We dig into the science of farming this week with a look at how agriculture can adapt to a changing climate, how scientists are striving to produce a perfect pea and a new initiative to turn native African fruit trees into the next commercial blockbusters. In Kitchen Science we use chromatography to reveal the colours concealed in chlorophyll, and in this week's news round-up, a new way to finger criminals using the trail of bacteria they leave behind, combating cancer with synthetic lethality, and how scientists have turned mosquitoes into flying vaccinators...
In this episode

01:40 - Targeting cancer's Achilles' heel
Targeting cancer's Achilles' heel
Cancer is a disease caused by gene faults - both inherited from our parents and picked up during our lifetime as our DNA gets damaged. And increasingly, researchers are working out how to use these faults to our advantage to fight the disease.
This week, research from Professor Alan Ashworth and his team at The Institute of Cancer Research, funded by Cancer Research UK, has revealed another Achilles' heel in cancer cells that we can try and target. They've just published their results in the journal Cancer Cell.
 The new research is based on the concept of synthetic lethality. In our cells, we have multiple ways of doing the same thing, which help to protect us if something goes wrong. It's a bit like a pair of trousers with a belt and braces holding them up - so if the belt breaks, then the braces still work to preserve your dignity.
The new research is based on the concept of synthetic lethality. In our cells, we have multiple ways of doing the same thing, which help to protect us if something goes wrong. It's a bit like a pair of trousers with a belt and braces holding them up - so if the belt breaks, then the braces still work to preserve your dignity.
But cancer cells have a lot of faults, and in many cases they're just relying on one mechanism to do something - to use our analogy, the belt is broken, and they're relying solely on their braces. So if we can figure out what they're dependent on, and target it with drugs - to snip the braces - then that might be a good way to treat cancer.
Professor Ashworth and his team looked at cancer cells that had faults in specific genes involved in repairing damaged DNA - genes called MLH1 and MSH2. Faults in these genes have been found in a number of different cancers, including bowel cancer.
Faults in either of these genes mean that cells tend to accumulate DNA damage, helping to turn them cancerous. But other pathways in the cell - the metaphorical braces - can still compensate for the lack of MLH1 or MSH2, so the cancer grows and spreads.
Here's where it gets clever. The researchers realised that molecules called DNA polymerases - which help to copy DNA when cells divide - were also repairing DNA, and compensating for the lack of MLH1 and MSH2.
The researchers used a technique called RNAi to knock out certain DNA polymerases in cancer cells lacking either MLH1 or MSH2. And they found that knocking out a polymerase called POLB could kill cells lacking MSH2, while targeting another polymerase called POLG could kill cells without MLH1.
We already know that this kind of approach works to treat cancer - we're starting to see very promising results from early clinical trials of new cancer drugs called PARP inhibitors, which work along the same principles, but targeting different pathways in the cell.
At the moment, the technique the researchers used to block POLB or POLG isn't easily transferable to patients (as we'll hear in my second news story). So scientists now need to find small chemicals that could be developed into drugs that would have the same effect. Although that's still some way away, this is a promising approach.
This research also highlights that we need to focus on the specific faulty pathways in cancer cells, rather than just the overall type of cancer. For example, faults in MLH1 and MSH2 are found in a subset of several different types of cancer, so they might all respond to polymerase-blocking drugs. We're starting to move away from thinking about treating "bowel cancer", or "breast cancer" etc., and towards treating "MLH1-deficient cancer cells", targeting the genetic faults that are specific to an individual patient.

05:14 - Bacterial fingerprint: a new way to catch crooks
Bacterial fingerprint: a new way to catch crooks
Scientists have found a new way to track down criminals - using the unique collections of bacteria they leave behind on things they touch.
The human body is plastered in a highly diverse community of bacteria that form a microbial fingerprint unique to each individual. So much so in fact that, even after a scrub with soap, after a few hours the same bugs are back on the average pair of hands.
 Now researchers have shown that this bacterial badge could even be used forensically. Writing in the journal PNAS, University of Boulder Colorado scientist Noah Fierer and his colleagues took swabs from computer mice and keyboards and were able to produce a genetic profile of the bacteria present and then pick out correctly - from more than 250 possible pairs of hands - the computer owner.
Now researchers have shown that this bacterial badge could even be used forensically. Writing in the journal PNAS, University of Boulder Colorado scientist Noah Fierer and his colleagues took swabs from computer mice and keyboards and were able to produce a genetic profile of the bacteria present and then pick out correctly - from more than 250 possible pairs of hands - the computer owner.
Although they caution that further investigation and validation of the technique will be necessary to establish its forensic credentials, the researchers think that it could be a powerful adjunct to existing methods. "This approach might represent a valuable alternative to more standard techniques," they point out.
"Given the abundance of bacterial cells on the skin surface and on shed epidermal cells, it may be easier to recover bacterial DNA than human DNA from touched surfaces."
08:13 - Gene switch technique shows promise in clinical trials
Gene switch technique shows promise in clinical trials
RNAi is a powerful technique used to switch off DNA polymerases in cancer cells grown in the lab. The technique has been shown to switch of genes in cells, and in small organisms like worms and flies. But until now we haven't seen that it can work in humans - it's really been a technique restricted to lab studies. Now research from Mark Davis and his colleagues in the US, published in the journal Nature, shows the first inklings that we might be able to get RNAi to work in patients.
RNAi stands for RNA interference. When a gene is switched on, it produces a little message in the form of RNA, a chemical that's similar to DNA. This RNA message then goes into the cell, where it's 'read' by the cell's protein 'factories', and the appropriate protein is produced. It's a bit like copying a recipe out onto a piece of paper, then taking the paper into the kitchen to bake a cake, rather than the whole recipe book.
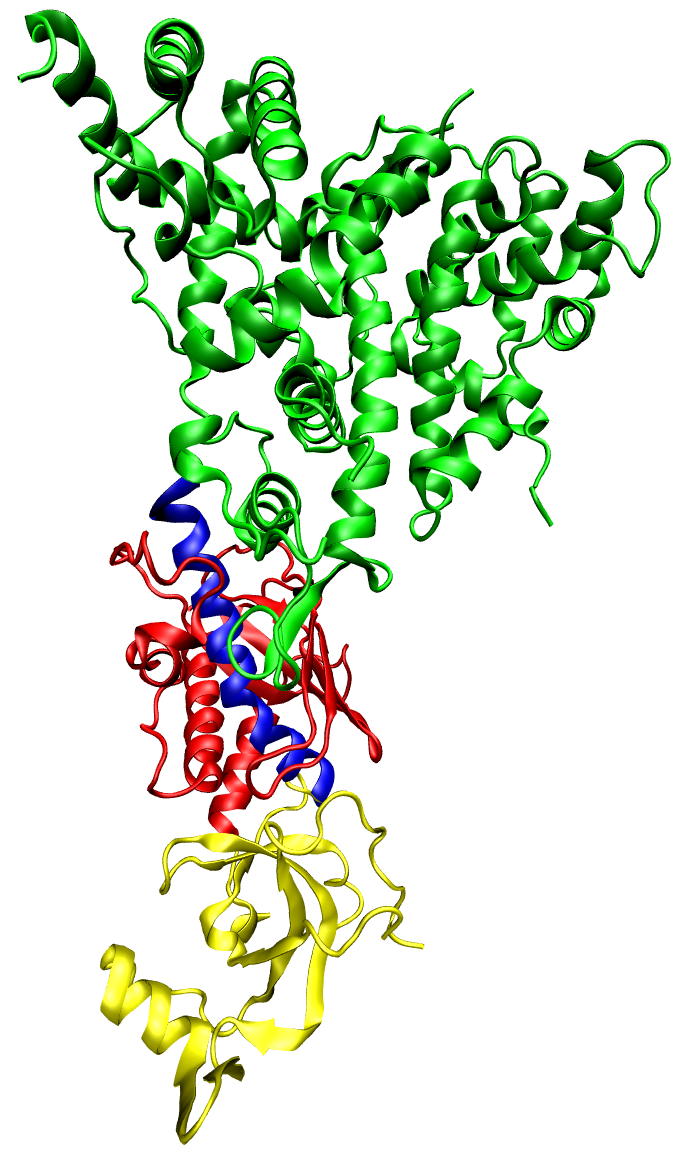 |
| One molecule of the Dicer-homolog protein. Dicer is an RNase that cleaves long double-stranded RNA molecules into short interfering RNAs (siRNAs) as a first step in the RNA interference response, and also initiates the formation of the RNA-induced silencing complex (RISC). © Opabinia regalis |
Researchers discovered that short reverse stretches of RNA could effectively silence RNA messages, and individually switch specific genes off. It's an incredibly powerful technique that helps researchers to switch genes on and off in the lab, as we heard in my earlier story, but it's not been clear whether it actually works in larger animals, or in humans.
In this case, the researchers were running a small-scale clinical trial to test RNAi in patients with cancer. They were using tiny nanoparticles to deliver the RNA to the tumours - for some reason that we don't understand, nanoparticles seem to be attracted to tumours.
The scientists discovered that the nanoparticles had effectively delivered their RNA payload to the cancer cells, and that the therapy was working as expected - the specific gene they were targeting, called RRM2, was getting switched off.
This is a very small-scale, early trial and in this paper, the researchers only present data from three patients, all of whom had melanoma skin cancer. And we don't know whether the RNAi actually helped to treat their cancer. Still, it's an impressive demonstration that the RNAi technique can work in humans, and bodes well for future research in this area.
10:58 - Turning mosquitoes into flying vaccines
Turning mosquitoes into flying vaccines
Scientists have found a way to use mosquitoes as mobile vaccinating machines!
Mosquitoes are ranked as the world's most dangerous animal because the diseases they transmit, including malaria, dengue and yellow fever, kill several million people per year. But now scientists having turned the tables and are working on ways to reprogramme mosquitoes to convert them from agents of illness into low cost flying vaccines!
 The work is based on the premise that when mosquitoes take a blood meal they first inject saliva, containing anticoagulants and immune-evading agents, around the blood vessel puncture site. This is what provokes the itchy inflammatory aftermath but is also responsible for transmitting infectious agents, which the mosquito regurgitates into the wound when it feeds.
The work is based on the premise that when mosquitoes take a blood meal they first inject saliva, containing anticoagulants and immune-evading agents, around the blood vessel puncture site. This is what provokes the itchy inflammatory aftermath but is also responsible for transmitting infectious agents, which the mosquito regurgitates into the wound when it feeds.
But Shigeto Yoshida, from Jichi Medical University in Japan, reasoned that it ought to be possible to exploit this unpleasant aspect of the insect's behaviour in a beneficial way. They set about genetically modifying Anopheles mosquitoes to make them produce in their saliva a protein called SP15, which is critical to the spread of another major disease-causing parasite, called leishmania.
Mice bitten repeatedly by these modified mosquitoes, the team found, developed antibodies to SP15, which other researchers have shown can protect against leishmania transmission.
"Following bites, protective immune responses are induced, just like conventional vaccination but with no pain and no cost," says Yoshida. "What's more, continuous exposure to bites will maintain high levels of protective immunity, through natural boosting, for a lifetime."
Now the team are testing whether mosquito-vaccinated mice really can be protected from leishmania infection; the same SP15 protein has been successfully tested as an experimental vaccine previously so the odds are that it should work.
But whether this flying vaccinator technology will take off in general is another matter. Some may feel slightly stung, however, by the idea of a natural and uncontrolled vaccination system delivering unmetred drug dosages and boosters indiscriminately, a sentiment prempted by the scientists themselves in their paper, published in Insect Molecular Biology.
"The concept of a "flying vaccinator" transgenic mosquito is not likely to be a practicable method of disease control, because "flying vaccinator" is an unacceptable way to deliver vaccine without issues of dosage and informed consent against current vaccine programs. These difficulties are more complicated by the issues of public acceptance to release of transgenic mosquitoes."

12:36 - Preventing Alzheimers Disease in Fruit Flies
Preventing Alzheimers Disease in Fruit Flies
with Dr Leila Luheshi, University of Cambridge
Kat - Also in the news this week, scientists have discovered a new molecule that can block the build up in the brain of beta amyloid. This is the toxic protein that causes Alzheimer's disease. But rather than working on human subjects, Dr. Leila Luheshi who's based at the Genetics Department here at the University of Cambridge made a discovery using fruit flies that were genetically engineered to develop the insect equivalent of Alzheimer's disease. Meera Senthilingam went along to meet her to find out more.
Leila - So it turns out that if you would like to study Alzheimers disease really you  need to have some sort of model of the disease in your own organism other than humans which you can try out new treatments on, and you can test new and rather crazy ideas. It turns out Drosophila are very good for this. They have quite complicated brains, a lot of the same genes that there are in humans and also, they have very short lifespans, so we can test lots of different treatments very rapidly in them. It turns out that we can give them something like Alzheimer's disease and then go out, and try and find different ways of curing it.
need to have some sort of model of the disease in your own organism other than humans which you can try out new treatments on, and you can test new and rather crazy ideas. It turns out Drosophila are very good for this. They have quite complicated brains, a lot of the same genes that there are in humans and also, they have very short lifespans, so we can test lots of different treatments very rapidly in them. It turns out that we can give them something like Alzheimer's disease and then go out, and try and find different ways of curing it.
Meera - So how do you set about giving them Alzheimer's disease?
Liela - So we take the gene that encodes for the protein that seems to be important in causing Alzheimer's disease in humans, and we introduce it into our fruit flies. Then, when that gene is expressed in the brain this protein, which is called amyloid, clumps together in the brains of our fruit flies, just like it does in the brains of humans with Alzheimer's disease. Then the fruit fly's brain starts to degenerate, and we can see that they can't move properly, they die much sooner, and their memory is impaired as well, just like people with Alzheimer's disease.
Meera - So, is it the case where all people that have the gene will develop Alzheimer's?
Leila - No. Actually, the amyloid protein that damages the brain in Alzheimer's disease is present in all of us. But under normal circumstances, it's present in small enough quantities, and in a form which doesn't seem to cause us any problems. In fact, what seems to happen in most patients with Alzheimer's disease that you produce a little bit more maybe of a slightly stickier than usual version of this protein. That starts to stick together in the brain, and then that starts the damage of the neurons.
Meera - So having given these flies Alzheimer's, you've set about finding a new molecule that should hopefully treat Alzheimer's in these flies, and hopefully in humans?
 Leila - Yes. So what we thought was, well, if we know there's a protein, this protein called amyloid, which clumps together in the brains, then if we can prevent this protein from clumping together, then hopefully the cells in the brain won't die anymore. And so, what our colleagues in Sweden did was they designed a new molecule which is called an affibody which binds to the amyloid protein that is important in Alzheimer's disease, and prevents it from clumping together. They first tested this in a test tube and they found this was very effective, and then they came to us and said, "Well, can you test this in your fruit fly model of Alzheimer's disease?" When we put this new molecule, this affibody, into the brains of our fruit flies with Alzheimer's, we found that essentially, the fruit flies were cured of the Alzheimer's disease, and in fact the protein which clumps together, this amyloid protein, was now completely cleared from the brain.
Leila - Yes. So what we thought was, well, if we know there's a protein, this protein called amyloid, which clumps together in the brains, then if we can prevent this protein from clumping together, then hopefully the cells in the brain won't die anymore. And so, what our colleagues in Sweden did was they designed a new molecule which is called an affibody which binds to the amyloid protein that is important in Alzheimer's disease, and prevents it from clumping together. They first tested this in a test tube and they found this was very effective, and then they came to us and said, "Well, can you test this in your fruit fly model of Alzheimer's disease?" When we put this new molecule, this affibody, into the brains of our fruit flies with Alzheimer's, we found that essentially, the fruit flies were cured of the Alzheimer's disease, and in fact the protein which clumps together, this amyloid protein, was now completely cleared from the brain.
Meera - How was this affibody created?
Leila - It's based on a protein that is found naturally inside bacteria. What our colleagues in Sweden did was they made mutations in this protein, then they screened thousands, in fact, millions of different mutations in this protein until they found a mutant form of this protein that bound very specifically to the amyloid protein that's important in Alzheimer's disease.
Meera - And how did you set about putting this affibody into the Drosophila then as well?
Leila - So we essentially did the same thing that we did to make our model of Alzheimer's disease in the first place. We made a gene for this affibody protein and we put the gene into the brain of our fruit fly, and then that gene expresses the affibody protein, so then we have one fruit fly that expresses the affibody, one fruit fly that has Alzheimer's disease, and when we breed those two fruit flies together their offspring, their children, have both proteins. They have the Alzheimer's related protein and they also have the affibody, and then we could see that when you have those two proteins together, the flies didn't get Alzheimer's disease anymore.
Meera - So, its' not the case where the fly had Alzheimer's and then you gave it this protein, and it actually treated the Alzheimer's that it had previously?
Leila - No. So right now, we have to do it with both proteins there from the beginning together so we prevent the amyloid protein that's important in Alzheimer's disease from ever clumping together. But what we would like to do in the future is to make our flies so that they actually develop Alzheimer's disease, so we let the protein stick together in the brains of the flies, and then put in affibody protein afterwards, and see if we can either help the symptoms or reverse them. But we haven't done that experiment yet. First of all, we have to go through some more tests in slightly more complicated models of Alzheimer's disease in our fruit fly to see if this kind of protein - for instance, if we inject it into the bloodstream, can even get into the brain? It's got to be able to get to the brain, really, to have any effect. So, we're still somewhere off seeing whether this kind of therapy would ever work in humans, but at least in principle, we know that if we can clear this amyloid protein from the brain that it might help the Alzheimer's patients. Assuming we get to them early enough in their disease.
Kat - That was Leila Luheshi from the University of Cambridge, talking to Meera Senthilingam there about a work she's just published in the journal PLoS Biology.

17:43 - Farming in a Changing Climate
Farming in a Changing Climate
with Professor Brian Thomas, Warwick University
Chris - The human population is estimated to be about 6.8 billion right now, and it's set to grow to over 9 billion by 2040. Supporting a population that big is a really big challenge, but it could be made even harder if you factor in the effects of climate change. Professor Brian Thomas is from Warwick University where he works on predicting how climate changes could affect future food production. He's with us now. Brian, Hello.
Brian - Hello.
Chris - Welcome to the Naked Scientists. Please tell us, if you would, in what sort of way, or how, do we think climate change will impact on agricultural production?
 Brian - I think the impact of climate change on production will be shown in a range of different ways. We're dealing with - first of all, the general changes in the climate, which you might think of increasing levels of carbon dioxide (which could be a key issue for agriculture), but also increasing temperatures and changes in water availability. So at one level, we can look at those factors and make assessments of how they might affect crop production and food production. And then, imposed on top of this, there are changes in particular types of weather conditions - sometimes thought of as extreme events, but when you get combinations of whether that in combination with a particular characteristic of the crop may lead to loss of production or crop loss in a particular year. We see that, at the moment, in general, crops in the UK are well-adapted to the general UK climate, but when we have a particular set of unusual weather then that will then often result in a particular problem in a particular crop, and we see that in a particular way. So, our work is really a combination of some general assessments of the impact of climate change on overall crop production, but also, trying to tease out which particular aspects might be changed, and therefore, really get some heads-up on the sort of research we need to do to modify crops to anticipate those problems.
Brian - I think the impact of climate change on production will be shown in a range of different ways. We're dealing with - first of all, the general changes in the climate, which you might think of increasing levels of carbon dioxide (which could be a key issue for agriculture), but also increasing temperatures and changes in water availability. So at one level, we can look at those factors and make assessments of how they might affect crop production and food production. And then, imposed on top of this, there are changes in particular types of weather conditions - sometimes thought of as extreme events, but when you get combinations of whether that in combination with a particular characteristic of the crop may lead to loss of production or crop loss in a particular year. We see that, at the moment, in general, crops in the UK are well-adapted to the general UK climate, but when we have a particular set of unusual weather then that will then often result in a particular problem in a particular crop, and we see that in a particular way. So, our work is really a combination of some general assessments of the impact of climate change on overall crop production, but also, trying to tease out which particular aspects might be changed, and therefore, really get some heads-up on the sort of research we need to do to modify crops to anticipate those problems.
Chris - Is it just a question of saying, "some bits of the earth are going to become a lot less propitious for growing anything. Some will become better suited to growing things that currently grow in different places"? And so, what we need to do is to have a re-jig - move things around a bit and we'll maintain productivity without actually having to sacrifice anything. Or are we facing a situation where actually we're not going to be able to feed everybody?
Brian - Inevitably, there will be shifts in production - it will often be "the art of the possible", but there are some big challenges there. For example, in some of the models I've seen for production, in China for example - which, obviously, in terms of its scale and its contribution to overall food production is quite immense. If you go back one step and say that one of the big problems is maintaining or sustaining production when temperatures increase, so much of the staple foods for temperate countries, the cereals, the impact of increasing temperature is usually to decrease yield, and that is partly to do with the fact that you get an acceleration of the growth of the crops so they have less time to photosynthesise and accumulate resources. The models suggest that a lot of that would be offset, or some models say fully offset, by the increasing fertilisation effect of carbon dioxide. But really, I think that the estimates are probably subject to quite a lot of uncertainty.
Chris - Could you just tell us, how is it you actually do what you do? Have you got a giant computer program where you're able to change various model parameters, tweak things in order to say, "Well, if we tweak this, this is the likely effect on this bit of the climate and this part of the world, and crops that grow there would change in a following way." And then you do that many, many times and look at many, many different bits of the world, and this gives you a global picture?
 Brian - Well, to be honest, we're not looking at a global picture. We're looking very much at UK agriculture and this work really is funded by Defra who are trying to use this sort of information to set their policy, although many of the principles we come up with would be applicable very widely. But the main approach we're taking is to use what is freely available output from computer models from UKCP09 who make predictions of the UK climate over the rest of this century, and they will give estimates of changes in overall rainfall and average temperature. But they also have the facility to take these averages and to deconvolute them into typical runs of daily weather. That would include things like day temperature, night temperature, rainfall. A lot of agriculture, a lot of production in agriculture, is sitting in quantitative models, and then you can use the output from the weather generator models, put it into the agricultural models and then say, "What is going to be the change in say, in 2030, or 2050, 2080?"
Brian - Well, to be honest, we're not looking at a global picture. We're looking very much at UK agriculture and this work really is funded by Defra who are trying to use this sort of information to set their policy, although many of the principles we come up with would be applicable very widely. But the main approach we're taking is to use what is freely available output from computer models from UKCP09 who make predictions of the UK climate over the rest of this century, and they will give estimates of changes in overall rainfall and average temperature. But they also have the facility to take these averages and to deconvolute them into typical runs of daily weather. That would include things like day temperature, night temperature, rainfall. A lot of agriculture, a lot of production in agriculture, is sitting in quantitative models, and then you can use the output from the weather generator models, put it into the agricultural models and then say, "What is going to be the change in say, in 2030, or 2050, 2080?"
Chris - Speaking of which Brian, what's your prediction for the southeast corner of the UK in the next 30 years?
Brian - Probably, it'll be warmer, it'll be wetter in the winter, dryer in the summer, crop production will probably hold its own just about, but crops that require a continual run of production are particularly vulnerable to periods of drought. Crops that require real cold in the winter like fruit trees are quite vulnerable to the increasing temperature.
Chris - Which is a worry because we've heard from Redlace Selene who's listening in at Second Life and says, "I was looking forward to the olive tree and the lemons in the UK garden." Never mind - better luck next time!

24:03 - Domesticating African Fruit Trees
Domesticating African Fruit Trees
with Roger Leakey, James Cook University; Tony Simons, World Agroforestry Centre
Meera - When many of us think of commercial crops coming from Africa, we think of coffee or cocoa. But the continent is home to some 3,000 species of wild fruit trees which have provided local residents with food and medicine for centuries. These trees are now being domesticated and harnessed in countries such as Cameroon and Kenya, as well as others, in order to boost the economy and meet the nutritional needs of the increasing population. Roger Leakey is a former director of the World Agroforestry Centre and adjunct professor of James Cook University in Australia. He helped develop this program of domestication and explained to me how his team set about doing this...
Roger - The beginning of that process was to go into the communities and to start talking with them and saying, "Which of all the species that you used to collect from forests would you actually like to cultivate?" The answer is nearly always some of the indigenous fruits and nuts. They have high levels of micronutrients, vitamins and minerals. Some of them are high in proteins and oils, so many of them have the dietary requirements that are not in starchy food crops. So I guess the thought is that they can be used to give a more balanced diet.
Meera - Well how did you set about getting farmers to actually start domesticating these and actually creating nurseries of particular trees?
Roger - Well that would turn out to be remarkably easy because they were so enthusiastic. Nobody had ever asked them before what they wanted to do and they instantly started to understand the possibilities once we explained a little bit of very basic genetics. It's quite easy to talk about heritability and the fact that children are different from their parents, from siblings are different from one another, and as soon as you mention that and say, "Well then you can propagate and make multiple copies of the best one..." They instantly cottoned on.
Meera - Another added benefit seems to be the fact that these various fruit trees can be harvested at different times of year. So, is the benefit then of this project also that you can have a continuous supply of various wild fruits?
Roger - Yes, certainly. And indeed, even within some species, we're now seeing that  we actually confined individual trees that fruit out of season. So, if you take the African plum for example in Cameroon, it normally fruits between May and September, but we've now got some cultivars which are fruiting around Christmas. So, we started to see that we can actually extend the productive season by selection as well.
we actually confined individual trees that fruit out of season. So, if you take the African plum for example in Cameroon, it normally fruits between May and September, but we've now got some cultivars which are fruiting around Christmas. So, we started to see that we can actually extend the productive season by selection as well.
Meera - So these fruit trees can be grown in rotation to supply fruit all year round. But what kinds of fruits are actually being grown and just how nutritious are they? Tony Simons is the current deputy director of the World Agroforestry Centre and he filled me in...
Tony - Well, fruit trees provide a number of things. They provide energy, vitamins, micronutrients, water, and also stimulants. And there are literally hundreds of different types of fruit trees. If you take the 33 trees native to the UK, there are only 3 that are essentially fruit trees. That's the crab apple, the hazel and the juniper and those are hardly mainstream fruit trees. Whereas in the Miombo woodlands in southern Africa, there are 75 different fruit trees that provide vitamin A, vitamin C, vitamin E, iron, micronutrients, energy for children etc, and thrse are tremendous contributions to household diets in rural areas.
One of those species is the African plum. The scientific name is Dacryodes edulis and edulis means edible. So this African plant grows in humid forests of west Africa. It is eaten by local people and collected from the wild, but now it's being brought into cultivation because the marketing of it - the growing urban populations love this particular fruit. It is traded across regional boundaries at the level of about $70 million US a year, and it's highly nutritious. It is prized for being eaten cooked and also eaten fresh, and the oil from the seed is also now being used in other food preparations.
 If we go to southern Africa, there's the species Sclerocarya Birrea. Scleo meaning hard, Carya meaning nut, and this particular species is the one that is used in the liqueur Amarula. But it's also used for beer making, it's used as a fresh fruit, and this particular species is also used, the oil of it is used in the food industry - it is a very good oil for sausage machines, etc, as a lubricant when you need a food crude oil. Other fruit species are Vitellaria paradoxa, that is the shea tree. It's traded much more in Europe both as cocoa butter substitute, and also for cosmetic and pharmaceutical products.
If we go to southern Africa, there's the species Sclerocarya Birrea. Scleo meaning hard, Carya meaning nut, and this particular species is the one that is used in the liqueur Amarula. But it's also used for beer making, it's used as a fresh fruit, and this particular species is also used, the oil of it is used in the food industry - it is a very good oil for sausage machines, etc, as a lubricant when you need a food crude oil. Other fruit species are Vitellaria paradoxa, that is the shea tree. It's traded much more in Europe both as cocoa butter substitute, and also for cosmetic and pharmaceutical products.
Meera - What stage is this domestication at at the moment? How much of an increase in production say, has there been, and what are the benefits been so far of this?
Tony - Let's take Uapaca kirkiana from southern Africa. This particular species had wild fruit that were about 10 or 15 grams each. The cultivars, the improved varieties, have fruit that are about 80 or 90 grams. So there's a 5-fold increase in the size of the fruit. There's also have been an increase in the thickness of the skin of the fruit so that it's not attacked by insects. And also, they have a much longer shelf life. So, rather than the three, four-day shelf life, they now have a two or three week shelf life. And we've seen a lot of growth in the horticultural industry in these countries, and Kenya now supply something like $100 million worth of mangoes to the international market, and about $60 million of avocado.
Meera - So that's quite an improvement in terms of both international trade and feeding the population, but what's the next step? And as a result, what's in the future for these African villages? Let's go back to Roger Leakey to find out...
Roger - We started out in two villages in Cameroon, something like 15 years ago now. It's snowballed from there so now we're working with over 450 villages, and more than 7,000 farmers in those villages. The next crucial step is some scaling up, if we're really going to have impact on global poverty, malnutrition, and hunger, then obviously it's got to be scaled up to millions and millions of farmers. I've recently been evaluating the longest running project to date and we found there that there were 31 positive impacts from the project already, ranging from obviously people making money, but people also saying that they're healthier, they're eating better diets, and they're able to send their children to school, and the most exciting one actually from my point of view, I think was young people saying, "We're actually going to see now and we have a future to stay in our village and make money in the villages rather than having to go to the town."
Kat - So, plenty of hope in the future of fruit then. That was Roger Leakey from James Cook University in Australia and Tony Simons, Deputy Director of the World Agroforestry Centre talking to Meera Senthilingam.

31:43 - Perfecting the Pea
Perfecting the Pea
with Dr Claire Domoney, John Innes centre, Norwich
Chris - The humble pea is something of a wonder plant, we all like to think - for one thing, it adds nitrogen back into the soil, and that reduces the amount of fertiliser that you need to add to the next crop that you want to grown on the same soil. But, some scientists think they can make it even better, and Claire Domoney is with us from the John Innes centre, to explain how. Hello Claire...
Claire - Hello, Chris.
Chris - Welcome to the Naked Scientists. Tell us if you would first of all, why is the pea a good crop?
 Claire - Well, we really want to look at it within the context of increasing the sustainability of agriculture. We know we have to cut our use of nitrogen fertilisers, so we're looking at the pea as really a model legume crop where we have excellent genetics, and we can use a lot of very powerful techniques - molecular genetics, biochemistry, metabolite profiling - to study seed quality. We've had lots of discussions with industries involved in growing peas and harvesting peas and so on, breeding peas, and one of the outcomes of these discussions, within what's known as the Pulse Crop Genetic Improvement Network which is funded by Defra, is that quality improvements are really greatly desired. At the moment, the industry use quite subjective methods for determining quality and so, better methodology for determining quality would really be very welcomed.
Claire - Well, we really want to look at it within the context of increasing the sustainability of agriculture. We know we have to cut our use of nitrogen fertilisers, so we're looking at the pea as really a model legume crop where we have excellent genetics, and we can use a lot of very powerful techniques - molecular genetics, biochemistry, metabolite profiling - to study seed quality. We've had lots of discussions with industries involved in growing peas and harvesting peas and so on, breeding peas, and one of the outcomes of these discussions, within what's known as the Pulse Crop Genetic Improvement Network which is funded by Defra, is that quality improvements are really greatly desired. At the moment, the industry use quite subjective methods for determining quality and so, better methodology for determining quality would really be very welcomed.
Chris - So let's look at that first of all then. So, are we defining quality in terms of how consumers see the pea on their plate and how the mushy pea goes down further up north on the coast or are we talking about it in terms of what yields the plant that grows from that pea actually produces?
Claire - We're talking first and foremost really about quality as perceived by the customer because there are a lot of issues to do with taste, flavour, mouth feel, a lot of customer determined traits, and on this basis, breeders are putting their late generation materials through breeding programs. They go to taste panels who sit down and score their products on all sorts of characteristics, which is done in a very subjective way as you can imagine. This is quite time consuming and they end up scoring the different lines on the basis of bitterness and sweetness, and lots of different characteristics like this. But it really is a very subjective way of determining quality. So we want to really add some biochemical knowledge to this and do metabolite profiling where we can look at all the range of compounds which are being determined by these taste panels, link those through to the genes that are involved in pathways that make these compounds, and through that, get hold of genetic markers that can be used by breeders. In that way, they'll be able to use their markers much earlier in breeding programs, and not do as they do at the moment which is reject valuable lines because at early stages, they can't do this type of screening.
 Chris - I see what you mean, so in the same way that people are making better, fatter turkeys that put on more weight in the right places, by knowing that the genes that tend to make those nice traits happen also to produce other visible changes in the birds, so you can, by picking the change in the bird that you can see, get other benefits that you couldn't originally see. You're doing that, slightly more intelligently and slightly more carefully. That will obviously produce improvements in quality that will make peas more popular, but what about the other thing we discussed which was the question of improving the soil? Because peas do have this one full ability to take nitrogen out of the air and shove it into the soil, don't they?
Chris - I see what you mean, so in the same way that people are making better, fatter turkeys that put on more weight in the right places, by knowing that the genes that tend to make those nice traits happen also to produce other visible changes in the birds, so you can, by picking the change in the bird that you can see, get other benefits that you couldn't originally see. You're doing that, slightly more intelligently and slightly more carefully. That will obviously produce improvements in quality that will make peas more popular, but what about the other thing we discussed which was the question of improving the soil? Because peas do have this one full ability to take nitrogen out of the air and shove it into the soil, don't they?
Claire - Yes, absolutely and of course, we get a value from not just the pea crop itself but also the value to the subsequent crops. So this can result in a huge saving in nitrogen fertiliser. And so, this is an incredibly valuable thing to think about, particularly these days with the increasing costs of nitrogen fertiliser as well as the greenhouse gas emission issue associated with nitrogen fertilisers. So we want to really encourage farmers to choose to grow legume crops more as part of their rotations. At the moment, we know that they're not taken up as much as they could be, and one of the things we want to do within this project is to do some predictive analysis based on having better systems for guaranteeing quality, and hence a high price return to the farmer. We want to look at what the impact of this could be on more frequent uptake of legumes as part of rotations.
Chris - So this is - it sounds like an awful pun, a vegetable analogy - this is more carrot than stick? If the pea tastes good, more people will buy it, therefore, more farmers will grow it. Rather than saying to people, "Just grow this because it's good for the soil". And in a climate change sort of equation, it's better for the environment too because you're not putting fertilisers on soils which we know contribute greenhouse gases and also the synthesis of those fertilisers in the first place eventually leads to the release of CO2.
Claire - Yes, absolutely. Of course, there has to be an incentive for the farmer to choose to grow the crops. The farmers won't grow it simply for public goods, they need an incentive. There are good quality markets that give a very good price, and if they can access those markets - that's not just the vining pea markets but also the canning markets give a good return to the farmer - and there are some excellent export markets. In some years, we don't meet those markets or the potential of those markets in the UK. So, for example, even for homegrown vining crops in the last, two to three years, we have had to import vast amounts from New Zealand because our stocks were so low. So there is a lot of potential for increasing production of these.
Chris - And just to finish off, Claire, where is the project now? Has it just got going or are you well into it? And what's the timeline?
Claire - We have just started at the beginning of February. Really, that was just the administrative start and we are now just starting to set out some plants to grow in plots. At the John Innes Centre, we have access to John Innes Germplasm Collection which is a collection of over 3,000 lines from around the world. So we'll be choosing among those for exotic lines which may give us some of the valuable genes that we want to introduce into breeding material.
Is fertiliser more damaging than buring fossil fuels?
We posed this question to Brian Thomas from the University of Warwick and Claire Domoney form the John Innes Centre...
Brian - Well I think it's not really a question of one or the other because I think as we've heard earlier, the manufacture of fertilisers involves a major amount of energy and contributes significantly to greenhouse gases, and therefore, you have to burn fossil fuels to obtain the fertiliser in the first place. I think in terms of fertiliser, it is possible to mitigate some of these problems. For example, as we've heard with plants that fix nitrogen, if we could extend that capability to others, that would help if we had a more efficient process for fixing nitrogen, more efficient than the Haber process, or we can get plants that use nitrogen more efficiently and there's a lot of work going on at the moment looking at the genetics of that particular aspect of crop production.
Claire - Yes and I think there is some debate as to how much of applied nitrogen fertiliser ends up as nitrous oxide. The IPCC estimates at about 1% ends up as nitrous oxide, but there are some papers in the literature which suggests that that's a three or four-fold underestimate, and it could in fact be much higher than that. So, I think there's a lot of discussion as to how much, but nitrous oxide is a greenhouse gas, much more potent than carbon dioxide by several hundred times.
Is a black eyed pea actually a pea or a bean?
We put this to Claire Domoney, from the John Innes Centre:
Claire - No. Actually, it's a member of the Vigna genus if I remember correctly and certainly not a pea.
[Other members of the genus Vigna include the Azuki Bean, Mung bean and Cowpea. Peas are Pisum sativum.]
Do environmental models consider handling?
We put this to Claire Domoney from the John Innes Centre and Brian Thomas from Warwick University:
Claire - I can say this isn't something we've come across in the lab, so this is news to me, but yes. It certainly sounds like an industrial problem that needs to be tackled. This is just one of the markets, of course, because the vining and canning peas are harvested at the immature stage so we don't have that problem with those, but the combining peas for animal feed and for marrow fats, these are the edible export markets. I guess that's the problem that's being refered to. But as I said, it's not one I've come across.
Chris - And Brian [Thomas], does this sort of thing get taken into account in models like yours? Obviously, it's easy to keep adding things but have we thought about how we get the crops in and how that may change in a warmer world?
Brian - Not particularly in the work that we've been doing. But I guess there is a lot of work, looking at total carbon footprinting of production systems going on at the moment. This would be one of the factors along with where you produce them and how you transport them around, and the logistics of that. So this whole "total energy balance" of how we produce crops is a live issue at the moment.
How do cancers spread between organs?
Well this is a process called metastasis and basically, it happens when, as cancers kind of evolve within the body, eventually the cells evolve the properties that they can start to breakaway from the starting primary tumour. They spread through the bloodstream or through the lymphatic system, and they set up home and start growing somewhere else. Often, this is in organs like the lungs, the liver and the brain. We're not entirely sure why they pick those areas. It's sometimes thought it's because they're areas of high blood flow. But there may also be biochemical properties of different parts of the body that different cancers like to spread to. So it's an area that's really under a lot of active research, and is an area that hopefully we can make progress in in the future because if we can stop cancer from spreading, that would really be the root to beating it properly.

50:42 - Do we absorb all the calories in food?
Do we absorb all the calories in food?
We put this to Susan Jebb from the Medical Research Council's Unit of Human Nutrition Research in Cambridge.
Susan - Not all of the calories that are actually in a food will be absorbed, digested and available for the body to use. What happens is that the calories which are in food, which will be released if we were just to burn it, as we might do in the bomb calorimeter in the laboratory, those calories cannot all be absorbed by the body. Some will be lost in the faeces and the remainder will be digested. About, perhaps, 10% of the total calories we consume might actually appear at the other end of the gut.
Once calories have been absorbed, again, they're not all fully available. Some will be lost in urine for example. The final loss of calories happens because some of the energy is fermented by the bacteria in the gut and so, it's not available to humans. It's actually burned off by the bacteria that are living inside us.
And so the consequence of all of that is that not all the calories that are actually in the food will be available for the body to use. But in fact, the losses are proportionately quite small. The calories that you see written on the back of a food pack have already had all of these adjustments made for the amount that will be digested and absorbed.
And so, the calories you see on the packet is actually not the total calories in that food. It's the so-called metabolisable energy, the amount of energy which is going to be available to the body...











Comments
Add a comment