Blowing out Candles Round Corners
In this festive episode, can you get drunk through your feet, the chemistry of cocktails, twelve marine critters of Christmas, the best food and drink combos to eschew indigestion, does a carbon fibre bike go faster, why are snowflakes different shapes and a way to impress your peers at the office party by blowing out candles round corners...
In this episode

02:07 - Putting the fun into fondue
Putting the fun into fondue
The Christmas edition of the British Medical Journal is a wonderful source of slightly silly science stories, as sensible researchers around the world cut loose. In this edition, there's a great paper from researchers in Switzerland and Germany, the traditional home of fondue - a tasty treat mostly made up of a bucket of melted cheese. But while fondue is a tasty treat, eating such a large amount of melted cheese can cause indigestion, or dyspepsia. Traditionally, fondue is eaten along with hot tea, which is thought to help prevent indigestion. But other people favour alcohol, in the form or wine or even schnapps.
 The scientists wanted to find out which drink went best with fondue and caused least indigestion. So they persuaded 20 volunteers to eat fondue, and gave them either white wine, or black tea, followed by either water or schnapps. And because this was a scientific experiment, they also added a small amount of a harmless tracer chemical.
The scientists wanted to find out which drink went best with fondue and caused least indigestion. So they persuaded 20 volunteers to eat fondue, and gave them either white wine, or black tea, followed by either water or schnapps. And because this was a scientific experiment, they also added a small amount of a harmless tracer chemical.
By measuring the amount of tracer in the volunteers' breath, the scientists could work out how fast the fondue was emptying from their stomach. They also measured the amount of alcohol on their breath, and asked the volunteers to rate their feelings of indigestion, bloating and nausea during the experiment.
The team found that alcohol slowed the rate of stomach emptying, compared to tea, and that adding schnapps also slowed it down. The combination of wine and schnapps also made people feel much fuller for longer - a phenomenon referred to by the researchers as a "cheese baby". But the type of drink made no difference to indigestion.
The scientists also think their findings may extend to the consumption of alcohol with other types of rich meals, such as the traditional Christmas dinner. I'm sure that the Naked Scientists team will be conducting our own rigorous experiments in this area over the coming weeks.
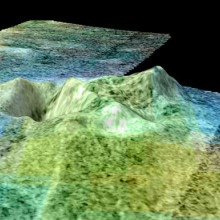
04:42 - Ice Volcano on Titan
Ice Volcano on Titan
Titan is a fascinating world, it is about as large as the planet mercury, it orbits Saturn and so the weak sunlight that reaches it means that it's surface temperature is about -189C. Despite this it has an active weather system with lakes and rivers, not of water but of liquid ethane and methane. The ground, pebbles etc is not made of rock but of water ice.
This strangely complete low temperature analogy to earth has now been taken a bit further, because Randy Kirk and colleagues seem to have found a volcano a kilometre 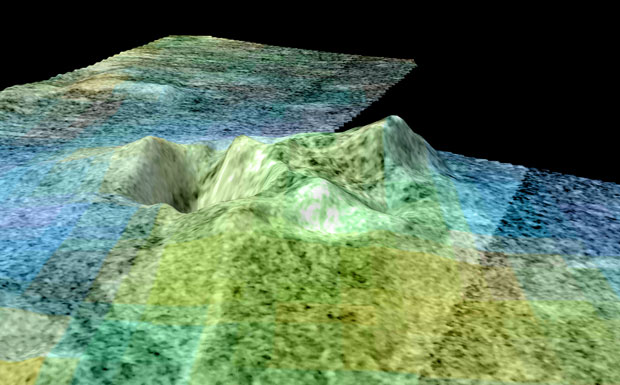 high on Titan. They have called it Sotra, and it is of course made of ice. It appears to have a different composition to the surrounding ice, and looks like it has spewed water based lava to form a volcano to one side. Right next to the volcano there is a hole about 1600m deep and nearby there appears to be a string of smaller volcanoes, like a volcanic region on earth.
high on Titan. They have called it Sotra, and it is of course made of ice. It appears to have a different composition to the surrounding ice, and looks like it has spewed water based lava to form a volcano to one side. Right next to the volcano there is a hole about 1600m deep and nearby there appears to be a string of smaller volcanoes, like a volcanic region on earth.
This of course makes Titan an even more interesting place to study, but also raises the possibly of molten water on titan and the interesting chemistry and possibly even life which could be associated with it.

06:34 - Getting Drunk Through your Feet
Getting Drunk Through your Feet
with Dr Peter Kristensen, Hillerod Hospital
Chris - Now, can you get drunk by immersing parts of your body in alcohol? Well, that was vaguely the hypothesis that a festive team of doctors from Hillerod Hospital in Denmark decided to investigate and Dr. Christian Hansen joins me from there now to tell us what this is. Hello, Christian.
Christian - Hello.
Chris - Well they say that Heineken refreshes the parts that other beers fail to reach. What were you trying to do?
Christian - Well, in Denmark, we have an urban myth that alcohol can pass through your feet if you submerge them in alcohol, vodka mainly. So, we thought that that was quite an important study to do because it's never been shown that alcohol can pass through your skin. It's been done from studies with cadavers and that didn't really show anything in particular.
Chris - It's hard to tell whether someone who's dead is drunk though, isn't it?
Christian - Exactly.
Chris - So what did you do?
Christian - Well, we bought some of the cheapest vodka we could get our hands on and we sat down for three hours, submerging our feet in this vodka at the hospital.
Chris - When you say submerging your feet, do you mean as in - when you have those stereotypical pictures of someone with a cold and they sit there in an armchair with their feet in a bowl of hot water, and a towel wrapped around their head, is that what you were sort of doing? You had your feet immersed in a bowl of vodka?
Christian - Yeah, except for the towel, that'll probably be a fairly good image of what we did and we had the lab examine our blood for ethanol for three hours - the duration of the experiment.
Chris - So blood samples were taken regularly during the experiment to determine what the concentration of alcohol was in the blood stream at any point?
Christian - Exactly, every 30 minutes and they were of course, rushed to the lab to make sure that the ethanol concentration didn't reach lethal levels.
Chris - And how many of you were participating in this study?
Christian - Three of us and we all were employed at the hospital so we had no students or volunteers in the experiment.
Chris - Did you find any alcohol getting into the blood stream?
Christian - None at all. Well at first, we felt quite confident and happy, almost intoxicated, but we actually measured if we had any spontaneous hugs occur in the stated hour of self confidence, but it didn't really show any significant changes.
Chris - So in other words, as well as measuring the level of ethanol in the bloodstream, you were also doing subjective measures of whether you were experiencing inebriation - Dutch courage or in this case, Danish courage, and speaking too much and speaking too loudly, that kind of thing, and everyone had that, but they didn't actually register any alcohol.
Christian - No, they didnt't.
Chris - Did anyone get tempted to drink the alcohol after the study when people's feet have been in it.
Christian - Yeah, it was kind of a difficult subject because we didn't really know what to do with the alcohol. It should have been disinfected and no bacteria should be present so it was quite difficult putting it in the toilet afterwards. So it was quite a shame, but the study was effective, I'd say.
Chris - So talking seriously for a minute, the implication is that you can't actually absorb alcohol in any way, shape or form, at least at the level of detection of your assay, in other words, how sensitive the lab's test is which is probably pretty good. And so, this suggests that people are actually going to have to take alcohol through their mouth, or potentially through other routes, in order to get into the body, but definitely not through the skin.
Christian - Yes, I say, through the mouth would probably be the golden standard, but we've only measured vodka with 38.5% of alcohol. So, there should be an experiment done with a stronger alcohol, I'd say.
Chris - So maybe onto the Calvados next year then?
Christian - Yes, probably.
Chris - Christian, thank you very much.
Christian - You're welcome.
Chris - Good to have you with us. That's Christian Hansen from Hillerod Hospital in Denmark and you can actually find the paper where they describe doing that experiment in the December edition of the British Medical Journal.

10:33 - Switch your Christmas dinner for burgers and oregano?
Switch your Christmas dinner for burgers and oregano?
In case you were thinking of shunning turkey for Christmas dinner this year and chowing down on hamburgers, researchers from the University of Arizona have some advice that could help to reduce the formation of potentially cancer-causing chemicals and inactivate food poisoning bugs in grilled meat.
 When you pop your burger on the barbeque - or under the grill in this weather - it goes nice and brown and crispy. But at the same time, chemical reactions that take place as the meat is heated produce chemicals called heterocyclic amines. These chemicals are thought to be partly responsible for the increased risk of cancer in people who eat a lot of meat. Cooking meat at a lower temperature can reduce the formation of the chemicals, but also increases the chances that bugs like E. coli - which can cause food poisoning - don't get destroyed.
When you pop your burger on the barbeque - or under the grill in this weather - it goes nice and brown and crispy. But at the same time, chemical reactions that take place as the meat is heated produce chemicals called heterocyclic amines. These chemicals are thought to be partly responsible for the increased risk of cancer in people who eat a lot of meat. Cooking meat at a lower temperature can reduce the formation of the chemicals, but also increases the chances that bugs like E. coli - which can cause food poisoning - don't get destroyed.
But the Arizona researchers suggest that a compound called carvacrol, found in the herb oregano, could help to solve both problems.
Carvacrol is an antioxidant, and the scientists think that it might reduce the formation of heterocyclic amines as meat is cooked. And as an added bonus, the compound also have anti-bacterial properties and can inactivate bugs like E. coli.
The scientists, led by Sadhana Ravishankar, are currently testing a range of plant compounds mixed with hamburgers, to try and find the most effective combination that still tastes nice. Yummy!
13:17 - Is a lightweight bike worth the extra expense?
Is a lightweight bike worth the extra expense?
Does owning a lightweight carbon fibre bike cut down your commuting time?  Contrary to expectations, a trial carried out by anaesthetist Dr Jeremy Groves from the Chesterfield Royal Hospital in the UK suggests that cyclists should pay more attention to the weight of the rider than the bike!
Contrary to expectations, a trial carried out by anaesthetist Dr Jeremy Groves from the Chesterfield Royal Hospital in the UK suggests that cyclists should pay more attention to the weight of the rider than the bike!
Writing in the 2010 Christmas edition of the British Medical Journal, the author documents a comparison between the time taken to complete a 43.5 kilometre commute to work using either an older, secondhand steel-framed bicycle bought for £50 and weighing in at 13.5kg, or a new £1000, lightweight 9.5kg carbon-fibre model purchased thanks to a "cycle to work" tax incentive scheme.
Groves compared his performance on each bike over a six month period, determining which bike to use on each day with the toss of a coin. Ultimately, thirty commutes were made on the steel-framed bike and 26 trips on the carbon fibre machine. The journey times were logged with a cycling computer, which clocked up 1144 kilometres over the course of the study.
Surprisingly, despite costing twenty times the price and weighing thirty per cent less, the carbon fibre bike was no faster than the steel-framed model. The top speed achieved - fifty-eight kilometres per hour - was the same on both bikes, and the average journey times were 1h48m21s and 1h47m48s respectively, although times were longer in the winter (owing to winds, heavier clothing and more cautious cycling on the part of the rider). Moreover, the author reports that the ride afforded by the carbon fibre bike was much less comfortable and the fear of falling off is higher in bad weather, leading to longer journeys.
Most importantly, Groves points out that although the thirty per cent reduction in bicycle weight achieved with a carbon-fibre frame sounds like a lot, this difference narrows to just 4% when the combined mass of the bike and rider is considered.
"A new lightweight bicycle may have many attractions, but if the bicycle is used to commute, a reduction in the weight of the cyclist rather than that of the bicycle may deliver greater benefit and at reduced cost."

16:39 - Unexpected Behaviour from the Sun
Unexpected Behaviour from the Sun
The sun is obviously incredibly important for earth as it provides virtually all the energy to drive the climate and life itself. We have been studying the sun for a long time, but almost all those studies have been done from the ground, so we have had limited information of how the output of the sun changes in frequencies which don't make it through the atmosphere. These are particularly important for the upper atmosphere as  if they are blocked by the atmosphere they will be heating it.
if they are blocked by the atmosphere they will be heating it.
Up until now atmospheric modelers have assumed that as the sun's output changes through the 11 year solar cycle all the different wavelengths change together. But a NASA satellite called SORCE the Solar Radiation and Climate Experiment has been studying the changes in the sun since 2003. And this has found that all wavelengths are not the same, the amount of Ultra Violet varies ten times as much as the average and Infra Red changes much less.
This could explain much larger than expected changes in the temperature of the stratosphere over the solar cycle, as this contains the ozone layer which is absorbing the UV.
It may also mean that variability in the sun has much less of an effect on climate than was previously assumed and it will probably make climate modellers life more complicated.
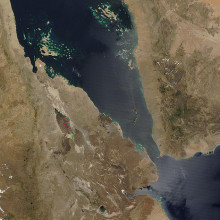
19:40 - Planet Earth - Afar Valley
Planet Earth - Afar Valley
with David Ferguson, University of Oxford
Kat - Now it's been subzero this week in the UK, so we're taking you to one of the hottest places on Earth, the Afar depression and that's a region of the Sahara Desert on the Ethiopian border. But it also contains a series of rifts that are slowly ripping the African continent apart to form a new ocean, and that's what makes it a dream destination for geologists like Oxford University's Dr. David Ferguson who explained to Sue Nelson the Afar Depression's appeal.
David - Well Afar is a geologically fascinating place because it's one of the few places on the Earth's surface where we can see the continental crust - and that's the rocky outer shell of the Earth that we live on - we can see it being ripped apart by the movement of tectonic plates, and hot 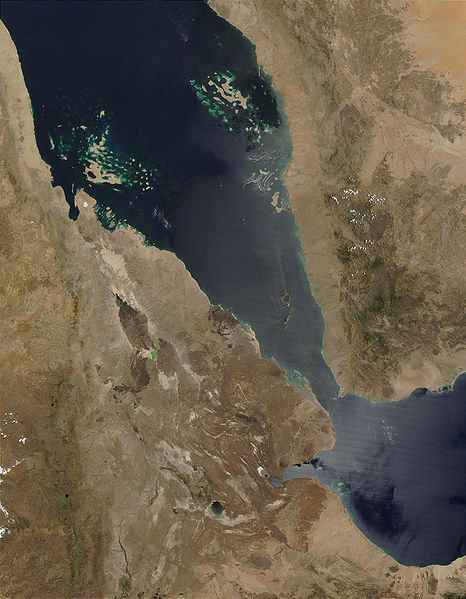 magma from the Earth's mantle, which is the region below the crust, wells up to fill the gap and creates a new ocean basin.
magma from the Earth's mantle, which is the region below the crust, wells up to fill the gap and creates a new ocean basin.
Sue - How long have geologists known about this particular region?
David - The first geologists to visit Afar were probably there during the 1960s and the 1970s, and it was recognised at this point that it was an exceptional place to understand how plate tectonics work, and firstly, due to the independence war in Ethiopia, we haven't really been able to visit the area for decades. So it's only in the last 10 years or so that scientists have been able to get back into Afar and been able to study, in detail, the processes that are going on there.
Sue - Before we go into the processes themselves, you've brought a couple of rocks here. Describe to me and to us, what the surface of this rocky remote place looks like.
David - So in this bag, we have a piece of the basaltic lava, it's one of the most common rocks on Earth actually and this is one of the newest rocks on Earth. This is just approaching its first birthday. This piece of the Earth's surface was underground a year ago.
Sue - It's almost like a hard, black grey piece of sponge, very porous.
David - Yes, so it's full of holes, so these are all the gas bubbles that escaped. It's quite spiky because it flowed across the surface and it's still young enough to preserve all these spiky bits. And if you look carefully, you can see it's full of shiny crystals, large, white, kind of glassy looking crystals.
Sue - Oh yes.
David - These grew underground in a magma chamber where this lava was stored before it erupted. We have another piece of rock here which is very different. There's no bubbles in this and this is a piece of volcanic glass.
Sue - Very shiny.
David - It's incredibly shiny.
Sue - Almost looks like black marble.
David - Yes, it's very heavy and it's composed almost just entirely of glass.
Sue - Let me just feel a bit. It's like the size of a bag of sugar, this bit. And actually, feels pretty much, probably a bit heavier than a bag of sugar as well.
David - Yeah, the difference between these two really is their chemistry and this magma was so viscous that the crystals that we see in this lava weren't able to grow and so, it just solidified into just a chunk of glass really.
 Sue - Now this great big rift, you say it's an ocean forming rift. Does that mean in the foreseeable future that the area, this part of Ethiopia that you're looking at, will be covered in sea, in hundreds of thousands of years time?
Sue - Now this great big rift, you say it's an ocean forming rift. Does that mean in the foreseeable future that the area, this part of Ethiopia that you're looking at, will be covered in sea, in hundreds of thousands of years time?
David - Yes, the crust that forms Ethiopia is being slowly stretched apart, at about 20 millimetres a year for instance. However, right now in Afar that process within the last five years have speeded up dramatically and it's moved approximately a metre a year, or two metres a year even, within the last five years. We think this probably goes in stops and starts and so, at the average speeds of maybe 20 millimetres a year, if this continues for 10 million years for instance, then we'd probably see a new ocean basin in Afar.
Sue - How normal is this for geologist to actually see this taking place?
David - It's incredible. It's pretty much a once in a lifetime opportunity. Tectonic plates move very slowly and it's not common that we get a chance to witness a furious stage of activity. There was one other well-documented phase which was in Iceland in the 1970s, but apart from that, this is a very rare occurrence. But we think in this part of Afar, it probably happens every 400-500 years.
Sue - So how often do you get to go out there because I know from reading, you kept a blog at one stage, that you had this hot date with a volcano. So how likely are you to go out there or do you go out there as soon as you hear that there's activity? Is it being like a fireman on-call or the geologist equivalent of a fireman?
David - Yeah, a little bit. We're volcanologists on-call and we go out for two reasons. We go out to collect rock samples and from older lava flows to perform experiments on. But yes, as you say, when we hear there's an eruption, we try and get out there as quick as we can because we want to collect samples, and we want to actually witness these eruptions in progress if we can to learn a little bit about it. We're always ready to drop everything and run out if we get the opportunity.

Where's the best place to put a heater to warm up your house?
Chris Smith answered this question...
Well, it depends what you actually want to do, because it's not just as simple as saying, "Well, we just want to heat the house."
Heat rises by convection, and convection currents will carry warm air upstairs in your house. But if you want to warm just the upstairs, it's actually better to take the fire upstairs and warm only your bedroom, if you're going in there.
And if you want to be downstairs and have the downstairs part of the house warm, put the fire downstairs but close the doors off so that you're just heating the room that you're in. That's probably the most effective way to do it.
On the other hand, if you do want to heat the whole house, open the interior doors and put the fire downstairs. What will happen is that the warm air will flow through the corridors and rise to the upstairs, displacing down the cold air that's up there. This colder air will fall downstairs, because it's more dense than the rising warmer air. When it reaches the ground, the cold air will get heated by the fire or stove up and then circulate upwards around the house.
Given enough time, and a sufficiently powerful fire, you could use convection currents to heat the whole house like this. This assumes of course that the fire can emit enough heat energy to outcompete the cold coming in through drafts and the heat that you're losing through the roof and radiation from windows and the walls...
Can we extract energy from the Cold?
Dave - That is a very interesting question. You can't gain energy directly from cold, but what you can do is get a lot of energy by moving heat from somewhere which is warm to somewhere which is cold. And that's essentially what a steam engine does or a car engine, any of the heat engines work exactly like that. However, the colder you get that cold into the system, the more efficient the process is. So if that cold end is absolute zero, then if you move a kilojoule of energy from something warm to there, then you've got a kilojoule of useful work out of that. If it's hotter than that, then it gets a lot less. So, once you collect energy from cold directly, you can get a lot of energy by transferring heat to somewhere very cold.

28:16 - The Chemistry of Cocktails
The Chemistry of Cocktails
with Darcy O'Neil, University of Western Ontario
Chris - Now, it is our festive Christmas Naked Scientists and I absolutely love cocktails. We were debating about what our favourite ones are earlier. I'm a Margarita person, but what's actually going on, chemically speaking, inside that cocktail shaker? Well to find out, I got together with the University of Western Ontario's Chemist and Cocktail Connoisseur, Darcy O'Neil...
Darcy - Early on, I studied chemistry and then ended up working in a refinery for six years and then I decided to move on to a different city. I didn't find anything that was really working for me career-wise so I decided to do some bartending, and then I ended up back at the University of Western Ontario and then I decided to take the science and apply it to the drinks that I was making.
Chris - How did that go down with the customers?
Darcy - It went down really well. Usually you think people just come out for a drink, but once they started understanding how the drinks were made and then bringing in some science to it, they really, really took to it.
Chris - Was it relatively easy to start to dig into the chemistry of cocktails? Did you actually find that it was pretty easy, from a chemist's point of view, to understand what was going on or is there still just a black art to cocktail making, and that "shaken and not stirred" is very much on the lips of the consumer, but there isn't much science behind it?
Darcy - Well, early on, I was probably one of the first dozen people to actually look at the science of cocktails, so there wass a lot of low-hanging fruit to work with. So, even just talking about ice, why things cool down, how they cool down, talking about how certain things mix. I mean, that was pretty simply early on. Now, it's actually getting more difficult because you're always trying to find new material or new and interesting facts that people want to hear.
Chris - Give us some examples.
Darcy - Well let's see. Right now, we are doing essential oils. There's been this perception that they're artificial flavours, which they're not. They're just distilled oils, much like ethanol. You distill off the ethanol, but if you continue distilling a great brandy let's say, you'd end up with cognac oil which comes off at about 110 Celsius. It's not artificial, but it's a very, very potent flavour compound that you can make a champagne flavoured non-alcoholic beverage. So, trying to get people to understand the distillation process, and all that background stuff gets more complicated as we go on.
Chris - But the interesting thing is, that we're now in a position where someone like you, who has a lot of chemistry knowledge behind you, can take your knowledge and explain what we're already doing. But could we turn the equation around and start saying, "Right. Based on what we know about chemistry, we could start doing something very unusual to make a whole new type of drink or cocktail, or a combination, or gustatory experience?
Darcy - That's actually starting to happen. It's following after molecular gastronomy which is the science of food, and what they called it now is molecular mixology, and it's taking science and doing something with the cocktail to create something completely unique. And one of the most interesting ones or early ones that really got a lot of media attention was caviar. It's not really fish eggs, but they look like fish eggs. So what it was, they would take sodium alginate and calcium chloride, and make a calcium chloride bath, and add a flavour to mix with the alginate, and then drop it into the calcium chloride bath and form these little spheres. One of the things you could do with these little spheres is to put them on a glass of champagne and they move up and down, like a lava lamp. People are quite fascinated by it.
Chris - I remember using that same trick to make immobilised enzymes in the chemistry class at school, but I never thought to put them in champagne.
Darcy - Yeah and it's funny, that's where all of the stuff's coming from. A lot of people, it's surprising have high level of education, but enjoy - food's a passion, drinks are a passion, and so, a lot of people bring or cross the information over.
Chris - Now, one of my favourite characters historically is James Bond for all kinds of reasons, but is there any science behind his shaken and not stirred claims?
Darcy - Yeah. Actually, last year, Tales of the Cocktail in New Orleans which is kind of a conference for high-end bartenders and industry people, looked at the science of shaking because we've always heard, shaken or stirred, or you know, which one is better and which one produces the different drinks. So they actually did a test. They took a cocktail shaker and put in a thermocouple, put different amounts of ice, they weighed the ice, and then they'd shake them and see what would happen. And what they figured out is that for shaking a drink, if you shake it 20 times or for approximately 20 seconds, that's as cold as it's going to get which is about -7 Celsius. And then they found with stirring and it takes a lot longer to bring the drink down to that temperature, so probably twice as much time, 40 seconds to get it to that plateau because it actually doesn't really go any colder than -7.
Chris - There was a company that were claiming that their ice was the best ice for cocktail making, wasn't there?
Darcy - Yeah.
Chris - So that basically blows them out of the water.
Darcy - Well it does. I mean, you know, there is still good ice and bad ice. You know obviously, ice picks up a lot of flavours, so using clean fresh ice is always the best idea. There is no such thing as the perfect ice for a cocktail. Any ice will do.
Chris - What about if you used dry ice? I don't mean that in a funny way, but that would presumably lower the temperature much further.
Darcy - Definitely and what happens is that it also carbonates the drink. You'll taste the acidity in it. So, for a lot of people who do a Halloween type drinks, they like to put the dry ice in it and they get froth and stuff. And if you've ever tasted it afterwards, it tastes much different.
Chris - You've tried that, have you?
Darcy - Yeah, working in a lab, I mean, there's always something I can try, so bring home dry ice and give it a shot.
Chris - One of the other things that I heard one person saying with the shaken not stirred business is that when you make a cocktail, some of the things mix quite well with stirring, but there are other heavier things that don't, and actually, shaking the cocktail up does make a very big difference to the way in which the combinations of chemicals mix around, and therefore, the flavour sensation they impart to your tongue and your mouth will differ between shaking and stirring.
Darcy - Well the funny thing is, one of the studies we've done at the university I work at, was about bruising gin. So, what they found was that it really doesn't change that much, but I think the oxidation potential goes up or something like that. It was a very slight difference, but never let facts get in the way of a good story because that's part of what drinking is. You know, you go to a bar and you want to talk about things. If you're a little bit too matter of fact, it becomes no fun to talk about it.
Chris - I could not conclude an interview with an expert on the chemistry of cocktails, and I suppose mixology, without asking you, what is your favourite tipple?
Darcy - I'd have to say the Manhattan. If you go to a bar, usually they'll get it right. It's not a drink that you mess up too much. Usually, it's about 2 ounces of whisky - not Scotch whisky, but American or Canadian whisky is the general preference. Half an ounce or two ounce of sweet vermouth and a dash of bitter, Angostura bitters works amazingly well, and just basically stirred as opposed to shaken, and then garnished with a maraschino cherry.
Chris - And served...
Darcy - In a cocktail glass, similar to Martini glass, actually we have a Manhattan glass which is a little bit shorter.
Chris - You got to have the right kit. It's very important that a chemist has got the right glassware, isn't it?
Darcy - Absolutely. Glassware is something I've got a little bit too much of and it's kind of a collection, and this drink has to have the right glass.
Chris - So none of those funky plastic tumblers like Dr. Kat's fond of when she goes out drinking. Just joking, Kat. That was Cocktail Chemist Darcy O'Neil talking to me from the University of Western Ontario, about his work as a molecular mixologist.

35:58 - The Marine Critters of Christmas
The Marine Critters of Christmas
with James Maclaine, Natural History Museum; Rob Jennings, University of Massachussets; Matt Gollock, ZSL
Chris - Now it is our Christmas Special, so let's take a seasonal dip into the underwater world. Helen Scales has been busy choosing the 12 marine critters of Christmas and here are just three of them to get you in a festive mood.
Helen - As a marine biologist, I think there's no better place to get in the  festive mood than among the weird and wonderful creatures of the ocean. So, instead of partridges in pear trees, I've been diving beneath the waves to track down the 12 marine critters of Christmas, and here are three of my favourites. First up is a denizen of the deep that comes complete with its own set of fairy lights...
festive mood than among the weird and wonderful creatures of the ocean. So, instead of partridges in pear trees, I've been diving beneath the waves to track down the 12 marine critters of Christmas, and here are three of my favourites. First up is a denizen of the deep that comes complete with its own set of fairy lights...
James - I've seen many, many weird and wonderful fish, but one of my favourites is a thing call the stoplight loosejaw which is a deep sea fish and it has some very unusual abilities. Like many of the fish that live at that depth it is capable of producing light. It has two different lights on its head, one behind its eye, and one a bit further down its jaw. One of these is blue which is not unusual. A lot of the fish and other organisms at that depth use blue light because it travels quite far through the water. But the really unusual thing about the stoplight loosejaw is that it also has a red light and it's one of only three kinds of fish that can do this. Hardly anything else can see it, everything's eyes are tuned to blue light. So the red light that's produced by the stoplight loosejaw is pretty much invisible. So it has its own private wavelength of light. We're still not totally sure what it uses it for. It could use it as a torch, to shine ahead of it as it's swimming around looking for prey, or it could even use it to communicate with other stoplight loosejaws and of course everything else would be oblivious.
Helen - James Maclaine there from the Natural History Museum in London with a fish that glows just as brightly as Rudolf's nose. For my second critter of Christmas, let's swim out into the big blue to catch up with an ocean drifter that's just about perfect for this time of year.
Rob - So, sea angels are a group of snails. They're not too distant relatives of garden snails that you would find on land in your backyard.  They are very beautiful animals and you know, they have these transparent bodies and these giant wings, they look a lot like angels. But one of the really interesting things is that in Antarctic waters, Clione antarctica has developed this evolutionary tactic to sort of make up for the fact that it doesn't have a shell. These sea angels have lost that protective ability, but instead they've evolved, or at least Clione antarctica has evolved, bad tasting compounds that it synthesises. So fish and other predators learn very quickly when they take a bite of Clione that it's not something that they want for a meal. This has led to actually a very curious interaction between sea angels and a totally unrelated group of animals, hyperiid amphipods, they're sort of distant cousins of shrimp and of krill. And these amphipods have learned that if they grab onto a Clione and essentially hold it hostage and swim around carrying this giant sea angel on its back, fish and other predators won't eat the amphipod because it's got this bad-tasting Clione carried along with it.
They are very beautiful animals and you know, they have these transparent bodies and these giant wings, they look a lot like angels. But one of the really interesting things is that in Antarctic waters, Clione antarctica has developed this evolutionary tactic to sort of make up for the fact that it doesn't have a shell. These sea angels have lost that protective ability, but instead they've evolved, or at least Clione antarctica has evolved, bad tasting compounds that it synthesises. So fish and other predators learn very quickly when they take a bite of Clione that it's not something that they want for a meal. This has led to actually a very curious interaction between sea angels and a totally unrelated group of animals, hyperiid amphipods, they're sort of distant cousins of shrimp and of krill. And these amphipods have learned that if they grab onto a Clione and essentially hold it hostage and swim around carrying this giant sea angel on its back, fish and other predators won't eat the amphipod because it's got this bad-tasting Clione carried along with it.
Helen - How crafty is that? Rob Jennings there from the University of Massachusetts, introducing us to the beautiful sea angels. Now, let's meet an organism for which one set of sex organs just isn't enough.
Matt - The Chimaeras are probably what I would describe as the forgotten cousin of the shark and ray. Chimaera in Greek mythology actually means a creature that's created from other bits of animals, which when you look at a chimaera you can actually sort of believe that. While chimaera is a sort of a group term for these fish, within that there's individual species that are known as rabbit fish, rat fish, elephant fish. So  there's a range of different names for different chimaera species. When you get to the head end, that's where it kind of gets really interesting because sharks, rays, and chimaeras, all use electrosense to detect their prey. And the chimaeras have some very unusual modifications of their snout. I still sort of have trouble imagining the mechanics of this but the males actually have a retractable sex organ on their head. They have traditional sex organs as well, but they seem to have this other one as well. And to my knowledge I don't know if anyone's actually seen it in use, but it is something that sort of slightly boggles my mind.
there's a range of different names for different chimaera species. When you get to the head end, that's where it kind of gets really interesting because sharks, rays, and chimaeras, all use electrosense to detect their prey. And the chimaeras have some very unusual modifications of their snout. I still sort of have trouble imagining the mechanics of this but the males actually have a retractable sex organ on their head. They have traditional sex organs as well, but they seem to have this other one as well. And to my knowledge I don't know if anyone's actually seen it in use, but it is something that sort of slightly boggles my mind.
Helen - A classic example of sex on the brain. That was Matt Gollock from the Zoological Society of London.
Chris - Helen Scales there with three festive Marine critters. If you want to find out a bit more about the rest of Helen's picks as the 12 marine critters of Christmas, you can find them on the special edition of Naked Scientists which is called the Naked Oceans podcasts, and you can find that at
nakedscientists.com/oceans.

Blowing out candles around corners
Why do Babies calm down when placed on a washing machine?
Kat - Yes, I think that there is definitely some truth in this because obviously the inside of a lady is not a very quiet and still place. People walk around, they're sort of constantly talking, joggling about, wondering around, all that kind of stuff. And so, I should think that the vibrations of a washing machine and perhaps a bit of the noise would help to calm a baby down. A friend of mine always used this, by sticking the kid just in the back of the car and driving around until it fell asleep. So, I'm sure there's probably some truth in that and it probably is the vibration that does it.
Why do Snowflakes differ in Size and Shape?
Dave - Snow is made up in the atmosphere and it's basically when water vapour goes directly from a vapour to a solid - straight to ice. It's sublimed into ice and there are lots of different ways it can do this. There are lots of different modes of growth. Some modes which produce long, straight crystals and make the crystals get longer and thinner. Some cause them to branch in different ways. Sometimes you can get plates forming and sometimes you get long straight needles as the temperature and humidity changes. So, if a snowflake moves around in a cloud both the temperature and the humidity will change while it's growing. And so, depending exactly how long it stays at one temperature, at one humidity, then another temperature then another humidity, you'll get different forms of growth. So, the snowflakes which you'll produce will be slightly, slightly different. They don't necessarily all have to be completely different, but there'll be lots and lots of different types.
What happens to all the salt put on roads in cold weather?
Chris - Well the answer for that is actually when the ice melts, the salt gets washed to the margin of the road, lots of it ends up in storm drains, so that ends up ultimately going to the sea, so that doesn't really matter. But on country lanes and things, and in residential areas, lots of that salt actually gets washed to the side of the road and then ends up in your garden. And there is a problem with salt contamination of soil because the salt make the soil become very, very saline over time, especially on busy roads that get very large amount of salt dumped on them, and this can actually have a problem for the wildlife, chiefly plants and things like that.
Do sunspots have anything to do with the Earth's magnetic field?
Chris - The answer is, Alan, that sun spots are an independent entity which occur on the Sun and the Earth's magnetic field doesn't make them happen. But they can interact with the Earth's magnetic field. And it's thanks to the fact that we have a magnetic field, that life is possible on Earth. The Sun is continuously giving rise to what's called the solar wind which is a million mile an hour maelstrom of charged particles that go whizzing past the Earth and are deflected around the planet by our magnetic field. And in fact, it's the interaction of those charged particles with the magnetic field that gives rise to phenomena such as the Northern Lights, the Aurora Borealis, and down in the southern hemisphere, the Aurora Australis. But there was one very, very important event that was documented in the 1850s. It was actually in 1859 and it's known as the Carrington event in honour of the British Astronomer, Richard Carrington who first saw this. What he spotted from his amateur observatory in the south of England was an enormous sun spot on the 1st of September 1859 and this coincided with a very dramatic event that happened on earth when observers, worldwide, recorded the sky turning blood red. There were bolts of lightning shooting down from the clouds like St. Elmo's fire for example and telegraph operators all over the world in countries that had a telegraph, were getting electric shocks. People were being killed and there were fires being started by all these currents surging down the telegraph connections. What was happening was that this sun spot was associated with an enormous coronal mass ejection and this is where the surface of the sun launches a huge amount of charged material as a plasma out into space, going very, very fast, with very high energy, and this would've impacted on the Earth, and the impact of these charged particles going into the Earth's atmosphere, and inducing electricity to flow in anything that could carry a current was what gave rise to the event. And the colour in the sky was because of the charged particles interacting with the atmosphere and with the earth's magnetic field. So, the sun spots do have an impact on what we see here on Earth, but they're not directly linked to the Earth's magnetic field. The Earth's magnetic field will interact with what they do and produce the events that we see.

51:38 - What makes someone photogenic?
What makes someone photogenic?
We posed this Question to Fhionna Moore from the University of Abertay, Dundee...
Fhionna - I think the thing about being photogenic is that you could be the best looking person in the world and just take terrible photographs.
There are certain things people can do to make themselves more or less photogenic, but there are certain things that some people are lucky enough to have which means that they'll take a good photo, and I think that's got a lot to do with maybe bone structure, and probably as well, being able to smile naturally and look natural in front of the camera which doesn't seem to something that everybody can do, which I know from personal experience.
The kinds of things that people can do to make themselves look more photogenic are things like, for example, I read a study recently where camera angle can make people look more or less attractive, depending on whether they're male or female. So a camera angle tilted looking down on a female face makes her look more feminine and therefore more attractive, whereas a camera angle sort of looking up at a male face makes them look more masculine.
There are things that women do, obviously like wearing makeup which tends to give you a healthier appearance, and we know that a healthy appearance is closely related to attractiveness. So things like having a healthy skin colour and skin texture, things like having blood profusion in the skin, so someone that exercises regularly has a lot of blood circulation, makes them look healthier.
Evidence recently from the perception lab at St. Andrews shows that these things are related to the diet you eat. If you've got lots of fruits and vegetables in your diet, you've got better skin colour and you look healthier and therefore, more attractive.
So there are things that people can do to enhance how photogenic they are, but I think ultimately, it comes down to how naturally people behave in front of the camera and perhaps very basic things like bone structure.
Kat - So the combination of genes, good health, cosmetics, and the skill of the photographer. But what makes a face attractive in the first place?
Fhionna - There are things like having a symmetrical face, so not having too much asymmetry in the two halves of the face tends to be associated with attractiveness and I suppose that's just good luck and good genes. We know that women with feminine facial features are treated as much more attractive, so that's things like having bigger eyes, and a smaller jaw. In men, it seems to be more complicated. Having sort of a feminine male face is attractive and so, is having a masculine male face.
So, I think a lot of it is down to your good genes!










Comments
Add a comment