name implies, they're muscle-like cells, and they're literally a cell that contracts and squeeze the milk out of the mammary gland. So our hypothesis is that the mammary gland is probably more complex than just milk-producing and muscle-like cells and it's our hypothesis that there's also stem cells present - mother cells present in the mammary gland that can generate both these luminal cells and myoepithelial cells. It's been basically our research for the last number of years now, where we basically try to identify mammary gland stem cells, trying to figure out what all the different types of differentiated cells in the breast and as well, other cells in between stem cells and the differentiated daughter cells. Cells we would call actually, progenitor cells.
Meera - How are you trying to understand how this pathway between stem cell to differentiated cell varies when somebody gets breast cancer?
John - The normal mammary gland is under quite strict control of homeostasis. So for example, a stem cell, would normally divide and produce a new stem cell, and it also produces more differentiated daughter cells. So cells would have some proliferative capacity but not as important as a stem cell. We call these cells a progenitor cell. Then these progenitor cells would produce their daughter cells. And this balance between a stem cell producing one new stem cell and a more mature daughter cell, we think is perturbed in cancer. Such that in cancer, there may be more a shift to, say, producing more stem cells. Basically, we believe that cancer is a disease of cells that have proliferative capacity, other stem cells or cells with stem cell-like properties. And the reason for this is that you have to keep in mind that cancer is multistep process. We don't just get a mutation, then get cancer. If that's the case, we would probably all be dead. In fact, you probably need about say, five or six mutations to go from a normal cell to malignant cell. But the body has developed pretty good methods for protecting its DNA and the probability of getting a mutation, a meaningful mutation of cells, is actually quite low. Analogous to like winning a lottery. So how is a cell supposed to get say, five mutations in a row, it's like trying to win the lottery five times in a row. But if you have a cell that has that possibility to generate lots and lots of daughter cell, such as a stem cell. So say, just by unlucky chance, a stem cell got a mutation. That stem cell can generate a million daughter cells. All of them will have that mutation. Now, the probability that a certain daughter cell will get a second mutation is quite low, but the probability that one of those daughter cells out of that, you know whole population will get a second mutation is quite high. And maybe the second mutation's a mutation that reduces DNA repairability. And therefore, the problem in getting a third mutation is not one in a million, but say, maybe one in a hundred. Basically, if you target cells that have the ability to grow, you can increase your chances of getting multiple mutations.
 Meera - Because whilst the probabilities are quite low, the fact that so many cells are being generated obviously makes that risk higher.
Meera - Because whilst the probabilities are quite low, the fact that so many cells are being generated obviously makes that risk higher.
John - Exactly.
Meera - So how are you going about actually researching this?
John - We get normal breast tissue from the hospital. This is human breast tissue say, reduction mammoplasty specimens. So women who had their breast surgically reduced. We bring them back to the lab and we basically mince them up and we incubate ithem in an enzyme mixture which basically degrades all the extracellular matrix but doesn't hurt the cells. And we basically make a single cell suspension of human breast tissue. We use a machine called the fluorescence activated cell sorter and by tagging the cells with fluorescent molecules, we can purify different subfractions of breast cells.
Meera - But now, with your team having separated out to find all of these different cells that are present within a breast, how are you then going to go about actually researching the cancer side of it?
John - So for example again, there are many different types of breast cancers. Are they all rising in a common cell type or they're arising from different types of breast cancer cells? The way how we're going to test that is, say for example, you have an oncogene, gene X, and you know that gene X is somehow an associate of breast cancer but you don't know how. Now what we're going to do is we're going to purify subfractions of normal human breast cells and then using specialized viruses, we can actually infect those cells, such that they overexpress oncogene X. And we can examine, okay, does oncogene X for example, cause stem cells to expand the stem cell population or does it cause a more mature progenitor cell to turn into a stem cell. And then we can also, instead of just introducing oncogene X, we can also get the oncogene X, Y, and Z and seeing what type of cell generates what type of tumour.
Meera - So having identified the cause of the tumour like a particular gene that's causing a tumour, you're inserting it into different types of cells that are made within a breast to see which of those cells that gene actually affects to become cancerous, essentially.
John - Yeah, exactly. The problems we've had is that there's been a lack of understanding of the normal cellular context in which oncogenes and tumour suppressor genes exert their actions. For example, does oncogene X, you know, does it cause this one population to expand or does it cause another population to expand or does it cause one population to generate another population?
 Meera - So you don't know whether it's causing one cell to just proliferate or if it's causing one cell to produce something that causes another cell to proliferate and so on?
Meera - So you don't know whether it's causing one cell to just proliferate or if it's causing one cell to produce something that causes another cell to proliferate and so on?
John - Yes. So we don't understand the underlying mechanisms of what is actually happening. We only see the end stage result and it's very important to understand the molecular pathways that regulate stem cell behaviour because you want to target these pathways in order to stop tumour growth. Now it has been demonstrated and pretty conclusively that there is such thing as a breast cancer stem cell. The challenge now is to figure out how abundant are these breast cancer stem cells, are they identical between different types of breast tumours, and they're probably not, and basically, how do we isolate them and how do we target them. And that's basically the stage we are right now.
Chris - Well, that was John Stingl who's from the Cancer Research UK Cambridge Research Institute and he was talking to Meera Senthilingam to explain how he and his team are investigating the role of stem cells and breast cancer formation and how those cells change their regulation and how that could be targeted as a potential therapy for cancer treatment.
Is the gene GSK3 owned by GlaxoSmithKline?
Sorry, but No. GSK 3 is short for Glycogen Synthase Kinase 3 - no link to the Pharma company whatsoever.
Does vitamin C treat cancer?
Kat - No, it isn't. This is something that Linus Pauling put around - the idea that you take massive doses of vitamin C and it can stop you getting cancer or treat cancer. And basically, there's no scientific evidence that this works. However, about a year or so ago, there was a paper that showed that injections of vitamin C may help some treatment. I can't remember all the details, but we certainly blogged about it on the Cancer Research UK Science blog. But it's important to stress that obviously, vitamin Cs are anti-oxidants and taking high doses of anti-oxidants may well interfere with some kinds of cancer treatment in ways that we don't really know and again, it's something that we have blogged about and it's an area that's really quite interesting because people do love to take vitamin pills.Chris - Indeed. There was also a Meta analysis by Goran Bjelakovic who's at the University of Copenhagen in Denmark. I remember this coming out last year and they looked at many, many thousands of people who'd all been in little trials on giving anti-oxidant vitamins like vitamin A, vitamin D, vitamin E, vitamin K, selenium, and that kind of thing, vitamin C, and compared that with people's outcomes if they didn't take vitamins. And in fact, in many cases, they found that some chronic vitamin treatments actually resulted in people having a higher mortality rate and morbidity rate than people who didn't take any of these supplements. A modest increase in risk, but at the same time, vitamin A and vitamin E did increase the risks. So, the chances are, yes, it's based on sound physiological principles, trying to take anti-oxidant but the outcomes don't necessarily fit the facts at the moment. So, needs more work I guess is the bottom line.

34:54 - Blocking Oestrogen in Breast Cancer
Blocking Oestrogen in Breast Cancer
with Professor Charles Coombes, Cancer Research UK, Imperial College
Kat - In case you haven't noticed, October is breast cancer awareness month across the UK. You've probably seen the explosion of pink in the shops and I do think it's a real shame that we don't have blue everywhere for men's cancer, so I think there's something to be done there, chaps, come on - get sorted. Anyway, breast cancer is still the most common cancer in the UK and it affects around 45,000 women and around 300 men every year. And although survival rates are improving year-on-year, there are thousands of people who still lose their lives to breast cancer. And often, this is because hormone blocking treatment such as tamoxifen or anastrozole stop working after a while. Now, Cancer Research UK's Professor Charles Coombes is a leading breast cancer researcher at Imperial College in London. And I went to find out more about the role of hormones in breast cancer and some of the promising new treatments, he and his team are developing.
 Charles - Hormones, mainly oestrogen, drive breast cancer cells to divide. So it acts as a sort of a fertilizer for breast cancer cells and tamoxifen has been the standard way that people try to block oestrogen action because tamoxifen is an anti-oestrogen drug. So, it prevents oestrogen from gaining access to the cells that cause the cells to divide. The problem is, that more than half of breast cancers eventually become insensitive to tamoxifen. And so, the first drug that we developed was - if you want, a drug that would work after tamoxifen has exerted it's action and worked. And that class of drugs is called aromatase inhibitors. And those drugs are drugs such as Arimidex, exemestane, letrozole, those sorts of drugs are all inhibitors of oestrogen synthesis. Under those circumstances, what happens is that a cancer cell becomes resistant to tamoxifen by over expressing the oestrogen receptor and the way to make those cancer cells die is to withdraw oestrogen completely. If you withdrew oestrogen after tamoxifen treatment, you could get a lot of cancer cells to die that had become resistant to tamoxifen.
Charles - Hormones, mainly oestrogen, drive breast cancer cells to divide. So it acts as a sort of a fertilizer for breast cancer cells and tamoxifen has been the standard way that people try to block oestrogen action because tamoxifen is an anti-oestrogen drug. So, it prevents oestrogen from gaining access to the cells that cause the cells to divide. The problem is, that more than half of breast cancers eventually become insensitive to tamoxifen. And so, the first drug that we developed was - if you want, a drug that would work after tamoxifen has exerted it's action and worked. And that class of drugs is called aromatase inhibitors. And those drugs are drugs such as Arimidex, exemestane, letrozole, those sorts of drugs are all inhibitors of oestrogen synthesis. Under those circumstances, what happens is that a cancer cell becomes resistant to tamoxifen by over expressing the oestrogen receptor and the way to make those cancer cells die is to withdraw oestrogen completely. If you withdrew oestrogen after tamoxifen treatment, you could get a lot of cancer cells to die that had become resistant to tamoxifen.
Kat - And that's what these aromatase inhibitors do?
Charles - Exactly. So, what we've been trying to understand is why do cancer cells, breast cancer cells, become resistant to aromatase inhibitors? This has been the next big challenge. And after about 15 years of work, we now think that we understand the mechanism. What happens is that various other signalling pathways in the breast cancer cell as it were, impinges on the oestrogen receptor. So, it increases its sensitivity. So, this is a hypersensitized oestrogen receptor. Now, aromatase inhibitors, although they stop the body from making oestrogen, and lower the oestrogen significantly, often by as much as 99%, they don't actually reduce it by 100%. But this low level of persistent oestrogen can be amplified - the effect of that can be amplified by various other enzymes which can be used to target in breast cancer. So, one enzyme that we recently discovered that does this is something called CDK-7 and we now, over the last two years have developed a specific inhibitor for CDK-7 which prevents the activation of the oestrogen receptor. So, that drug is now being optimized and will be - we hope available for women whose cancer cells have become resistant to aromatase inhibitors. Another route that we have gone down and which has already produced a drug which is now being tested throughout Europe is in looking for other sources of this residual tiny percentage of oestrogen that remains after aromatase inhibitors have been used. It turns out that breast cancer cells can bypass the aromatase pathway by using a storage form of oestrogen.
 Kat - So it's a bit like having a spare tank of oestrogen hidden away.
Kat - So it's a bit like having a spare tank of oestrogen hidden away.
Charles - Exactly. In a storage form called oestrogen sulphate. And there's an enzyme called oestrogen sulphatase which cleaves the sulphate off, thus liberating the oestrogen. And so, it's a very clever way that the breast cancer cells use this enzyme to liberate the oestrogen which can then sit on the receptor, even when aromatase inhibitors are being used, and then drive the cancer cells to divide.
Kat - So, how do you try and combat the actions there?
Charles - Right. Well, it's taken again about 15 or 20 years work mainly by chemists, again, Cancer Research UK supported, at Bath University and at Imperial College, have made the first inhibitor of this enzyme. There hasn't been any inhibitor up to date, but this new drug has now been tested by our group here and has been found to completely abolish the enzyme activity and this is the drug that's in trials, in patients with breast cancer whose cancers have become resistant to aromatase inhibitors.
Kat - And that was Professor Charles Coombes from Cancer Research UK at Imperial College, talking about the promising role of enzyme inhibitors that alter the activity of oestrogen on breast cancer.
Can anti-perspirant cause cancer?
Kat - Absolutely none. This kind of information goes around and round on the internet but no, there's been a lot of scientific studies done with large - huge, thousands of women and basically, there's no difference. Using an anti-perspirant will not increase your risk of cancer. Neither will having underwire in your bra that's too tight or any of those things that go around the internet in hoax emails.Chris - I suppose it's a flaw in statistics, isn't it? They're saying, if you look at people who use underarm deodorants, they're more likely to get cancer. But then everybody uses an underarm deodorant so therefore, by sheer fluke, you're going to see people having breast cancer because it's a common disease.Kat - Also, in people who don't use underarm deodorants, you still get a case of breast cancer. I mean the best thing is to check out something like cancer help UK to find out whether this stuff is actually true and try and find out.
Why do cancer and AIDS patients lose weight so rapidly?
Chris - Actually, this is a phenomenon that's very well described. People who get malignancies very often lose dramatic amounts of weight and people who get HIV also lose enormous amounts of weight. What they both have in common is that the immune system is often driven into overdrive. It's pushed very hard. But scientists didn't really know, aside from saying, it must be some kind of production of signalling hormones in the immune system. They couldn't really tell exactly what the culprits were. But then about a year - no, a couple of years ago now almost- a paper was published in Nature Medicine. It was by Haiko Yonan et al and what they did was to find one of the factors that seems to cause this dramatic weight loss. They were using mice and they had mice in which they implanted a prostate cancer sample. And those mice went on to lose dramatic amounts of weight and when the scientists compared the levels of various chemicals in the blood of the mice with healthy mice, they found that one chemical in particular, MIC-1, Macrophage Inhibitory Cytokine -1 was at very high levels. And when they gave antibodies to that particular chemical, the mice didn't lose any weight.So it looks like it's the immune system, trying to respond to the cancer or being manipulated or thwarted by the cancer and it makes these abnormal signals that it shouldn't make. And they then cause the appetite centre in your brain's hypothalamus to go off kilter and it causes people to lose their appetite and stop taking in the right amounts of food. So, they're burning off lots of energy, but they're not replacing the calories. And as a result, they end up getting too thin and they lose all that dramatic amount of weight.The fact they've identified one of these factors is really good news because it kind of suggests that we might be able to reverse the process.
Is it possible to identify the first cell to mutate in cancer?
Kat - No. It probably isn't. Cancer cells are so mutated, it would be virtually impossible to go all the way back and in some cases, the original cell may not even be there.

51:45 - How do sharks make blood?
How do sharks make blood?
We put this question to Mark Briffa, lecturer in marine biology at the University of Plymouth:Mark - In vertebrates, a major component of the blood is the red blood cell or erythrocytes and this can make up the half volume of the blood and it's these cells that contain haemoglobin and do the job of transporting oxygen around the circulatory system. Now in adult humans, and other mammals these red blood cells are made in the red bone marrow and this is the soft tissue found inside the hollow bones of tetrapods or four-limbed vertebrates which as well as the mammals include amphibians, reptiles and birds. But red bone marrow isn't the only site where red blood cells are produced and in bony fish and cartilaginous fish that don't have bones like sharks and rays, the main places where red blood cells are made are in the spleen and in the front section of the kidneys. And some sharks also have the unique organ called Leydig's organ which is actually absent in the other vertebrates, and this is a large organ that's wrapped around the oesophagus. And although it was discovered way back in 1857, very little is known about exactly what it does, but it's thought that it might play some additional role in producing red blood cells and also in the immune system. So, although bone marrow is important for making red blood cells in humans; in fish, the most important site seems to be the spleen.

Where is an elephant's bone marrow?
Chris - This is a really interesting question. When I was in Africa, I found an elephant's leg bone and they're like a solid piece of rock.
An elephant is so heavy that it needs to have almost solid bone, with no marrow cavity in the middle, because the bones otherwise would not be strong enough to support the elephant's weight.
So an elephant shunts all of its blood formation up into its pelvis, which is also an important place in humans too, but it means its legs are largely solid bone with virtually no marrow in them.
Unlike a human, where most of our blood is made in our long bones - our femurs...
- Previous Aspirin and a Healthy Recession
- Next Eyes, Aliens and Goosebumps!










Comments
Add a comment