Could plastics be polluting your body? This week, we hear how hormone-mimicking chemicals leaching from plastics can cause coronaries, strokes and diabetes. Even the plastic mineral water bottle isn't safe - snails grown in them produce more offspring. Also, how oestrogen in lakes can feminize fish and cause their populations to plummet, Meera takes a trip to the sewage works to see how we clean up our act and, in Kitchen Science, Ben and Dave play with mud to find out how a water filter works. Plus, the hot news this week: how sperm get turned on, recreating colourful dinosaurs and understanding how mosquitoes smell the world.
In this episode
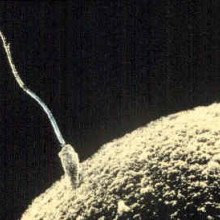
00:16 - How sperm get turned on
How sperm get turned on
Scientists have discovered the mechanism that starts sperm swimming once they exit the male...
Most people regard sperm as tiny swimming cells that vigorously dash about in search of eggs. But, in reality, they only begin behaving like this once they enter a female. In the male they remain quiescent so as not to wear themselves out before the "great egg race". How this was achieved though, no one knew.
Now researchers at the University of California San Francisco have found that an ion channel (a specialised pore) in the cell membrane allows protons - hydrogen ions - to flood out of the sperm at the right moment, and this is the trigger that starts them swimming.
Polina Lishko and her colleagues made the discovery using a technique called "patch clamping". A tiny pipette was applied to the membranes of individual sperm cells to measure the electrical currents flowing into and out of the cells under different conditions.
The work, published in the journal Cell, showed that, in response to alkaline conditions, a drop in zinc concentration, or the presence of one of the body's own cannabis-like chemicals - a substance called anandamide - the ion channel - called Hv1 - opened. The exit of hydrogen ions through the channel raises the pH inside the sperm, activating other metabolic processes including switching on the sperm flagellum, which it uses to swim.
The discovery sheds new light on an old mystery but may also hold the key to treatments for some forms of infertility and even the next generation of contraceptives: by blocking the proton pore with a drug scientists are hoping they can switch off sperm to induce a state of reversible infertility; it may also be possible to give the motility of sperm a boost in people with poor sperm function.

The markings of a dinosaur mapped out
By comparing tiny pigment particles between modern-day birds and fossils, researchers have rediscovered the colours of a dinosaur that existed 150 million years ago. And they weren't just ginger.
 Reporting in the journal Science, the latest team, led by Yale University, follow beautifully from last week's news of the flame-haired, feathered Sinosauropteryx.
Reporting in the journal Science, the latest team, led by Yale University, follow beautifully from last week's news of the flame-haired, feathered Sinosauropteryx.
This latest study analysed colour-making structures called melanosomes from the whole fossil of a single dinosaur and managed to map out a great deal of the pattern of its markings.
The dinosaur in question was Anchiornis huxleyi which had wings and a Mohawk-like crest on its head. From their reconstruction the palaeontologists think it would have had a grey body, its crest would have been reddish-brown, it had facial speckles (or freckles) and white feathers on its wings and legs, with black stripes.
They way they did this was rather ingenious. Jakob Vinther (also from Yale) was studying the ink sac of an ancient squid and realized that microscopic, grainy features within the fossil were actually melanosomes - and these contain melanin, which provides pigment in animals. But previously some scientists had thought these granules were just some rather unexciting ancient bacteria. Not anymore.
The team looked at 29 feather samples from the fossil and they measured and mapped out these melanosomes. They then compared these with the types of melanosomes known to create particular colours in living birds, using data compiled by a group at the University of Akron. The analysis allowed them to say with about 90 percent certainty that these are the colours of individual feathers.
The finding hints that, as these dinosaurs were flightless, colourful feathers may have arisen for aesthetics and attracting mates.
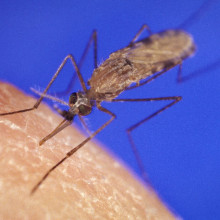
05:25 - What do mosquitoes smell?
What do mosquitoes smell?
Scientists have discovered the specific odour receptors used by the malaria-spreading mosquito species Anopheles gambiae to hunt down humans.
A paper in this week's Nature sets out an ingenious strategy  whereby a US-based team tested, one at a time, each of the 70-plus genes that encode mosquito odourant detectors in order to work out what chemicals each receptor could pick up.
whereby a US-based team tested, one at a time, each of the 70-plus genes that encode mosquito odourant detectors in order to work out what chemicals each receptor could pick up.
Yale researcher Allison Carey and her colleagues painstakingly inserted each gene in turn into a family of mutant fruit flies with a defective sense of smell. In these flies, known as the "drosophila empty neuron system", one of the nerves in their antennae lacks any of the olfactory receptors that are needed to detect odours.
Consequently, adding a mosquito receptor gene to this class of nerve endows the antennae of the recipient flies with the mosquito receptor, sensitising their antennae to any chemicals to which that receptor responded. And by studying just one gene at a time, the full spectrum of odours detected by that receptor could be identified.
To find out what odourants each responded to, the team recorded the electrical activity from the modified flies' antennae as they were exposed to more than 100 different consecutive smells, including chemicals known to be produced by humans and the bacteria that live on human skin.
The result was the discovery of 27 olfactory receptors that responded particularly strongly to components of human sweat.
Now, having mapped the "smell spectrum" detected by Anopheles mosquitoes and determined which receptors play the most important role in enabling the insects to home in on humans, the researchers are turning their attention to finding more effective repellents and trap-attractants.
"We're now screening for compounds that interact with these receptors," says co-author John Carlson. "Compounds that jam these receptors could impair the ability of mosquitoes to find us. Compounds that excite these receptors could help to lure mosquitoes into traps or repel them. The best lures or repellents may be cocktails of multiple compounds."

10:10 - Bisphenol-A and Human Health
Bisphenol-A and Human Health
with Professor Tamara Galloway, Exeter University
Chris - Now this week's show is all about the pollutants that get into water and, therefore also into our bodies. And in particular, chemicals that can leach out from the tonnes and tonnes of plastic that we're using every single day. Tamara Galloway is Professor of Ecotoxicology at Exeter University and she's been looking at the effect of just one of these plastic based chemicals...
Tamara - We've been interested in a compound called bisphenol-A for some time now because it's highly controversial. It was first produced around about the 1950s and at that time, it was produced as an artificial oestrogen. But it was then found that actually, it was a very useful monomer for making a range of plastics, particularly a kind of plastic called polycarbonate. Now, this is a hugely useful compound because it's makes very clear, rigid plastic which is good for re-usable containers. Previously, it was thought that this compound was extremely safe. However, more recently, results from animal health studies and laboratory studies have suggested that it could be having oestrogenic effects. So that is, it's acting like a hormone in the body.
 Chris - Where would we find this stuff, out there in the world or in the environment around us?
Chris - Where would we find this stuff, out there in the world or in the environment around us?
Tamara - Well, polycarbonate plastic is found in a range of rigid containers that are used for things like re-usable drinks bottles. Bisphenol-A is also used to make epoxy resins and these are most commonly encountered as the lining of tin cans. So, these are the two places that probably most humans are exposed to bisphenol-A.
Chris - And the evidence is that this stuff being in the environment and ubiquitous around us, and in these food containers, can get into our bodies?
Helen - Yes. More recent research done in America by the Centre for Disease Control have shown that something like 95 percent of the population has measurable levels of bisphenol-A in their bodies. They worked this out by measuring bisphenol-A in urine samples because what goes in to your body, you have to pass out.
Chris - What do we think this stuff does when it comes out of a food container or whatever, and gets into our bodies? Is it there in meaningful amounts?
Tamara - Well, the evidence has always suggested that it passes through our bodies very quickly. So we rapidly metabolise it and excrete it out in the urine within a matter of hours. So that, combined with earlier studies that suggested it was very safe meant that it wasn't really much of a public health issue. But more recent studies have suggested that, at very low concentrations it could be having an effect as an oestrogen in many animal model studies.
Chris - And why is that bad?
Tamara - That's bad because - oestrogen is a good hormone, it promotes growth and it promotes healthy reproduction - but if it's there at the wrong time and in the wrong place, then it can lead to adverse consequences.
Chris - Such as?
Tamara - Such as, if it's there at the wrong time in development, oestrogen can lead to feminization. So, if there's not enough testosterone and there's more oestrogen, you get feminizing effects.
Chris - And how are you trying to find out, when people are exposed to these things, what those health consequences could be?
 Tamara - Well, we were very interested to know, not just what happens in early life, but what happens in the general adult population. We were able to take advantage of a study done in America called the Nutrition and Health Examination Survey and this is a really fantastic program where tens of thousands of American volunteers have had all aspects of their health and nutrition studied. Since the 1980s, this has included the measurement of a whole range of different chemical compounds. And since 2003, it's also included bisphenol-A. Now one of the problems, if you're trying to look for very small effects is that you need very large populations to be able to see them. So this large population offered us the opportunity to search for adverse health effects.
Tamara - Well, we were very interested to know, not just what happens in early life, but what happens in the general adult population. We were able to take advantage of a study done in America called the Nutrition and Health Examination Survey and this is a really fantastic program where tens of thousands of American volunteers have had all aspects of their health and nutrition studied. Since the 1980s, this has included the measurement of a whole range of different chemical compounds. And since 2003, it's also included bisphenol-A. Now one of the problems, if you're trying to look for very small effects is that you need very large populations to be able to see them. So this large population offered us the opportunity to search for adverse health effects.
Chris - So what you're basically doing is - you've got a very big cohort of people, you can then stratify them according to potential exposure or the amount of bisphenol-A that's in them. You can measure that from urine for example...
Tamara - That's right, yes.
Chris - ...and you can then marry that up with medical history. Have they got condition X, Y, or Z, and see if there's any relationship?
Tamara - That's perfectly correct and we had a lot of history on the actual health conditions of the people who were suffering, but also a range of different biochemical measurements and laboratory measurements on the individual proteins and hormones in their bodies.
Chris - And when you do that study, what sort of relationships emerge?
Tamara - Well, quite surprisingly, when we did this study, what we found was that there was a strong association between increased exposure to bisphenol-A, as evidenced by measuring concentrations in the urine, and the presence or the reporting of cardiovascular disease, of diabetes, and of changes in liver enzyme function.
Chris - Is it not possible that people who just eat a lot and therefore being incidentally exposed to large amounts of bisphenol-A, because they're getting a lot of fast food out of food packets and things, that accounts for the fact that they have large amounts of it in their body. And because they put on a bit too much weight, that elevates their stroke, diabetes and cardiovascular risk?
Tamara - Absolutely. Well of course, that was our first thought, but we went back and controlled the data extremely carefully for body mass index, for diet, for socio-economic position, for all of the usual things that might confound this kind of result. And these associations remained robust throughout. So what that meant was that the 25 percent of the population with the highest exposure to bisphenol-A had over a two-fold increase in risk of cardiovascular disease compared to those in the bottom 25 percent. That's quite a big increase in risk.
Chris - It certainly is - obviously this is an association, it's not causal, you don't prove that one thing leads to the other.
Tamara - When you report this kind of association, in scientific terms the most important thing to do next is to show that you can see the same association in a completely separate population. That's called replication and it makes it much less likely that the results that you saw first time were the result of chance. So we went back. The American study had published a second wave of data in September of this year [2009] and we went and examined this study again, to see if we could see the same associations. And we did, so that makes it very unlikely to be a matter of chance.
Chris - This presumably, when people hear this, they find quite alarming, don't they? And they're thinking, "Well how do I avoid getting exposed to these chemicals? Have I been exposed? What can I do to minimize my exposure and is it too late?"
Tamara - Well, there's a second good news part of this story and that's between the first study and the second study, the level of exposure in the general population in America had fallen by 30 percent. We're not entirely sure, we can't say exactly why that is, but it's most probably because of changes in manufacturing processes from companies who are saying; "Well you know, there's all this concern over bisphenol-A. It's not being regulated against at the moment, but let's change our formulation so that the compound is used less." The other good new story is that bisphenol-A does seem to be rapidly metabolised in the body. So if you remove the exposure, it will quite rapidly disappear from the body. So it's not saying, if you've been exposed, then that concentration will always remain within you.
Chris - Which is reassuring. That was Tamara Galloway. She's at the University of Exeter and although they aren't sure yet what the mechanism for these health effects, the heart disease, diabetes, and so on could actually be, they think it could be related to the hormone mimicking effects that we know these chemicals can have. One important point that Tamara also went on to tell me is that they're also very concerned about the amount of plastic that's actually going into, and has gone into, landfill in past because these chemicals could now be leaching out into the water courses, and potentially also groundwater, and therefore, potentially exposing us to trouble in the future.

17:50 - Oestrogen in the Water
Oestrogen in the Water
with Karen Kidd, University of New Brunswick
Diana - Professor Karen Kidd from the University of New Brunswick studies what effect oestrogen-like chemicals have on populations of fish. Hello.
Karen - Hello.
Diana - Good to have you on this show with us, Karen. Where do these hormones, like oestrogen, come from?
Karen - Well, they're excreted by women. We excrete a number of natural oestrogens and we also excrete the oestrogen that's used in the birth control pill. So, these oestrogens are going into our municipal waste water works or treatment works, and not being completely broken down in the treatment process. And we're finding very low, but effective concentrations of the oestrogens in the waters being discharged into our rivers.
Diana - Once the oestrogens are in the water, what kind of effect can they have on the wildlife in there, on the fish?
Karen - Well, we know from a great body of work that's been done in the UK that these oestrogens are very effective at impacting or impairing reproduction in fish. In particular, there were a number of studies showing that male fish living downstream of these discharges were becoming feminised - and when I say feminised, I mean the males were producing early stage eggs or producing egg proteins, or developing smaller gonads, and there was a great deal of detective work to try and figure out what it was in the river waters that was causing this feminisation. These researchers at Exeter and Brunel and the EPA discovered that it was indeed these natural and synthetic oestrogens that women were excreting that was causing the feminisation in the male fish.
Diana - So this wasn't just affecting juvenile fish. This was affecting adult male fish.
Karen - Yes. It was impairing their ability to produce sperm and to fertilise eggs. It was also changing their external maleness, so they were less macho-looking, I guess you would say.
Diana - So, what was the effect on this particular species of fish and then other fish that perhaps fed on them?
Karen - Well, one of the questions that was outstanding was, what does it mean to have feminised male fish in the population? And there have been a number of studies since then - that work in the UK really motivated a lot of work in North America as well - where we found male fish in a number of our rivers that have become feminised because of the oestrogens. But the big question that we wanted to answer with our study was; what does that mean to have feminised males in your population? Can they still successfully reproduce or are you going to have fewer fish in the rivers because of this continuous low inputs of oestrogens and oestrogen mimics?
Diana - So, how did you do your specific study?
Karen - Well, there's a research station in Canada. It's run by the federal government and it's quite a unique facility because we have a series of lakes that we've set aside for research purposes. So there's no development in the watershed, there's no commercial or sports fishing. And what we can do is study the lake in its natural state for a few years and then add in chemicals or some other stressors and look specifically at what those stressors do to the ecosystem. In this case, we did a whole lake experiment where we took the oestrogen that's used in the birth control pill, we added it into the lake at levels that are very low, but what's been measured in municipal waste waters, and then we followed how the fish responded at the biochemical level, at the tissue level, and the organism and population level.
Diana - So you found obviously the male fish were becoming feminised. So first of all, what happened to that species of fish where the males were becoming feminised and then what happened to the predator fish that ate them?
 Karen - Well, right away, the male fish in the lake started to respond to the oestrogen, so they started producing egg proteins then they developed early stage eggs. And then only after two summers of additions of the oestrogen, we saw reproductive problems; the fat head minnow, which is a small-bodied fish in the lake, stopped reproducing and the numbers of individuals in the lake basically collapsed. And so, we showed that it didn't take very long for this very short-lived species to respond to small amounts of the oestrogen. And then what was surprising to us was not just how quickly these fish responded to the oestrogen, but also the impacts that the loss of these fish species had on others in the lake. In this case, what happened is the bigger predators in the lake, their numbers also started to decline, and it wasn't because they were getting exposed to the oestrogens. It was because the bigger fish were losing their prey species. So, they're becoming skinnier and they stopped reproducing as a result. This is showing us that you can have impacts of oestrogens on fish directly that interferes with their reproduction, but also, you can impact fish populations indirectly through the loss of their food source.
Karen - Well, right away, the male fish in the lake started to respond to the oestrogen, so they started producing egg proteins then they developed early stage eggs. And then only after two summers of additions of the oestrogen, we saw reproductive problems; the fat head minnow, which is a small-bodied fish in the lake, stopped reproducing and the numbers of individuals in the lake basically collapsed. And so, we showed that it didn't take very long for this very short-lived species to respond to small amounts of the oestrogen. And then what was surprising to us was not just how quickly these fish responded to the oestrogen, but also the impacts that the loss of these fish species had on others in the lake. In this case, what happened is the bigger predators in the lake, their numbers also started to decline, and it wasn't because they were getting exposed to the oestrogens. It was because the bigger fish were losing their prey species. So, they're becoming skinnier and they stopped reproducing as a result. This is showing us that you can have impacts of oestrogens on fish directly that interferes with their reproduction, but also, you can impact fish populations indirectly through the loss of their food source.
Diana - So, once the population have gone into decline, what did you do then? Did you remove the oestrogen and find out what would happen as soon as the levels went back to normal?
Tamara - We did. We added the oestrogen for three summers and then stopped, and followed the lake through recovery. The first two summers after we stopped adding the oestrogen, the fat head minnow population numbers were still very low. Then in the third year of recovery, they rebounded. The numbers of fat head minnow in the lake were back to what they were before we started the experiment, and this was great news because, like in the case of bisphenol-A, it's showing that once you remove the exposure, your effects go away basically. So, there can be recovery in systems once you take oestrogens out.
Diana - So they can recover very quickly, but what do you think the solution might be? Currently, sewage works don't remove oestrogen from wastewater. What do you think we could do to improve the situation?
Karen - Well, they do remove quite a bit of oestrogens. They know probably 80 to 90 percent of the oestrogens can be taken out when the wastewaters are treated with at least secondary treatment. The challenge is that in some cases, there's accidental overflow of systems when wastewater treatment works get overwhelmed with storm waters. Some areas, there's only very rudimentary treatment of wastewaters and it also seems to be more of a problem when river flow is made up primarily of wastewater discharge. So, the more people you have along a river, the more municipal wastewater that's being discharged, the greater the likelihood of impacting fish health from oestrogens is. So, we do know that it can be removed and the more you treat it, the more effective you are at removing oestrogens from the wastewaters.
Diana - Let's hope it happens more all over the globe. Thank you very much, Karen. That was Professor Karen Kidd from the University of New Brunswick, explaining how chemicals that mimic the effects of hormones like oestrogen have an impact on aquatic ecosystems.
If you reverse the polarity, do the sperm go backwards?
Silverwing was referring to this news story.
Chris - I'm not sure if they tested that, but my thought would be probably not because they're turning the sperm from something that's inactive to something that's active. But it's a good thought!

26:14 - How Safe is our Mineral Water?
How Safe is our Mineral Water?
with Marin Wagner, University of Frankfurt
Chris - We're talking about environmental pollution and how these chemicals can impact on human and animal health this week. Martin Wagner is at the University of Frankfurt and he and his colleagues have been looking at an oestrogen-like chemical that can leach out of bottles that are made of the substance PET, polyethylene terephthalate. That's the sort of bottles that you buy mineral water in. People are obviously worried as mineral water has become very, very popular in recent years. What could be the impact on their health? Hello, Martin.
Martin - Hello.
Chris - Welcome to The Naked Scientists. Could you tell us a bit about the studies you've been doing to try and find out how these sorts of plastics could be affecting people?
Martin - Yes. Basically, we used an in-vitro bioassay for detecting oestrogenic activity. It's a pretty simple system where you have a genetically modified yeast that expresses the human oestrogen receptor, and thereby, you can use it for detecting all compounds that bind to and activate our oestrogen receptor. Normally, you're using this for effluents of sewage works and just to detect the level of oestrogenicity in the environment that's sampled.
Chris - I see. You have genetically modified a yeast cell so that the yeast cell can see oestrogen and then presumably change colour or something, so that you've got a readout of whenever there's oestrogen, or something that had oestrogen-like effect in water, you can see it?
Martin - Yes, exactly. That's how it works. And since everybody was using it for environmental water samples, we thought, "why don't we test all our favourite mineral  water brands and have a look into that?" So it was, in the beginning, more like a fun experiment we did at the lab and of course, we didn't expect to find some estrogenic activity in mineral water because of you know, mineral water is just minerals dissolved in water! But then in the first experiment, we had really highly positive samples. This meant that we were able to detect oestrogenicity in these mineral water samples, and then we took a deeper look into that and we got funding and continued our work here.
water brands and have a look into that?" So it was, in the beginning, more like a fun experiment we did at the lab and of course, we didn't expect to find some estrogenic activity in mineral water because of you know, mineral water is just minerals dissolved in water! But then in the first experiment, we had really highly positive samples. This meant that we were able to detect oestrogenicity in these mineral water samples, and then we took a deeper look into that and we got funding and continued our work here.
Chris - So there are some brands of mineral water that come in plastic bottles. There are others that come in glass bottles. Some come in both. So that would presumably give you a quite neat way to compare, wouldn't it? You could look at the same brand of water in both plastic and glass.
Martin - Yes. What we did was, we just went to the supermarket and we bought 20 samples of 20 brands of mineral water here in Germany, and just looked at the oestrogenicity. Half of it was packed in plastic and the other half was packed in glass bottles.
Chris - What did you find?
Martin - It was quite surprising to us that we found really high oestrogenicity in these mineral water samples and in almost all mineral water samples, you can detect significant oestrogenic activity, 60% of the samples were positive. What was striking to us was that, in most of the water coming from plastic bottles, we detected estrogenicity whereas we only had three waters from glass bottles where we could detect some levels of oestrogen activity.
Chris - So, the assumption is that the oestrogen-like chemicals are coming out of whatever is in the plastic. They're getting into the water and your yeast is seeing them. What does this tell you about how likely they are to have relevant effects in humans. Because obviously, it's one thing to detect a chemical, it's another to demonstrate that it's actually having a physiological effect, in other words, a meaningful effect in the body?
Martin - Yes. That's the Achilles heel of our work that we've done so far. We have only detected oestrogenicity, the yeast can't tell you if it's bisphenol-A for example (it's not very likely that it's bisphenol-A) or other chemicals. It just tells you something activates the estrogen receptor, some chemical. It can be a mixture of different chemicals for example. So far, we haven't identified the chemicals causing this oestrogenic activity in mineral water yet. We're still working on it and it proves, I must say, really hard because you're looking for really low levels, low concentrations of these chemicals. In fact, we don't know what we are looking for, so we have to do an effect-directed chemical analysis, and that's what we're trying to do right now.
Chris - Can you not take the contents of the mineral water bottles and then expose them to an animal which is a bit bigger than a yeast, and therefore see whether they do have at least some effect in animals?
 Martin - Yes. We conducted the second series of experiments where we're asking the question whether the plastic bottle is really releasing some oestrogen-like compounds. So, we emptied all of our bottles and put oestrogen-free water in every plastic bottle and in every glass bottle. And then we inserted small little snails, New Zealand mud snails, inside the bottles and we cultured them and took care ofthem for eight weeks. And the thing with the snails is that they are really sensitive to oestrogens. So, if they are exposed to low levels of oestrogen-like compounds, they change their reproductive patterns and we can look at it.
Martin - Yes. We conducted the second series of experiments where we're asking the question whether the plastic bottle is really releasing some oestrogen-like compounds. So, we emptied all of our bottles and put oestrogen-free water in every plastic bottle and in every glass bottle. And then we inserted small little snails, New Zealand mud snails, inside the bottles and we cultured them and took care ofthem for eight weeks. And the thing with the snails is that they are really sensitive to oestrogens. So, if they are exposed to low levels of oestrogen-like compounds, they change their reproductive patterns and we can look at it.
Chris - So you get one group of snails, they're in water in glass bottles.
Martin - Right.
Chris - You got another group of snails in the same water, put into a plastic bottle and kept for two months.
Martin - Yes.
Chris - What happens to the snails?
Martin - After that, we looked at the reproductive output of our snails and we found that the reproduction of snails living in glass bottles was completely normal. But compared to that, snails living in plastic bottles had double the amount of embryoes. So, they doubled their reproduction, just by living in the plastic bottle, and that was for us, at least a strong evidence that some of these oestrogen-like compounds leached out of the plastic packaging material.
Chris - Which is a worry, isn't it? Just to finish off, Martin. Can you comment therefore - obviously, a snail isn't a person - but can you comment on the relevance on this to humans because one thing we're seeing in the world these days is that girls are going into puberty and they're starting menstruation at younger and younger ages all the time. The average age now is about 10. It used to be about 12 and this is within the last 50 years. This is not a genetic change. This is something environmental. Now obviously, people are better fed, but you can't explain all of it on the basis of nutrition.
Martin - I think our mineral water study provides a good example that we're exposed to lots of these endocrine disruptors, these oestrogenic compounds, from different sources. If it's in mineral water, I'm asking myself, is it not also in cheese maybe packed in plastic for example? So there's a lot of exposure going on and we don't know a lot about it. But there could definitely be a causal link there with reproductive problems...

34:00 - Keeping our Rivers Clean at the Sewage Treatment Works
Keeping our Rivers Clean at the Sewage Treatment Works
with Sara Rowland and Nicola Marvin, Anglian Water
Meera - This week, for some true scientific glamour, I have come along to the Cambridge sewage treatment facility located on Cowley Road in Cambridge. With me is Sarah Rowland and Nicola Marvin, both from Anglian Water, and they're going to take me through the various steps, processes and biological treatments that take place here in order to provide Cambridge with clean water. So, first of all Sarah, tell me a bit about this site.
Sarah - Well, sewage treatment has been carried out on this site since 1895. Before that, and we're talking back in Victorian times, everything used to go straight out into the river Cam untreated. Of course, you had dysentery, cholera, the plague, all sorts of nasty things, and Cambridge wasn't a very pleasant place to live in. But come 1895, the Victorians got a real hang of the idea of sewage treatment and they started pumping it here into what was called a sewage farm. They used to put it on the land and literally grow crops on it. We do use natural processes still today, but it's a little bit more sophisticated, and we're treating for a population of about 155,000 people every single day. That's the equivalent of about 5 million toilet flushes every single day, coming in here to be treated before it goes out clean into the river Cam.
Meera - We're currently standing at the inlet works which is very high up and I've got a good view of the entire sewage works. It's a big site, 200 acres. So what are the various stages that take place here?
good view of the entire sewage works. It's a big site, 200 acres. So what are the various stages that take place here?
Sarah - Well the first thing we do is screen out all the rags, all the disposable things that people have put down their loo that should be going in the dustbin. Items such as sanitary towels, condoms, anything that people think that they can get down the toilet and seem to like trying to do so, should be going in the bin. We take those out first, and then what we'll start doing is separating out solids from water. We've got some settlement tanks where everything heavy settles to the bottom and that goes for separate treatment. If you follow the effluent, the runny side of things, it gets digested using biological processes, using microbes, using bacteria that take out different elements of the sewage. We then settle it again to separate out the microbes or bacteria from the liquid. We put it through sand filters on the far side of the site which is a final polishing, just to make sure that the effluent has got a crystal clear quality to it, and then it finally goes out in the river Cam.
 Meera - Thanks, Sarah. Now, Nicola, you're one of the service delivery scientists here at the sewage works. You're going to take me over now to one of the trickling filters which is where the first stage of the bacterial processes takes place.
Meera - Thanks, Sarah. Now, Nicola, you're one of the service delivery scientists here at the sewage works. You're going to take me over now to one of the trickling filters which is where the first stage of the bacterial processes takes place.
Nicola - That's right, yes. Up until now, it's just been all physical processes, so that's where all the guts of the treatment happens.
Meera - So we've come alongside one of the trickling filters now. Their name is pretty much what they do, there's a large pipe moving along the top of a large bed of stones and it's trickling water onto the stones.
Nicola - That's right. The water comes through from the top surface and it's stones all the way down to through the filter bed. And these are probably about 1.8 to 2 metres deep. The water works its way down through and on the stones, we've actually created an environment that the biomass likes to live in. The biomass is bacteria. It's made up of hundreds or thousands of different types of bacteria and animals that eat the bacteria. It's a whole ecosystem on its own. So things like the carbonaceous treatment happens at the top, so basically, that's where the guts of the carbon is removed from the water. As this works its way down through the bed, the nitrifiers then proliferate. They love it down at the bottom because they get to eat all the ammonia that comes from urea. Urine breaks down to urea which then breaks down to ammonia. So things like nitrosomas and little bacteria like that, they live down near the bottom.
Meera - So, looking at the bottom and the water coming out now, what kind of things do we have left in here?
Nicola - As the water moves down through the bed, the natural control mechanism for the bacteria in the bed itself tends to get knocked off. By the time it comes out the bottom, we can see that it looks quite dirty, but actually, it's fully treated. The bacteria have done most of the treatment job here. They've taken out things like ammonia that will cause impact on the water course by causing eutrophication later on. If there's too much ammonia and phosphorus in the environment, you get algal blooms and things like that.
Meera - What then happens to just finally give it a good clean?
Nicola - Straight after the biological treatment, they all have their own settlement tanks and that's where we take the biomass, the bacteria, out of the system. We're separating basically the solids from the liquid.
Meera - What are the levels that you consider acceptable then for the water to contain of particular contaminants when it's released back into the Cam?
Nicola - Now the standards here at Cambridge are 20 [mg/l] for total suspended solids - that's the amount of 'bits' basically left in the water, 15 [mg/l] for BOD, Biochemical Oxygen Demand, and 5 [mg/l] for ammonia.
Meera - As you mentioned, one of the first stages is primary settlement where some of the more solid waste is settled out and removed. Various biological processes also take place with these solids as well.
Nicola - That's right. They have to go through a digestion stage so that we can actually fully utilise the sludge that's produced and actually make it reusable. So let's take a walk down to the far end of the site where the digesters are.
...
Meera - We are now surrounded by lots and lots of tanks. They're bigger than two or  three stories high, some of them up to six stories high. So, what is taking place in all of these tanks?
three stories high, some of them up to six stories high. So, what is taking place in all of these tanks?
Nicola - Well, we have to blend all the sludge that we create on-site, the indigenous sludge. So, the primary sludge, solids that we take out of the final settlement tanks from the filters, and then those in turn are blended into the tank that we're standing next to which is a feed tank for the monsal plant and the monsal plant is made up of six large green tanks. The first stage of those is at 42 degrees so, mesophylic digestion. Digestion has to happen without air, so this part of the process is all anaerobic. There's three stages: there's hydrolysis, breaking down the long carbon compounds into smaller chains, then there's acetogenesis which is where the bacteria have changed it into a food source for other bacteria, for the methanogens. Then the methanogens will actually produce methane. We start off at 42 degrees and then we push it through the rest of the monsal plant at 55 which kills all the pathogens, because sewage has got nasties in it. Viruses, bacteria that we don't want, that cause diseases, so we have to kill them off. We then push through into the two big digesters, the six-story high ones where we gather most of the methane that's produced by the sludge process. Before it can get to their final stage, we actually have to do what we call de-watering. The sludge comes out as a lovely, crumbly, what we call sludge cake or biosolids.
Meera - And this sludge cake is then used on agricultural land.
Nicola - That's right. The farmers love it. It's a really good soil conditioner and it's got phosphorus and nitrogen in it.
Meera - The methane that's produced is also used to help power the site?
Nicola - That's right. We run a combined heat and power unit and some boilers off the methane gas that's produced, and we can actually back feed into the national grid.
Meera - Well now, having understood what happens to the solids that's removed and how the water's treated, let's go join Sarah who's on the furthest part of the site, where the effluent that's actually created enters the river Cam and we can have a look at how clean it is.
...
Meera - Right. Hello again, Sarah.
Sarah - Hello.
 Meera - So, we're now by the final effluent that actually enters the river Cam. First of all, I have to comment on the fact that it smells actually quite nice here!
Meera - So, we're now by the final effluent that actually enters the river Cam. First of all, I have to comment on the fact that it smells actually quite nice here!
Sarah - Well if you think this is our finished product, we don't want this to have any nasty effect on the river.
Meera - So we did see the water at its original murky stage. Now, how long does it take for it to become this clean?
Sarah - From coming through the front door, to going out of the backdoor, shall we say, it takes anywhere between 8 and 10 hours. We've literally squeezed every last drop out of it. We've got rid of all the pollutants that we can, we've harvested all the energy in terms of methane, we've harvested a valuable soil conditioner for the farmers, and we're putting back that final bit, the effluent, in a nice clean state, back into the river Cam. We've come on a long way since the Victorians, let's face it!
Can you get mercury poisoning from too much sushi?
We posed this to Karen Kidd from the University of New Brunswick:
Mercury has been very well studied and we know a lot about its effects on human health. There's some great advice available on the web in terms of the types of fish that are safer to eat. For example, we know that fresh tuna has a lot more mercury in it than canned tuna, swordfish is another one that's high in mercury. We know that bigger, older fish are higher in mercury, so what I would recommend that everyone do, is take a look at that advice on the web and choose fish that we know are lower in mercury.
The Food Standards Agency have some advice online here.
If human males eat feminised fish, will they become more feminine too?
We put this question to Professor Karen Kidd:
There's a lot of concern about oestrogens in the environment and what they're doing to our health, but in this case, we've looked for estrogens in fish muscle and have found no evidence that they concentrate in the muscle or any parts of the fish that we eat. So, in this case, it's not a risk to human health.

How safe is the wax on apples?
This was answered by Yor_on on our forum... "If you walk into an orchard, pick an apple from a tree, rub that apple on your shirt, you'd notice that it shines, and that's because you've just polished off the natural waxes and also yeasts that the apple produces in order to protect its high water content. And without that wax, fruits and vegetables would end up going all dry and nasty.
After they've been harvested, apples get washed and brushed to remove leaves and field dirt, and then they get packed in cartons for shopping to your market. This process removes some of the fruits original wax coating that actually protects the fruit.
So the apple packers re-apply a commercial grade wax, and one pound of that wax can cover as many as 160,000 pieces of fruit. So in other words, two drops of it on each apple. The waxes have been used on fruits since the 1920s. they're all made from natural ingredients certified by the US Food and Drug Administration as safe to eat and they come from natural sources such Carnauba that wax, the leaves of the Brazilian palm, Candelia wax, which is derived from a reed-like dessert plant of the genus euphorbia and also food grade shellac."

52:33 - Have the seasons ever moved? ?
Have the seasons ever moved?
?
We posed this question to Dr John Nudds from the University of Manchester:
John - To answer this question, we firstly have to understand why we have seasons today. We have seasons today for a very simple reason, simply because the Earth is tilted.
If you remember the globe on your geography teacher's desk, the rotational axis of the Earth actually tilts an angle of about 23 degrees. Now if you imagine our tilted Earth revolving around the sun, when the northern hemisphere is tilted towards the sun, the southern hemisphere will be tilted away. But when our Earth gets around to the other side of the sun, the northern hemisphere will be tilted away from the sun, and the southern hemisphere will be tilted towards it. In the northern hemisphere, we have our summer in June, while the southern hemisphere has its winter in June. While the tropical regions around the equator will remain in a pretty constant distant from the sun all the time and therefore, experience little seasonal difference. So that's why we have seasons today.
Now, we have no reason to believe that this situation is ever any different to this. As far as we know, the Earth has always been tilted at this angle, so in the geological past, for example in the time of the dinosaurs, we can assume that the temperate areas of the globe experience the same four seasons that we experience today.
So to answer the question, if you impose our calendar on the Jurassic year, those dinosaurs living in temperate regions in the northern hemisphere as we do, experience their summer in June. Those living in the southern hemisphere experience their winters in June, and those living in the tropics experience little seasonal change. The one thing that was different however, is that the Earth is slowing down on its rotational axis. So, within the time of the dinosaurs, the days were actually shorter, but there were more of them in a year.
Diana - And a long year with more days in it would mean our calendar wouldn't settle perfectly over the dinosaur year. That said, they probably experienced the same annual temperature changes that we do today.
What is Aspartame?
Well aspartame, the sweetener, works because it's about 200 times sweeter as a molecule than sugar is. But because it contains virtually no calories compared with a large number of calories from sugar, you can use it to replace the sweetening things in food that would normally be sugar, and therefore, cut the number of calories in food. So that's how it can be used to help people lose weight. The worry is, whether or not it gets metabolised or broken down in the body into something toxic. So if you look at the molecular structure of aspartame, it consists of two amino acids. One of which is called phenylalanine - perfectly healthy there, no problem with that. Another one called aspartic acid - again, very common, no problem there. The two are linked together though with a bridge molecule which is a methyl group, a carbon atom attached to a couple of hydrogens. But when this molecule goes into your digestive tract, the acid and other chemicals in that environment actually break apart the two amino acids, the phenylalanine and the aspartate, and they get absorbed and used in the body in the same way any other amino acid would. But the methyl group actually gets turned into a methanol, and the other name for methanol is wood alcohol. We know that methanol can be harmful to health when it goes into your body - although in itself, methanol is not harmful, it goes to the liver. There, enzymes break down alcohol, ethanol, ultimately into ethanoic acid, vinegar, and it's just excreted. These enzymes also turn methanol initially into formalin, which is formaldehyde - a fixative - the same stuff you use to embalm bodies. Ultimately, the formalin gets oxidized to make formate or formic acid. This is the same stuff that ants squirt out their back end, which makes you sting. That's not good because it's very toxic to mitochondria, the powerhouses in our cells. Mitochondria supply energy to cells and if nerve cells don't have enough energy, they can die.
So if you have an intense ingestion of methanol, then the methanol can turn into formalin and fix your body internally, and also into formic acid which will deactivate your mitochondria, and that means nerve cells can die because they don't have enough energy.
The amount of methanol you get from aspartame is very, very low - a daily intake of probably less than a few milligrams or possibly 10 milligrams of methanol. So probably trivial, probably not going to harm you, but the jury is out. We just don't know, is really the answer.











Comments
Add a comment