What happens to bruises when they disappear? Why are some batteries better than others? Would a siphon work in a vacuum? We take on your science questions this week as well as find out about steampunk and how this online movement has led to the development of the music genre of chap hop! Plus news of active volcanoes on Venus, a new species of early human and a potential new drug to fight tumours. We also solve a ping pong problem in Kitchen Science and explore how worms cope with the rain in Question of the week!
In this episode
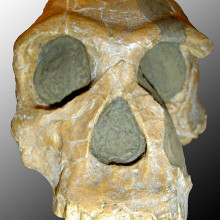
01:31 - New Species of Early Human – Australopithecus or Homo?
New Species of Early Human – Australopithecus or Homo?
A new species of early human has been described this week - Australopithecus sediba. And it looks like it's a key member of the family as it links the more archaic, small-brained 'australopithecine' with the more modern species of Homo. Sediba was unearthed two years ago at the somewhat aptly named site of 'Cradle of Humankind World Heritage Site' and its discoverer is Professor Lee Berger from the University of the Witswatersrand.
Two Sediba have been looked at by Berger and an international team of 60 researchers. The adult female and juvenile male are actually quite complete for such early specimens; they allowed Berger and his team to study their pelvis shape, spine, ankle and skulls. What's interesting is that they have a mixture of old and new traits.
Like modern humans they had quite an upright stance, small teeth, an advance pelvis and relatively long legs. They were, however, just over 4ft tall in adulthood and had quite small brains.
The researchers have used a combination of dating techniques to get as accurate as possible an age on these creatures, including uranium-lead isotopes and palaeomagnetism. From this they believe Sediba would have lived around 1.95 million years ago. And it's this period when the climate record changes dramatically, Africa becomes more arid. So perhaps the development of Sediba and later humans owes something to these climate changes

03:18 - Active volcanoes found on Venus
Active volcanoes found on Venus
Despite Venus being very similar to the earth in size and general composition its crust appears to be very different. Earth has a crust made up of moving plates, which collide to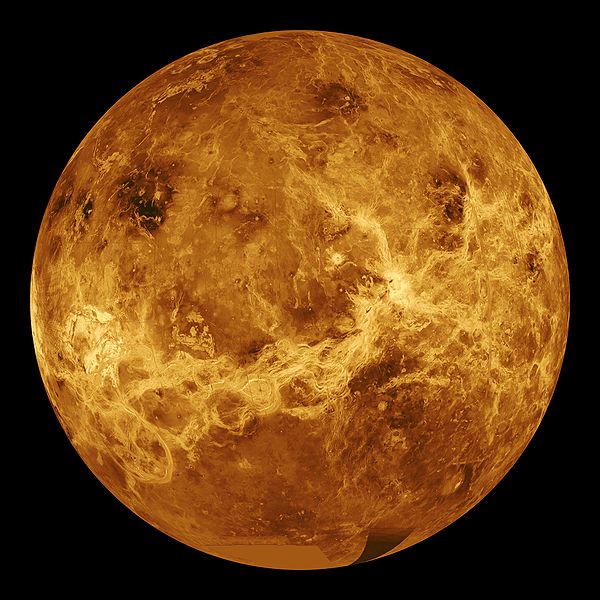 form mountains, and have volcanoes around their edges. Venus has definitely had some form of vulcanism in the past as there are far too few impact craters on the surface so something must have been destroying them, but until now there has been no evidence for active volcanism happening now, not least because seeing the surface through the thick sulphuric acid filled atmosphere is very difficult.
form mountains, and have volcanoes around their edges. Venus has definitely had some form of vulcanism in the past as there are far too few impact craters on the surface so something must have been destroying them, but until now there has been no evidence for active volcanism happening now, not least because seeing the surface through the thick sulphuric acid filled atmosphere is very difficult.
Suzanne Smrekar and colleagues have been studying Venus using an instrument on the ESA Venus Express mission which can look through narrow infra red transmission windows in Venus' atmosphere. They have particularly been studying structures which look like hot spots on earth - these are places like hawaii which are associated with upwellings in the mantle and lots of vulcanism.
They haven't seen flowing lava, but they have seen rocks which are very good at emmitting Infra red, possibly rocks like basalt on earth. On many planets this wouldn't help very much, but on Venus the atmosphere is so reactive that the high emmisivity minerals such as ferrous silicates will react with carbon-dioxide and sulphur dioxide to form a thin crust of minerals like calcite, or quartz dolomite on the surface, which are much worse at emmitting infra red.
They don't know how exactly long this would take, but experiments indicate that it could be in the region of a few years. So it looks like Venus might be the first rocky planet other than earth found with active vulcanism. It is also important because understanding how venus works helps us understand the earth.

06:03 - Sushi-digesting marine gene turns up in human intestines
Sushi-digesting marine gene turns up in human intestines
Scientists have discovered that bacteria inhabiting the intestines of Japanese sushi-eaters have picked up seaweed-digesting genes from marine microbes!
Writing in Nature, University of Victoria, British Columbia, researcher Gurvan Michel and his colleagues explain how they first identified, in a marine bacterium called Zobellia galactanivorans (a member of the Bacteroidetes family), a new class of enyzmes called porphyranases. These break down sugar-based molecules called porphyrans, which are present in large amounts in certain seaweeds, including the one used to wrap up sushi.
Having identified the gene sequences encoding the enzymes, the team then searched the international DNA databases for any other similar genes.
Surprisingly, the same sequences turned up a having been found in bacterial samples from human intestines. In this case the organism harbouring them was part of the normal human flora and known as Bacteroides plebeius.
But, intriguingly, the samples in which these bacteria had been identified were all from Japanese people; the genes are absent from the guts of people in America.
This suggests that the Japanese liking for seaweed (the average daily consumption is 14.2g per person) has at some point carried the DNA from a marine bacterium with seaweed-digesting genetic know-how into the guts of aJapanese sushi-eaters whereupon their own bacterial flora have grabbed the genes and incorporated them into their own metabolic repertoire.
In this way they've equipped themselves with the chemical equivalent of a new knife and fork to help them to deal with an unusual fodstuff.
As Stanford scientist Justin Sonnenburg puts in in commenting on the paper, - "next time you take a bite of an unfamiliar food, think about the microbial inhabitants you may be ingesting, and the possibility that you will beproviding one of your ten trillion closest friends with a new set of utensils..."
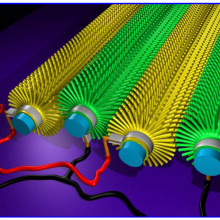
09:23 - Boron Carbide Nanowires
Boron Carbide Nanowires
One of the hottest areas of materials science is the development of composite materials, that combine the useful features of two or more pure materials. It is often useful to mix the materials as thoroughly as possible but this can be difficult as you get smaller, because the particles often stick together rather than mixing.
Xinyong Tao and collegues have come up with an interesting solution, the humble cotton 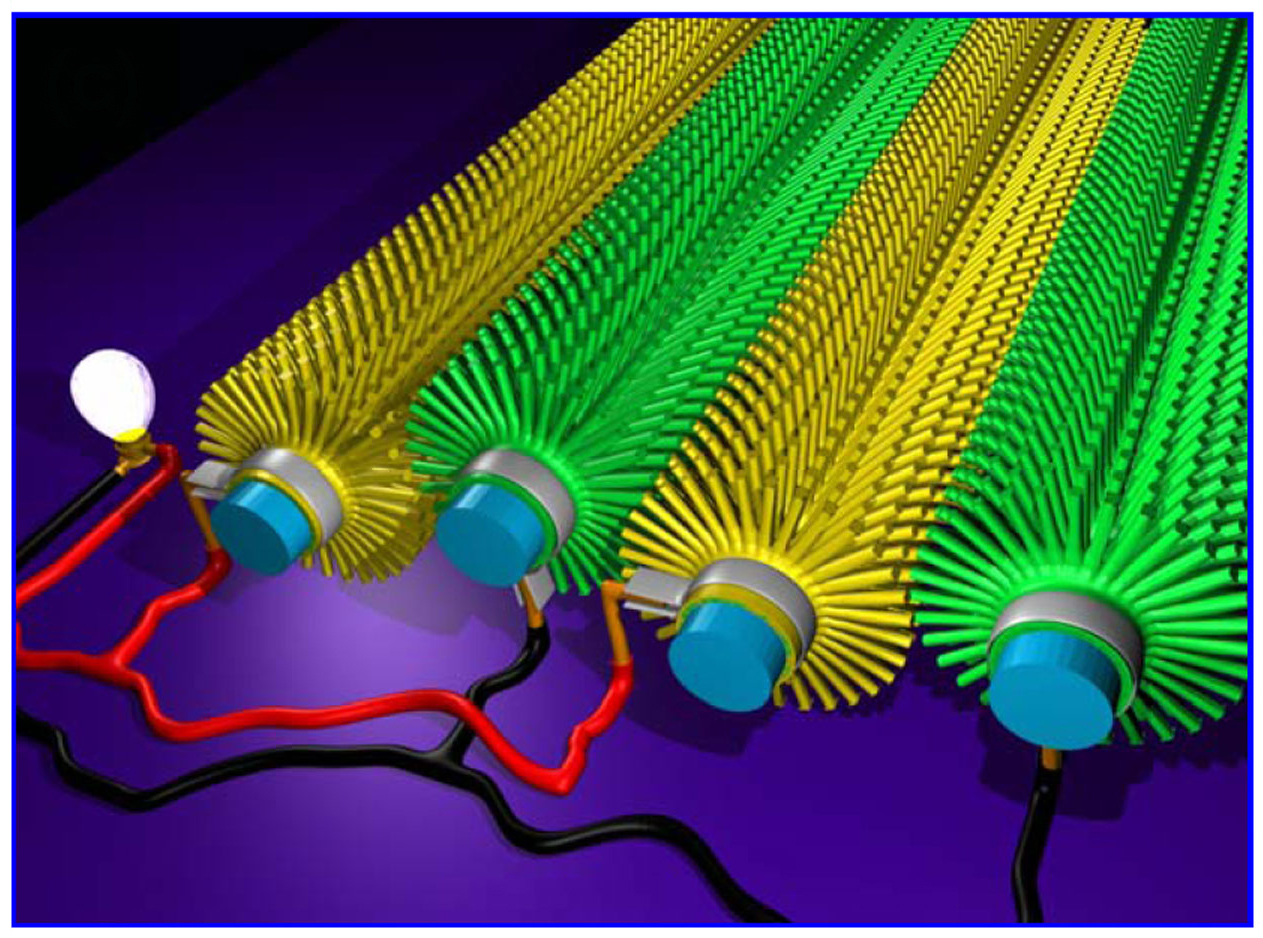 T-shirt. Cotton is particularly well suited to this purpose because each cotton fibre is covered with tiny hairs, these give it a huge surface area, which gives cotton its remarkable ability to absorb water. These hairs are beautifully distributed and don't aggregate, so if you can convert them into the material you want to distribute they are ideal.
T-shirt. Cotton is particularly well suited to this purpose because each cotton fibre is covered with tiny hairs, these give it a huge surface area, which gives cotton its remarkable ability to absorb water. These hairs are beautifully distributed and don't aggregate, so if you can convert them into the material you want to distribute they are ideal.
They first carbonised the t-shirt, then surrounded it in boron and heated it up. The carbon then reacts with the boron to form boron carbide nanowires. Boron carbide is the third hardest material known, and is often used in body armour, with other useful properties, such as absorbing neutrons, and temperature resistance. These wires can then be mixed in with a polymer and they showed make it significantly stronger.
The final material they have made is not especially strong, but it is a very interesting way of producing this sort of material, and adding other nanowires such as silicon carbide, or titanium carbide, in a relatively cheap manner.
11:16 - New Peptide to Fight Tumours
New Peptide to Fight Tumours
with Professor Erkki Ruoslahti, University of California, Santa Barbara
Researchers in California have shown how a new drug, which they call iRGD, can help to fight tumours. It's done by boosting levels of chemotherapy agents that can get inside the cancer. And to explain more how they've done this, we're joined now by Professor Erkki Ruoslahti who is from the Stanford-Burnham Insitute at the University of California, Santa Barbara...
Erkki - Tumours are supplied by blood vessels, and anything that is introduced into the circulation, of course, will then get into the tumour. That thing includes anti-cancer drugs. The problem is that tumours have a high internal pressure and the tissue fluid is actually from the tumour to the surrounding tissue, which means that it is difficult for drugs to penetrate into tumours. And they only get a couple of cell diameters from the blood vessels, which leave some of the tumour cells with a supple optimal amount of drug that contributes to recurrence and also the resistance that tumours usually develop.
Chris - And so, of course, physicians try to compensate by increasing the concentration of the drug in the blood stream. But then that, of course, has a knock-on effect for healthy tissue because it begins to generate side effects?
Erkki - That is correct. So one can only go that far using that approach.
Chris - So if you've got a way of carrying chemicals selectively into tumors far further and far more easily, in other words at lower concentrations in the blood than previously, you could potentially hit the tumour much harder where it hurts, leaving healthy tissues spared and, therefore, minimize the side effects.
Erkki - That is right. And that is what we are able to do with iRGD.
Chris - How does iRGD work? What is it and what does it do?
Erkki - iRGD is a peptide. We originally found it using screening of huge peptide libraries in live mice. It is possible to make libraries of billions of peptides. And we started screening them in vivo for their ability to go to tumours. And one of the peptides we found turned out to be quite special. It has the RGD sequence, that's arginine-glycine-aspartic acid, which my laboratory found 25 years ago as a key sequence for cell attachment. Now, the receptors for this sequence are up regulated, present at high concentrations in the blood vessels of tumours, but not in normal tissues. So the RGD sequence makes the peptide concentrate in tumour blood vessels. It then gets cleaved there by an enzyme which takes away most of the RGD activity, but exposes another receptor-binding sequence that now transfers the peptide to another receptor called Neuropilin-1. And when a peptide binds to Neuropilin-1, it activates the transport system. And that transport system can take the anti-cancer drugs deep into the tumour.
Chris - And so, doing some simple experiments to work out, how much better it is if you give this agent to the tumour alongside some kind of anticancer agent, how much higher concentrations of anticancer drugs can you get in the tumours when you do that?
Erkki - Well, it depends on the time when we look at the drug concentration. If we look at it fairly soon after the single injection, we can get seven to 40 times more of the drug into the tumour. Then, if the treatment is long-term, let's say, several weeks, then the difference becomes somewhat smaller. But it persists even after weeks of treatment.
Chris - And is this with the iRGD protein linked chemically to the drug or with the drug just given separately and at the same time into the blood streams so the two molecules are washing around together, and the iRGD drills a hole in the tumour and the drug then goes in?
Erkki - We originally would couple our homing peptides to the drug and that makes them more effective. But we then discovered, and that's the message of this newest paper, that we didn't have to couple the two together. We could just give them at the same time. And as you say, what happens is that the peptide activates this transport pathway, and it's a bulk transport pathway such that it takes in and through the tumour anything that is around when the peptide is there and has activated the system.
Chris - And, lastly, you've obviously showed, this as you said in mice, will this work in men so we've got something that works in mice and men?
Erkki - Yeah. Well, so far we know that we can grow human tumours in mice and the system works. We have not tested anything in humans yet, that requires a lot more preliminary work. We're just tooling up to start those studies.
Chris - Well, we wish you luck and thank you very much for joining us. A wonderful study. If you'd like to read it, it's actually published in the Journal Science this week...
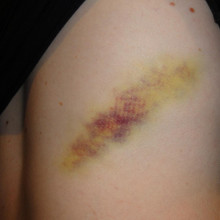
What happens to bruise as it disappears?
A bruise is indicative of trauma. So, some kind of trauma has to happen which allows blood to escape from a blood vessel into what's called the extravascular space. So, it spills out and splurges out around the subcutaneous tissue in the body.
It can also happen in other tissues. You can get bruising of your internal organs, contusions of organs, including the brain, for example.
But once blood leaves a blood vessel, the first thing that happens is that it starts to lose the oxygen that's locked up in the haemoglobin. And when haemoglobin is full of oxygen, it's a nice red color. So, that's why the injury first looks red.
As the haemoglobin gets deoxygenated, it goes a bluey hue and then purplish. And that's the darkening that's happening.
The next step is that the inflammation and damage also attracts the immune system. So, white blood cells come in, trigger some more inflammation and they begin to eat up, engulf the red blood cells.
And when they do that, they break open the cells. And out of the cells comes the haemoglobin, the red pigment. And the protein part of that gets chewed up inside the cell, turned into amino acids and they get recycled or metabolised.
And this leaves just the central core of the haemoglobin called the haem group, which is what's known as a porphyrin ring. It's a series of chemical carbon rings which have got locked away in their centre, an iron atom. That's why it's red because of the iron.
Now, what happens is an enzyme then kicks in called microsomal haemoxygenase, and this breaks open that ring, and it releases the iron which then goes off into the tissue and gets locked up with something called haemosiderin. And that looks an orange colour. So there's the orange colour in your bruise.
But the ring of haemoglobin, the haem group, initially gets turned in something called biliverdin, which is a green color, so there's the green in your bruise.
And that very quickly gets reduced to bilirubin, the yellow stuff. And then that's why you also see yellow in your bruise.
And as the bruise ages, it's also affected by gravity because these materials begin to spread out in the tissue doing a, sort of, human chromatography experiment. They spread out and different molecules get stuck to different things and travel at different rates. And so, the bruise spreads out and you see the separation of colours and the evolution of the colours.
But, eventually, all those substances just soak away and are taken away by cells in the bloodstream, and that's why the bruise eventually fades away and then you get better. So there's your answer!

How are fossils dated?
Diana - Well, actually, as I mentioned in the news story earlier, the Sediba skeletons were dated using paleomagnetism and uranium-lead, which is an isotope. With paleomagnetism, certain rocks they will have magnetic polarity which tells you what the magnetic polarity of the Earth was at the time that they were exuded from the crust. And this changes over time. So, you can work out when this rock was made. With uranium isotope dating, there's uranium-238 and uranium-235,which both decay to lead over time. So, you can check the relative proportions of uranium and lead and work out how much has decayed into lead. These isotopes have half-lives of a million to even 4.5 billion years, so you can actually go quite far back with your fossils. These are absolute dating methods, but there's also relative dating, which is the simplest method really. You just look at the layers of the rock and say, "Well, this layer is below that one and we know that layer is so old. So this must be even older." And that's how a lot of fossils are dated.
Chris - So, I guess what you're aiming to do is to use a number of different methods. And it's a bit like drawing a series of lines to see where they all converge. And you take the best agreement between all the different methods and say, well, that one seems to agree with everything and, therefore, it's likely to be that old.
Diana - Yeah, that's right. And the uranium-lead method is quite popular and it is actually quite accurate. They can get down to 0.1% with the actual date, which is pretty good.
Dave - The original way which doesn't give you an absolute date but does give you a relative date is that you look at the other fossils which are around it, because if you've got something like a dinosaur bone, around it there's also tiny things like beetles and little tiny snails. If you know these fossils only appeared at a certain date and went extinct another date, you know that the fossil must be from within those two dates. You've got 40, 50 different fossils there, so you can get a really quite accurate date.
Chris - Yeah. As long as you get the other ones right, you're right.
Why do Gums recede?
The answer is because there is a very close relationship between the tooth and the gum. They talk to each other. The gums talk to the teeth, the teeth talk to the gums and also to the bones, and there's a big interplay between all of those tissues, which has what's called a 'trophic' or growth promoting effect. And so, if you loose teeth, you also loose bone and gums, and vice versa. So, it's because one thing is there to protect and support the other. And if you take one away, the other one realizes that its role is now a bit more redundant. So, the gums thin.
Could a straw be used to suck greenhouse gases into space?
Dave - That sounds like a lovely idea. The problem is, there's nothing actually around the Earth holding the atmosphere in. There's not like a great big greenhouse holding the atmosphere in. If you keep on going up, you just hit the vacuum of space anyway. So what's holding the atmosphere down? It's just gravity. The Earth has got enough gravity to hold even the tiny molecule of oxygen down on it. And the reason why there's so much air pressure pressuring on us now is all of the air above us is getting pulled down by gravity and it's pushing down on us about 10 tons per square meter. So, if you put a big straw up into space, all you would do is have a straw with air in the bottom and there wouldn't be any air at the top. I guess you could possibly pump the air out. But you'd have to push it a long way up for it to get blown away.
Chris - If you could make a straw 50 miles high, you presumably have got the technology to deal with it another way would be my argument, wouldn't it?
Dave - And it would probably take more energy than not burning the fuel in first place.
Chris - But an impressive sight, though, wouldn't it?
Dave - It would.

Are microwaves safe?
Chris Smith answered this question...
Chris - Well, I think there's two ways to look at this. There's the simple, does a microwave as a form of light, a radiowave relative if you like, have any impact on the chemistry of food? And then, the other aspect of this is, does it have any impact on the chemistry of the other things that you put on the microwave like the container that the food is in?
So, first of all, let's look at the food angle. The reason people think microwaves are safe is that the energy in a microwave is too low to physically break the bonds that join atoms and molecules together. It's called non-ionizing radiation. And for that reason, we believe that microwaves are not unsafe. They will not rearrange or mutate things in your food. And they shouldn't, therefore, pose a threat.
But what microwaves could do is to interact with other things you put in the microwave, like plastic containers. And not all containers are necessarily safe to be heated up to the kinds of temperature that they might get to in a microwave. Because one of the things that a microwave does have is hotspots and cold spots because of the way the waves work. And that's why you have to have a turntable. But that also means that when you put something in the microwave, you could end up with the plastic being in a hotspot and getting very, very warm and some chemicals that are added in making plastics - these include chemicals called plasticisers - can have unwanted effects.
There are now big studies going on around the world to see if some of these chemicals are a health risk at the kinds of concentrations at which they are leaching out of plastic bottles. And we did a show a couple of months ago on this subject, and we looked at various things. There's one called Bisphenol A, which is in certain type of plastic, and also some polycarbonate bottles can also release these chemicals. So, the bottom line is, at the moment, we don't know for sure. There is evidence suggesting that plastics can produce chemicals. It may be unsafe if you heat them up. And, therefore, the best option and the safest option is probably to use a container which is safe in a microwave, either certified as safe, as such, or use something made of porcelain because that, we know, doesn't undergo any kind of changes. Would you concur with that, Dave?
Dave - Yeah. The one other thing which affects food is if you've got these hotspots (I mean, it depends on your microwave and there are more modern microwaves which have less severe hotspots) is that you can overheat the food and destroy some of the goodness in it. And so, although it hasn't been hot for very long, if it gets up to maybe 120 for a short period of time, it could destroy some of the vitamins and things.

28:13 - Steampunk
Steampunk
with Chris Vallance, BBC Technology Correspondent
Diana - Now, it's time to find out what's been happening in the world of technology as we join Meera Senthilingam who is going back to the age of the steam engine in this month's tech update.
Meera - So it's been a while since we've had a tech segment, and so I've come down to Television Centre here in London. And it's a lovely day, so I'm joined outside in the grass area with our resident tech expert, Chris Vallance. Hello, Chris.
Chris - Hi, Meera.
Meera - This month, Chris, you're taking us into the past but using modern technology.
Chris - Well, I thought I'd talk about something a little aesthetic. It's a bright sunny day. Let's talk a bit about art perhaps more than technology. I'll talk about a very popular online movement called "steampunk." It's a science fiction fantasy sub-genre. Very popular online. Essentially, it imagines what would modern technology be like if it had the aesthetics, the feel of Victorian technology, the classic age of steam? And so you get devices, creations that are essentially technological things but rendered in a Victorian style. Lots of brass, they have dials; they have levers. It's all a bit Jules Verne and H. G. Wells. It's 20,000 Leagues Under the Sea. There are lots of metal. And these two things, they're often just artistic things. Sometimes they function; like people will, for example, recreate computer keyboards but make it look a bit like old typewriters or they'll have USB keys with little pistons and little brass styles. It's a big event a couple of weeks back in London where it's one of the big annual jamborees for people interested in steampunk. And I went along and I spoke to some of the artists and steampunk fans who turned up.
Cyberfi - Oh, hello, I'm Cyberfi.
Chris - Cyberfi? Ok, now I'm going to describe your costume. You're entirely silver. You're surrounded by a halo of what look like hypodermic syringes. Your face is covered in rhinestones. What's it all about?
Cyberfi - Well, I tonight - you see, it's a great exhibition here today, so I'm exhibiting my latest invention, which is a tremendously exciting craniometre, which I believe will transform society. You see, a craniometre is the secret to eternal youth and beauty!
Chris - I suppose it's loaded with Botox. That's what you're saying?
Cyberfi - Well, it's somewhat pre-botox. It's pre-botox. It's a little bit more painful than Botox. It does involve mild electric shocks.
Jaz - My name is Jaz Delorean. I'm the writer and lead singer of Tankus the Henge. We're a band from London and we have a steam piano and an accordion and lots of eclectic influences.
Chris - Steam is your career, your vocation?
Jaz - Yeah, it is. It certainly is. I drive a set of steam yachts from Carters Steam Fair and there are only two of those in the world.
Chris - What is a steam yacht?
Jaz - A steam yacht is a fun fair ride. The one I drive is from 1901. And it's the nearest thing that mankind has got to building something that's alive.
Mr B - My name is Mr B the gentleman rhymer. I'm re-connecting hip hop and its ilk and other parts of popular culture to the Queen's English manners . Basically I've invented something I call Chap-hop.
Chris - Chap hop? What is chap-hop?
Mr B - Well, chap-hop is exactly that. It's hip hop but I'm an English gentleman, I enjoy cricket and I enjoy smoking my pipe and we have to keep it real. So I can't be rapping about the ghetto and things like that.
Chris - You're straight out of Purley
Mr B - I'm straight out of Surrey. Shady old Surrey, that's where I'm from.
Chris - Very good. I should describe your outfit, a very fine, double-breasted suit - no, a single-breasted suit, with a nice waistcoat, a nice narrow tie, a wonderful moustache. You must have spent quite some time curling that.
Mr B - I have no wax on it whatsoever. It's curling up but I can curl it down. I can go a little bit biker with it if I wish.
Chris - It's a little bit semaphore.
Mr B - Exactly, yes. I can do moustache semaphore with it, you know. I can land planes with this little thing.
Chris - Some steampunk enthusiasts and artists.
Meera - Is this a relatively new movement, Chris, or does it have quite a big following already?
Chris - It's been around for a few years and it does have quite a big following. I mean, there are steampunk authors, there are films with a very strong steampunk aesthetic. There is a lot of Victorian technology that can be quite easily updated. And people love doing that. I mean, if you think about the early computer, for example, Babbage's difference engine, you know, it's all cogs it's all brass, very easy to imagine that as, you know, something that might have persisted into the future. So, yes, it does have a big online following.
Meera - Now, as well as seeing these things online, are there any future events where people can see some of these objects and these gadgets in the flesh?
Chris - There's a lot of activity going on throughout the year. You can see exhibits of work, you can watch the bands that tour and, perform in a steampunk style. It isn't as big as some science fiction genres, it isn't as big as some literary and artistic genres, but it does have a sizeable following online. And there are even people who sell steampunk stuff. So, you know, there's a lot of stuff to explore out there.

Why do some batteries last longer than others?
Fundamentally, a battery is a chemical reaction going on, and part of that chemical reaction is it pushes electrons from one side of the battery to the other side...
So, actually, you have two halves of a chemical reaction: one which absorbs electrons and another that gives them out. And the only way that the chemical reaction can carry on is when these electrons are going around and getting back to the other side through your circuit and they can do work when it's doing that.
Now, there are lots of different chemical reactions you can use and so, basically, the number of atoms - the number of molecules which can be active or which can move electrons across is important. The more you can get in the battery, the more current - more charge - you can store in the battery.
You've got to have lots of complex things that hold it together, which take up space and take up weight - and, normally, it's only on the [internal electrode] surfaces where these things can react. So, any kind of centre of an electrode which isn't able to react with things is useless and doesn't work very well.
So, basically, the way they last longer is by having more of the battery which is active; you can do different chemical reactions which can store more energy, take up less space. You can also use different chemical reactions if to produce different voltages. So, a lithium ion battery is at 3V, whereas a standard one and a half volt alkaline cell is 1.5V, and a rechargeable battery is 1.2 volts.
Different batteries have different properties. An alkaline cell will last for a very, very long time. It doesn't lose its charge. It could just sit back and it will last for several years. It has a shelf life of several years. Whereas a rechargeable battery will just discharge itself in maybe a month or so. Also, a rechargeable battery can give out much more current. So, if you've got a high-current application, a rechargeable battery works a lot better. So, yes, there are different circumstances where some are better than others.

How do some orchids mimic insects?
There was a wonderful paper written by a lady called Jennifer Brodmann, who is a researcher at the University of Ulm, and she was on the Chinese island of Hainan looking at an orchid called Dendrobium sinense.
Now, this is a really interesting orchid because no one knew what pollinated it. It makes these beautiful flowers. It's a white flower with a red centre, but it's "rewardless". In other words, the flower doesn't give anybody anything if they come and visit it.
So she decided to do a stakeout and she watched this flower - 121 hours of footage - to see what came by. And 35 insects paid a visit, of which the majority - over 30 - were a kind of hornet. And she thought, "That's interesting."
A closer inspection revealed that these hornets didn't come in and spend much time loitering there. They flew in and pounced on the flower and then abruptly left.
But when they looked more closely, they saw that, as the hornet was doing the pouncing, it was actually depositing a bit of pollen on the orchid, fertilising it, and also picking up some pollen to take to another flower.
So they thought, "There must be something which is attracting this hornet to this flower." So they made extracts of all the chemicals that come out of the flower and they found one really interesting one. It's eicosen-1-ol. And this particular molecule is a pheromone made by bees. And, in fact, it's an alarm pheromone that bees make when they want to tell other bees about something exciting going on.
What they realised is that this hornet species eats bees, and it feeds the bees to its young hornet larvae.
So what the orchid is doing is making itself smell like a bee to attract a hornet, to get itself fertilised. And it's doing it by making the same chemicals that the bees would and, thereby, fooling the hornet, so a wonderful example of sexual kind of subversion going on.
The point is that the plant has evolved to have the same genetic pathway, or the same synthetic pathway, that can produce these chemicals because this is the way in which it gets itself pollinated, and very effectively too by the look of it. If you want to read it, it was actually published in Current Biology, last year, Jennifer Brodmann, a wonderful bit of science.
In the case of the bee Chris asks about, it's the same. The plant has evolved to smell like a bee and, ultimately also to look like a bee, to favour its own pollination. And because plants that do this more effectively will produce more offspring, those plants that are best at fooling insects in this way will slowly dominate the gene pool. So plants cannot "see", but they can adapt by genetic mutation, which slowly confers upon the plant the best set of tools to subvert insects into helping it to reproduce...

Would a siphon work in a vacuum?
Dave Ansell answered this question...
The simple answer to that is, no, it wouldn't work. The general way that siphons work in general is that, essentially, you've got air pressure pushing down on both ends of your pipe. One end is higher than the other, then that can push - push it on both sides, push the water up to the top and then it carries and going.
If there wasn't any air pressure, then you could form a bubble at the top of the tube and that bubble would expand and expand until the two levels of water are same level as that their two tanks.
Okay, so that's the simple answer. The somewhat more complicated answer is that water has something called cohesion. The water molecules do stick together as related to the surface tension. And, therefore, as long as you don't form any bubbles, water can actually be pulled and actually will stay stuck together even at a negative pressure, even at zero pressure, or even as you can pull it apart, which is the reason why trees can actually go higher than about 30 meters which is the air pressure which will push the water up, or 10 meters, which push the water up. And so even with a very, very thin tube and until you get a bubble, it would work for a bit but not for very long.
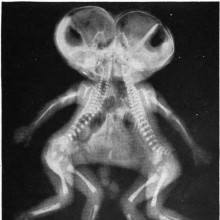
Have there been conjoined twins in the past?
Diana - Well, as far as I'm aware, no, and I think it would be quite big news if they had.
But there are all sorts of historical examples, and there was one example in some art made by the Moche, which was a group of people living in coastal Peru in South America around the turn of the first millennium.
But, basically, in about 300 A.D., they made some pretty pictures of them. But there are also some examples about - I think it was 965 AD, there were some European Siamese twins were recorded in history, but as I said, as far as I know, no skeletal evidence.
Chris - Presumably, because it would have been so hard to give birth to a Siamese twin?
Diana - Yeah.
Chris - Probably many perished and so did many mothers, and it would have just been the end of story.
Diana - That's the thing. And, also, neonatal bones of these - bones of babies really don't survive very well at all.

Mysterious Movements - Surface Tension and a Ball
How are calories in food calculated?
Chris - I'll start with the simple aspect to this, probably good for me, which is that the way scientists work out how many calories are in food is quietly literally by burning it. You use something called a bomb calorimeter. And you put the food inside a container, you use an electrical element to ignite the food, and that's because you can log exactly how much electricity you fed to the element. So you can work out how much energy it took to get the food ignited. And you soak up all of the heat that comes out of the burning food by water, and you measure the temperature rise in the water. We know how much energy you have to put in water to make the temperature rise by a certain amount, so we can work at how much energy comes out of the food. So if you subtract how much energy you have to put in to light in the first place and how much came out at the end of the day that tells you the gross amount of energy you could get out of the food. But it is a bit more complicated, isn't it, Dave?
Dave - Yeah. The problem is that you don't absorb all the energy which you get from burning a food. So things like dietary fibre which you can't digest - it goes straight through you and you don't get energy out of it. Some things take more energy to digest than others. So, I think, actually when they work it out, they do it from a great big table of ingredients and they work it out. I'm not exactly sure how they work out how much food is absorbed from each ingredient.
Diana - Yeah. I think when we had Susan Jebb on to answer the question about how many calories are actually absorbed from food, she said that a lot of it does end up in your urine and your poo. And also that bacteria will end up digesting a lot of calories in your gut and that the amount of calories that bacteria will get through actually varies between people's guts because they have different species in there.
Why does toothpaste reappear?
Dave - I get in trouble with this all the time with my girlfriend because I'm pretty clumsy when doing my teeth as well. What's going on here is to do with the reason why T-shirts go fairly transparent when they get wet. Basically, the reason why you can see a white object is actually, if you look at it under a big microscope it's transparent crystals or lumps. When light hits it, it gets bent because light goes slower in it. It gets bounced off in all sorts of different directions because the surface is very rough. If you cover it with water that smooths out the surface and so light can get through much better so it looks dark. As soon as that water dries out - so you've actually cleaned up most of the toothpaste but not quite all of it, then you can see those little particles, light bounces off them and it looks white.
Chris - A bit similar to why paint looks lighter when it dries.
Dave - Exactly the same thing.
Why do spiders have such potent venom?
Chris - It's not just spiders. There are many examples of organisms in the natural world that make toxins that are really, really toxic. As one researcher put it to me, it is a case of overkill, and they do it incredibly well. And the reason is because these animals have evolved to use and exploit a whole range of different prey rather than just one. And this means that because the prey animals are different, they therefore need to make a range of different venoms, which have action against the range of different prey. If they just use one, it would be very easy for one prey to evolve away from being sensitive to that particular venom. By using a range of different venoms and therefore targeting a range of different prey, there's no chance that it will start working. And over time, they've just continually updated and adjusted their repertoires so they have this amazing example of all these different molecules, some of which will work really well against one species, others less so. And when they sting us, some of them absolutely take us to the cleaners. And it's not just spiders, there are many, many examples that is going all around nature.
Dave - Well, I guess also, some prey will evolve to be more resistant to them so that doesn't work as well. So they have to get stronger and stronger.
Chris - Yes. But also, it means that some of these genes do, at the same time, become redundant, some of these venoms. And so they actually lose the ability to use them and therefore those genes stop working. They're still hiding in the genome, but they just don't turn those genes into products anymore. But yes, they're very interesting.

49:16 - What do Worms do in the Rain?
What do Worms do in the Rain?
We put this question to Kevin Butt from the Earthworm Research Group at the University of Central Lancashire...
Kevin - Okay, worms are quite amazing creatures really. Unlike many other creatures, they obtain their oxygen through their moist skin. They don't have anything like lungs or gills unlike other animals. This means they can get the oxygen they need from the air which is the normal situation. But equally, they can get their oxygen from water because obviously water is H2O containing oxygen. So if the earthworm is underwater, it can get oxygen just as easily as if it was in the air. And experiments have been undertaken keeping earthworms underwater for days on end, and they come to no harm. Some people think that if a worm is buried and gets flooded, then they would seek to escape. But as I just mentioned, they don't need to because they can get the oxygen they need from the water. However, quite often we see earthworms on the soil surface or on pavements seemingly trying to escape from inundation of water. But perhaps, it's not that. Maybe it's something slightly different - that the animals are actually trying to make use of the moist conditions in order to move away from their burrows, if you like, to pastures new so that they can mate with individuals that are not closely related to themselves. Not only can the earthworms survive well in the wet conditions, they can survive well under dry conditions in the soil, if it's really, really dry. And they do this, perhaps, by creating a spherical chamber in the ground, lining it with their own mucus, curling up almost tying themselves in a knot and just sitting there and waiting for better conditions to come along. And many species of earthworm do this. Others like the lobworm, the big worm that is in Britain, burrow deeply down into the soil and just avoid the dry conditions near the surface.
- Previous Do Worms Drown?
- Next Animals, Hair and Hot Flushes!










Comments
Add a comment