From anti-ague to anti-Alzheimer's agent: over the 112 years since it was first trademarked, Aspirin has evolved from popular painkiller to powerful preventative against heart attacks, strokes and even cancer. In this week's show we trace its history from the extraction of aspirin-like chemicals from willow bark to the creation of the drug itself. Plus, in the news, how the chemistry of life could have come to Earth in a meteorite and why we need to be careful with stem cells: a new study finds they have an above-average mutation rate. Also, a new technique to etch graphene sheets with single-atom precision, an insight into how our drugs are made and how painkillers hit pain where it hurts...
In this episode
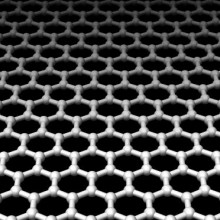
- Graphene Lithography Atomic Precision Etching
Graphene Lithography Atomic Precision Etching
with Professor James Tour, Rice University
Kat - Also in the news this week, researchers at Rice university have developed a new way to etch structures into stacked piles of graphene, the marvellous material consisting of a single layer of carbon atoms. This could allow manufacturers to make computer chips from graphene in much the same way they currently do for silicon. But sometimes in science, you make a great discovery entirely by accident - and this was just one of those cases, as Professor Jim Tour explains..
Jim - What we set out to do was to convert graphene to graphein, and what that is, is taking the carbon structure of graphene which is a bunch of 6-member rings in a plane and attaching hydrogen to it. That makes then graphein, and that would make an area that would be non-conductive. We thought if we could pattern zinc upon graphene, we could then use the hydrogen which is generated from the zinc reduction reaction from where zinc is treated with acid to hydrogenate the graphene to graphein. But what happened was, it turned out that wherever the zinc landed, it removed the graphene layer, but left the underlying layer completely intact.
Chris - It's really reminiscent of what people do with silicon and lasers to make microchips, isn't it? But you're doing this using zinc and graphene which is interesting because people are talking about using graphene as a material to make the next generation of microchips.
Jim - Precisely and so, this constitutes lithography. Lithography is the way we make computer chips. You take a big silicon wafer and you chip away at it using chemicals and light to make the small features, namely transistors and wires for example. So one takes a mask and has certain areas as holes from the mask and then shines light through those holes and that will develop what are called "resists" on top of the silicon to develop and build up these structures that we've seen on chips. But now, to be able to do this in graphene brings graphene one step closer. There is a huge difference between a monolayer and a bilayer of graphene. A monolayer of graphene is a metal. It doesn't have a band gap. It's not something that's easily made into a transistor, but if you have two layers of graphene, it opens up a gap and it becomes a little bit more like silicon where you can make it into a transistor. And so, to be able to have one layer or two layers, or three layers which can be more conductive then you can have different devices next to each other, and that's what you want. You want to have heterogeneity in devices. You don't want all devices to be exactly the same. So it's a new tool and a toolbox for making graphene into electronic chips.
Chris - Do you actually know though, Jim what the zinc is doing? Why this actually works to strip away the single layers and leave the one underneath untouched?
Jim - I think we have a reasonable idea. So what happens is the zinc is spattered on the surface so that will cause zinc atoms to fly up from a chunk of zinc metal, and to hit the surface that we're trying to pattern. You make a mask and wherever you want the metal to go, you have holes in the mask and it hits the surface. So what happens is about 0.5% of the zinc atoms, come with enough energy to actually knock out a carbon atom from the graphene and substitute in with the zinc atom. The zinc metal has a very high oxidation potential, so it's really begging to oxidise rather rapidly and that's going to then leave, cause the zinc atom to come out and you'll get oxygen-carbon bonds. So wherever a zinc atom had been, now the carbon atom is knocked out and the surrounding carbon atoms become oxygenated. So what you end up with is, instead of a sheet of graphene now, you have a sheet of graphene with holes in it. Then what's done is we put it into acid and acid then strips away the zinc, and in stripping away the zinc, it generates hydrogen. And that bubbles and helps to wash away the small pieces of graphene that have now been diced up on the surface. But the zinc atoms that hit never had enough energy to go through one layer and affect the second layer below. And so, it turns out to be quite a selective technique that's not only shown now with zinc. We showed we could do it with aluminium as well. So that's a reasonable understanding that we have now of the mechanism.
Chris - And once you cut through by substituting oxygen onto some of these area so they may be removed or floated off, the residual graphene from the layer where you've got - say, a step is the bit that remains behind stable or will that chemically deteriorate with time?
Jim - Everything that we have seen at the step edge, where you have one sheet of graphene that is one step higher than the sheet below it, is stable. We haven't seen that curling up. We haven't seen that undergoing any problem. It undoubtedly has different atoms at the edge. There's going to have to be - they're hydrogen atoms or oxygen atoms at that very edge. But no, there is no delamination that occurs. The other fascinating point about this is lithography is always done in industry but we have hit the ultimate in lithography. It is single atom layer precision. It will never get better than this. So in other words, in a thousand years after doing lithography, they can't do better than this. We are stripping off a single atomic layer. You can't cut an atom in half. That's as thin as you're going to get. This shows that we can have precision that you could never have in silicon - single atom resolution.
Chris - And you think that this will be a practical way to make, if you had to - a microchip of the future using graphene as a base material.
Jim - It's certainly a new wrench in the tool box. There was no way to do this before, so if we are going to make large scale patterns out of graphene, this is definitely a way to do it. And it uses methodologies that are commonly used in silicon manufacture.
Kat - ...which will make ousting silicon from its position at the heart of the microprocessor industry that bit easier. That was Professor Jim Tour, from Rice University - he's published that work in the journal Science this week.

- Is Aspirin a hangover cure?
Is Aspirin a hangover cure?
We posed this question to Professor Peter Rothwell from the University of Oxford...
Peter - Aspirin versus paracetamol for hangovers is very difficult. It's an individual thing really.

01:52 - Key component of life's building blocks could have come from space
Key component of life's building blocks could have come from space
The origins of life on earth are a hotly debated topic among scientists. One theory suggests that, even if whole organisms didn't come to earth carried by meteorites, then maybe meteorites brought some of the chemical building blocks for amino acids - the molecules that make up proteins. Now tests on a meteorite with the catchy name CR2 Grave Nunataks 95229 provide more evidence that meteorites might have brought these building blocks to earth, kickstarting the chain of events that led to the evolution of life here.
CR2 Grave Nunataks 95229 is a type of meteorite called a carbonaceous chondrite - meteorites that contain a range of organic chemicals including amino acids. Because of this, some scientists think that they might have 'seeded' earth with these chemicals when they fell from space, providing the primitive building blocks for the formation of DNA and proteins, which ultimately led to life as we know it. But studies of similar meteorites haven't come up with solid evidence for this, as the chemicals they contain are a real mix of all kinds of things - most of which couldn't easily be used to create molecules of life.
 The Grave meteorite spun off from an asteroid and landed in Antartctica in 1995. Researchers, led by Sandra Pizzarello and her colleagues in the US, analysed the chemical makeup of the meteorite using high pressure water at a temperature of 300 degrees centigrade - conditions designed to mimic both the asteroid where the meteorite was made, and the conditions on the early earth.
The Grave meteorite spun off from an asteroid and landed in Antartctica in 1995. Researchers, led by Sandra Pizzarello and her colleagues in the US, analysed the chemical makeup of the meteorite using high pressure water at a temperature of 300 degrees centigrade - conditions designed to mimic both the asteroid where the meteorite was made, and the conditions on the early earth.
Publishing their results in the journal PNAS this week, the team discovered that their asteroid contained surprisingly high amounts of ammonia - a chemical precursor to amino acids. These levels were much higher than might be expected on earth at the time.
Given the chemical makeup of similar meteorites, and the fact that most of these only contain compounds built of rings of carbon atoms, this is an unusual find indeed, and the first of its kind. Further analysis showed that the ammonia in the meteorite could only have come from the original asteroid it came from, and suggests that there was a lot of ammonia around in that environment.
Nitrogen, which is a key part of ammonia, is the fourth most common reactive element in the universe. Here on earth, it's a vital component of proteins, as well as DNA and RNA - the genetic information within living cells - and it's indispensible for life. Ammonia plays a key role in many chemical reactions, including the reactions that create the molecules of life.
But from what we know of the conditions on the early earth, it's been hard for scientists to figure out how this might have worked back then. For a start, evidence suggests that the early earth's atmosphere wasn't relatively rich in ammonia. And we know that sunlight can also break down the chemical, which would have been a big problem at the time.
But the discovery that meteorites can actually contain relatively large amounts of ammonia suggests an alternative route for the chemical to turn up and get involved in the chemical reactions that might have led to the generation of the molecular building blocks of life all those millions of years ago. And it adds weight to the idea that at least some of the molecules that kickstarted life on earth may have come from space.

05:36 - Stem cell mutants
Stem cell mutants
Stem cells made by reprogramming a patients own cells have the potential to revolutionise personalised medicine. But are they safe?
A paper in Nature this week urges caution after finding that current techniques involving the addition of up to four genes to "reprogramme" adult cells so that they revert to a stem cell state can also leave the cells harbouring potentially harmful mutations, some capable of causing cancer.
 Study author Kun Zhang and his colleagues genetically sequenced 22 lines of so-called iPS cells - induced pluripotential stem cells - produced by seven different laboratories using several different techniques. They then compared the resulting genetic profiles with the corresponding sequences of the parent cells from which the stem cells had been made. They were surprised to find tenfold more mutations (DNA changes) in the stem cells than would be expected normally for cells kept in culture, and these changes appeared to be permanent, remaining present when the cells were grown for over 40 generations.
Study author Kun Zhang and his colleagues genetically sequenced 22 lines of so-called iPS cells - induced pluripotential stem cells - produced by seven different laboratories using several different techniques. They then compared the resulting genetic profiles with the corresponding sequences of the parent cells from which the stem cells had been made. They were surprised to find tenfold more mutations (DNA changes) in the stem cells than would be expected normally for cells kept in culture, and these changes appeared to be permanent, remaining present when the cells were grown for over 40 generations.
They were also not a random smattering of changes but instead were more than twice as likely to be "missense" mutations that would directly alter the structure of the protein encoded by the affected gene rather than silent "synonymous" changes that don't actually alter protein structure.
This indicates that strong selection is at play when the cells are being produced and that those that tend to grow best might be the ones that have more of these sorts of changes.
The researchers aren't sure yet what renders the cells more vulnerable to mutation during the reprogramming process, or when the changes occur.
"These studies look at two different aspects of stem cell mutations," says Zhang, "but their take-home message is the same - things can go wrong at the genome level when reprogramming and growing reprogrammed cells. So, to maximise safety, before we put these cells back in the human body for therapeutic purposes, we must be sure that the cells contain the same genome as the recipient, with no cancer-causing or other serious types of mutations."

09:15 - Etching Graphene
Etching Graphene
With a breakthrough that could hasten the arrival of a new generation of even more powerful microprocessors, scientists have discovered how to etch single sheets of graphene...
Seen down a microscope this material resembles a flat sheet of six-membered rings of carbon atoms which, stacked up into many millions of layers, makes graphite. But, peeled apart into individual atom-thick sheets, graphene has a range of interesting properties including being highly deformable as well as electrically conductive and yet sufficiently thin to also be transparent.
Consequently, technologists think it could hold the key to the next generation of computer displays and might even be fashioned into extremely small transistors for microchips.
But the problem has been how to "etch" or cut stacks of graphene in order to create the required architecture. Now, writing in Science, Rice University researcher Jim Tour and his team think they have stumbled on the solution.
Setting out originally to make graphane - in which hydrogen atoms are attached to the edges of a piece of graphene, rendering it non-conductive - the team "sputtered" metallic zinc onto a sheet and then dissolved this off with acid, reasoning that the resulting hydrogen gas would add itself to the graphene where the zinc was, making graphane.
Instead they were shocked to see that the zinc dissolved away leaving perfect holes in just single sheets of the graphene. And by applying a mask to some graphene so that only some areas became zinc coated, the team found that they could precisely etch away individual parts of the graphene sheets producing any pattern they desired, including even making the nano-equivalent of a linocut owl!
The technique works because the zinc atoms have sufficient energy to knock out and replace some of the carbon atoms they hit, also causing oxygen to be added to the edge of the sheet where the zinc lands. When the zinc is then dissolved with an acid solution the hydrogen that results from the reaction prises free the individual oxygen-flanked sheet, floating it away.
"This offers precision engineering at the resolution of the individual atom," says Tour. "Even in a thousand years' time you won't see people getting better than that!"
15:56 - Foetal immunity protects females
Foetal immunity protects females
The female genital tract uses a foetal gene to protect itself from infection during adulthood, new research has found.
Mucosal surfaces, like the genital tract, mouth, gut and eyes, are important portals of entry for pathogens attempting to penetrate our immunological defences. They are known to be defended by a class of antibodies known as IgG as well as a number of other chemical armaments, but exactly how the IgG antibodies reach the skin surface where they can neutralise attacking microbes, no one was sure.
 Now, writing in PNAS, University of Maryland researcher Xiaoping Zhu and his colleagues have discovered that adult cells use a gene normally found working in the placenta to do the job. The gene in question is called FcRn and it's role during pregnancy is to grab antibodies from the mother and add them to the baby's bloodstream. This temporarily provides the newborn with so-called "passive protection" from the mother while its own immune system gains momentum after it is born.
Now, writing in PNAS, University of Maryland researcher Xiaoping Zhu and his colleagues have discovered that adult cells use a gene normally found working in the placenta to do the job. The gene in question is called FcRn and it's role during pregnancy is to grab antibodies from the mother and add them to the baby's bloodstream. This temporarily provides the newborn with so-called "passive protection" from the mother while its own immune system gains momentum after it is born.
Reasoning that the same gene might be at work in the genital tract, the Maryland team tested a range of cultured cells include uterine, cervical and vaginal lines, confirming the presence of high levels of FcRn activity.
Next, to confirm that the gene was actually functional, they incubated cultured cells that were expressing FcRn with IgG-class antibodies. Intriguingly the antibodies were picked up and passed from one side of the cell to the other, a process which ceased when the researchers disabled the FcRn gene.
They were also able to confirm the same results in mice, including demonstrating that chemically-labelled antibodies are transported onto the mucosal surfaces of the genital tract by the FcRn gene. When this was genetically "knocked out" from the mice, the antibody movement was arrested. These knockout animals were also highly susceptible to infection with a strain of herpes virus that transmits genitally.
Knowing how this process now works, the researchers argue that it may be possible to exploit this system to produce better vaccines capable of boosting immune defences at mucosal surfaces and hence better protect patients against a range of infections, not least HIV and other sexually-transmitted diseases.

18:25 - Planet Earth Online - Eyeing Up Earthquakes
Planet Earth Online - Eyeing Up Earthquakes
with Brian Baptie, British Geological Survey
Kat - Every week, somewhere in the world, an earthquake occurs that's big enough to cause serious damage to people, roads and bridges as we've heard from New Zealand. Seismologists monitor this activity to try to learn more about the geological processes underlying the phenomenon. Richard Hollingham went to visit one part of the network of UK earthquake monitoring stations - which was hidden beneath a lecture theatre at the Royal Observatory in Edinburgh - with Brian Baptie from the British Geological Survey...
Richard - So we've just opened up the hinge of a large trap door of the floor to reveal - it's like Harry Potter this - a staircase that goes down beneath the lecture theatre! So let's head down the stairs.
Brian - It's the first door... second door... finally, this is the entrance to the vault....
Richard - Okay, so we've come in to what appears to be a concrete lined room with a bit of water dripping from the ceiling and there are three hefty concrete blocks on the floor like enormous pieces of Lego.
Brian - Well these are what we call piers and they're built directly onto the rock of the hill. This hill is an old volcano and these piers are built directly onto it and if you look around, you'll see that the floor is actually suspended, so that when we're walking around on the floor in here, we don't actually come into contact with the piers. And on the piers, on each of the piers, there's a seismometer which looks like a very large tin can, but they're actually incredibly sensitive devices. They can pick up vibrations that are around thousanths of a millimetre.
Richard - What are you picking up on these seismometers?
Brian - Well we pick up earthquakes from all around the world, so large or even moderate sized earthquakes from the other side of the world. We also pick up vibrations that are rather closer to home and we actually detect between 100 and 200 earthquakes that come from the British Isles and immediate offshore areas every year.
Richard - So 200 a year in the UK?
Brian - Up to 200 a year.
Richard - Now this is part of a network, isn't it? This isn't the only one. There are secret vaults around the country.
Brian - Yeah. We have about 100 instruments all around the country. Not all the vaults are as big as this one. Some of them are in underground tunnels or old bunkers. Some of them are just buried in holes in the ground. The data from all those instruments is transferred automatically using the internet, back to our offices in Edinburgh and then we can analyse that data and use it to work out exactly where the earthquake was, when it occurred, and how big it was.
vaults are as big as this one. Some of them are in underground tunnels or old bunkers. Some of them are just buried in holes in the ground. The data from all those instruments is transferred automatically using the internet, back to our offices in Edinburgh and then we can analyse that data and use it to work out exactly where the earthquake was, when it occurred, and how big it was.
Richard - Well we made it out of the vault and into the offices of the British Geological Survey. Now Brian, this office was where you used to analyse the sounds of earthquakes.
Brian - That's right. This office is what was called the replay lab and although nowadays we record all our data digitally and analyse it using computers, 10 years ago, all our data used to be recorded on analogue magnetic tapes. Our analysts would listen to these tapes, speed it up about 50 to 100 times and then they would use their ears to tell the difference between different types of seismic events.
Richard - And you've got some of the recordings of earthquakes, some of the things you can hear.
(Sound)
Brian - So that was a magnitude 5.4 earthquake from North Wales, recorded somewhere in Britain. It was actually the largest earthquake that we have a recording of in our archive. You can tell when you listen to it, it's actually quite a distinctive double sound and that's because there are two seismic waves that propagate when there's an earthquake through the Earth, and those travel at different speeds and arrive at the receiver at different times.
(Sound)
Brian - The next one is an underwater explosion. So typically, we pick up quite a few of these underwater explosions every year. It's when the navy, quite often, they find old world war II mines or perhaps the navy are doing some kind of naval exercise from where they're setting off explosions in the water, and we can pick them up.
(Sound)
Richard - Sounds like a shot being fired.
Brian - That's right. So, much, much higher pitch, completely different type of signal. We didn't have the distinctive double bang. We just have this kind of relatively high pitched pop, if you like, which you can think about in terms of something that will pop in the water,creating a huge bubble.
 Richard - And the final one.
Richard - And the final one.
Brian - So the final one is a magnitude 6.9 earthquake from Japan. So the vibrations from this earthquake have travelled all the way through the Earth and have been recorded here.
(Sound)
Richard - So let's get this straight, that was a recording made here of an earthquake in Japan, on the other side of the world?
Brian - That's right. When you have really large earthquakes, huge amounts of energy is released and that energy travels all the way through the Earth and we can pick up the vibrations from that earthquake.
Does immunity gained from a foetus explain better immunity in mothers?
Chris - The answer is no, because the same gene which is used by a baby is used by these women because they carry it anyway. They're just activating it in a different set of tissues to get the same effect as the gene would have in a baby developing inside the mother, but at a totally different site.
That's what's wonderful about the huge genetic recipe book we have. We can redeploy the genes and their functions all over the body to do the same job in different places or even by tweaking the gene a bit, a totally different job, using almost the same gene.
24:53 - The History of Aspirin
The History of Aspirin
with Sarah Castor-Perry
On the 6th March 1899, the Beyer pharmaceutical company officially registered Aspirin as a trademark, following their chemist Felix Hoffman's successful synthesis of a stable form of acetylsalicylic acid - the chemical name for aspirin - in 1897.
Best known as an analgesic against aches and pains, aspirin can also be used as an antipyretic to control fever and as an anti-inflammatory to reduce inflammation. It also has the effect of making the blood less likely to clot, known as anti-coagulation.
It was one of the first 'non steroidal anti inflammatory drugs' to be discovered (another example being ibuprofen), and it had the additional benefit of masking pain without impairing consciousness; nor was it addictive like the opiate painkilling alternatives, like laudanum.
 By the time of the discovery of aspirin, medicines based on similar chemical compounds called salicins that were derived from plants, including meadowsweet and the bark of willow trees - Salix in latin, hence the name of the drug, had already been in use for agues, aches and fevers for over 3 and a half thousand years.
By the time of the discovery of aspirin, medicines based on similar chemical compounds called salicins that were derived from plants, including meadowsweet and the bark of willow trees - Salix in latin, hence the name of the drug, had already been in use for agues, aches and fevers for over 3 and a half thousand years.
One proponent was a priest from Chipping Norton named Edward Stone, who's credited with conducting one of the world's first clinical trials. Having found willow bark to be beneficial for his own aches and pains, in the 1760s he prepared further extracts that he administered to fifty parishioners, whom, he later wrote in a report to the Royal Society in 1763, gained relief from a range of agues and intermitting disorders".
However, it was only much later, during the 1820s and 30s, that chemists in Italy and Germany finally managed to purify the active salicin from the plant remedies. Salicin, it turns out, is chemically very similar to aspirin except that it contains a sugar molecule where aspirin has a short chain of carbon atoms. When ingested, salicin is oxidised to its active form, salicylic acid.
But although the medicinal use of salicin and hence salicylic acid grew throughout the mid 1800s, it did have several drawbacks, not least the fact that it caused severe irritation to the lining of the stomach and even ulceration and bleeding.
Around the time that this was happening, an industry began to grow in Germany to investigate medicines that might be made from the aniline cloth dyes that were being produced from coal tar.
And surprising as it might sound given the starting point of coal tar, a lot of compounds were discovered that could be used to reduce fever and pain. One German dye firm, called Friedrich Bayer and Company, began to expand to investigate some of these potential medicines further, seeing that there was money to be made. It was this company that a young chemist called Felix Hoffman joined in 1894, to work with two more senior scientists, Arthur Eichengrün and Heinrich Dreser.
 In 1897, Eichengrün instructed Hoffman to find an alternative form of salicylic acid that would be less irritating to the stomach, but would still produce anti fever and pain effects.
In 1897, Eichengrün instructed Hoffman to find an alternative form of salicylic acid that would be less irritating to the stomach, but would still produce anti fever and pain effects.
In his lab book entry for the 10th of October 1897, Hoffman declared that he had synthesised a pure form of acetylsalicylic acid by refluxing salicylic acid with acetic anhydride. This ended up producing a much purer and more stable form of acetylsalicylic acid than had been produced before using other techniques. Clinical trials suggested that it was just as effective as salicylic acid but far more stomach-friendly and with fewer of the unpleasant side effects.
Unfortunately the company couldn't patent their potential new pharmacological blockbuster because, it turned out, the acetyl-salicylate molecule had been made and published previously. A French chemist called Charles Frederic Gerhardt had described the synthesis of the substance in 1853, but had never followed up on his discovery.
Instead, Bayer elected to trademark the agent, constructing the name, it's claimed, from the A for acetyl, the SPIR from the meadowsweet plant they extracted the salicylic acid from, Spirae ulmaria, and the IN came from the fact that ending drug names with IN was the in thing to do at the time.
The drug was initially produced as a powder and then in pill form from 1915.
Aided by the 1918 'flu pandemic, where aspirin was very successful in controlling pain and fever of flu victims, its popularity skyrocketed. However, the 1956 launch of the alternative agent paracetamol, followed by the entry of ibuprofen in 1969, together with mounting evidence that aspirin could occasionally cause a potentially fatal brain-swelling disorder called Reye's syndrome in some children, dented its popularity. It is now advised not to be given to children under 16.
However, the tarnished image of aspirin was reversed when its powerful anticoagulant effects were shown in the 1970s, and after this aspirin came back into mainstream medical use and it remains today the preventative mainstay measure against strokes and heart attacks by reducing the likelihood of a blood clot. More recent studies have also confirmed that this simple agent can also prevent certain forms of cancers and stop them recurring after treatment, and can even reduce the risk of Alzheimer's Disease.
However, with a discovery of such magnitude and with money and fame to be had, there is unsurprisingly a lot of controversy surrounding the discovery of aspirin. Eichengrün released a paper in 1949, three years after Hoffman's death, arguing that he should be credited with the discovery of aspirin and that Hoffman had just been following his instructions. This account of events was not supported until 1999, when a researcher at Strathclyde University examined the case and came out in support of Eichengrün. Bayer, the pharmaceutical company that Hoffman and Eichengrün had been working for at the time, dismissed the findings and stood behind Hoffman. As of 2004, the controversy is still unresolved.
The events that led to the production and widespread use of aspirin are an important chapter in the history of medicine. It was the first modern painkilling drug that could be used without affecting a person's day to day activities and has brought relief to millions of people over the years. Although less in use as a painkiller now, it still plays a vital role in medicine.

Is hot water heavier than cold water?
Chris - Hot water is actually a little bit heavier than cold water because, as Einstein told us, E=mc2. So if E, the energy in the water, goes up because it's hotter, then mass, m, must also go up to keep the equation balanced [c, the speed of light in a vaccuum, doesn't change]. So there will be a very subtle and very tiny increase in mass of the hot water, compared to the cold water.
The reason the ice floats is actually because it's a lot less dense than the water. The ice is made of water but, because water expands when it freezes, the ice is pushing a bigger volume - and hence a bigger mass - of water out of the way than the ice itself weighs. For this reason the ice is actually feeling a bigger push "up"(called buoyancy) from the water underneath than the ice weighs itself, which makes it float.
31:43 - Aspirin as Preventative Medicine
Aspirin as Preventative Medicine
with Peter Rothwell, University of Oxford
Chris - Aspirin was touted originally as treatment for pain; but, 100 years on, we now know it's much more powerful than that. Peter Rothwell is Oxford University's Professor of Clinical Neurology and he's with us to discuss what else aspirin can do. Hello Peter.
Peter - Hello.
Chris - Thank you for joining us on the Naked Scientists. So tell us, what else can aspirin do, apart from be a very good pain killer?
Peter - Well as you say, it's still a good painkiller and a lot of people use it for that reason, particularly patients with migraine for example. But it's probably used more widely now as what's called an anti-platelet drug or an antithrombotic drug to reduce the risk of blood clots, particularly in people at risk of heart attacks and strokes. It was realised slowly in the 1950s and 60s that people who took aspirin regularly seem to have a lower risk of recurrent heart attacks in particular, and then it was found that the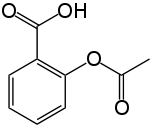 mechanism of this was by inhibiting platelets which were one of the crucial cells that form blood clots. Therefore, people started to do clinical trials in the 1970s, to see whether in randomised trials, it really would be effective in preventing heart attacks and strokes. And generally speaking, these have all been positive. It reduces the risk of a stroke or a heart attack if you've had them already by about a third.
mechanism of this was by inhibiting platelets which were one of the crucial cells that form blood clots. Therefore, people started to do clinical trials in the 1970s, to see whether in randomised trials, it really would be effective in preventing heart attacks and strokes. And generally speaking, these have all been positive. It reduces the risk of a stroke or a heart attack if you've had them already by about a third.
Chris - It's quite surprising that someone made the observation that the aspirin users were having fewer heart attacks and strokes because they didn't initially suspect that might be the case, so they wouldn't have been looking for it.
Peter - No. it's one of those fortuitous observations as things often are in medicine. In fact, it was at the very far-sighted general practitioner that noticed in his patients.
Chris - That's a bit like the cure for smallpox, I suppose. The vaccine for smallpox [was discovered by] another GP, Edward Jenner in that case. So tell us a bit about how aspirin actually works. How is it doing what it's doing, both to cure a migraine as you mentioned, but also, to do these other things to stop blood clotting quite so keenly?
Peter - In relation to blood clots, what it does is inhibit one of the processes, whereby platelets become activated. Of course, we've all got platelets going around in our blood all the time and for the most part, they don't become activated. But when they come across various stimuli such as collagen, they become activated and clump together and form blood clots. One of the mechanisms that this happens is via an enzyme called cyclo-oxygenase, which is part of the pathway that synthesises prostaglandins. Aspirin irreversibly inhibits the cyclo-oxygenase enzymes and therefore, blocks that particular pathway. The platelets still work via other pathways, but they just work less effectively.
Chris - What about the side effects though? Because, as we heard from Sarah's history piece just now, people had realised that there were these chemicals in nature, they took them from meadowsweet and willow bark and things which were very good at taking away the painful problem. Presumably, they're also going to have some of this anti-thrombotic effect, but they also have the side effect of making people have very bad stomach ache. So, why does aspirin not do that as much as salicylate, but still, nonetheless, does cause a problem?
 Peter - Well it still does this unfortunately and there are essentially, two mechanisms involved. One is that the anti-platelet effect itself means that when the stomach lining is damaged, you're more likely to bleed. But in addition to that, aspirin does seem to inhibit the healing process that maintains the endothelium, the protective surface of the stomach lining.
Peter - Well it still does this unfortunately and there are essentially, two mechanisms involved. One is that the anti-platelet effect itself means that when the stomach lining is damaged, you're more likely to bleed. But in addition to that, aspirin does seem to inhibit the healing process that maintains the endothelium, the protective surface of the stomach lining.
Chris - So, apart from the fact that it does stop blood from clotting and had a very dramatic effect on strokes and also on heart attacks, saving literally, millions of lives. How is it now emerging that it's doing all these others things because the benefit don't stop there, do they? As we've alluded to, there are benefits for cancer and also, potentially for dementia.
Peter - Certainly the benefits for cancer are becoming more clear cut. I think there's still a lot of uncertainty about whether it has a beneficial effect for dementia, but it's certainly a possibility. What's clear is that the effect of aspirin, particularly on this COx2 enzyme system, occurs in most tissues that express the enzyme - which is the majority of tissues in the body. And so therefore, it has a broader anti-inflammatory effect. In fact, that's one of the mechanisms as it works, in terms of reducing fever and reducing pain, as an anti-inflammatory drug. Inflammation is a very important process in the development of several diseases including cancers. It looked to us for two or three decades now as though, from the epidemiology, aspirin potentially could prevent at least some of the more common cancers. In recent years, people have now followed up patients who were in trials of aspirin many years ago, to see whether there is any evidence of reduced risk of cancer. Over the last years, several studies have shown that in fact, that's the case.
Chris - Does the same mechanism apply probably, if it's going to, in the anti-dementia protective effect?
Peter - Certainly there's a link between the inflammation and dementia, inflammation and depression, and certain other neurological diseases. So, one might expect a anti-inflammatory effect to have some benefits in terms of neurological diseases. But there are a whole host of other things that aspirin does which could also be involved, and it's not clear if there is an effect, what the mechanism is.
Chris - Do you have any feeling, Peter, for when someone should start to take aspirin? At what age would you need to initiate it in order to get all these benefits or is that a very hard question to answer?
Peter - It's hard to be absolutely certain about it for an individual, but I think what we can say is that the risk of heart attacks and strokes, which is obviously one of the things you're trying to prevent, increases with age. When you get into your mid-50s, particularly if you have a family history or any other risk factors, trials have already shown that the benefits of aspirin in terms of prevention of heart attacks and strokes outweighs the risk of bleeding. It's just that it's a relatively small benefit for the individual. But the more recent data showing that aspirin also reduces the risk of death due to several common cancers when added to that existing benefit, really does tend to push things towards significant benefit. The complication is that the risk of bleeding increases with age. And so, when you get to your mid-70s, certainly, the risk of bleeding probably outweighs the benefit. So one might argue that people should consider taking aspirin from the age of about 50 to 75.
Chris - Because there was a paper that was published a year or so ago from a group in Edinburgh and they were actually saying that quite a few people do actually self-treat with aspirin. They've just read the newspaper reports and various publications, saying that aspirin has all these beneficial effects. They therefore put themselves on it and they were saying, that actually, the balance is that it could be bad to do that because the people who don't need it will be doing it equally, alongside people who do need it, and so, one could balance out the other and there'd be some people who will suffer at the hands of taking aspirin they didn't need and there'll be others who do benefit of it.
Peter - That's absolutely right and as always, with self-treatment, it often tends to be the fairly health conscious people that think about taking aspirin. And often, they're the people with a relatively low vascular risk because they don't smoke, they have few other risk factors, they exercise regularly and have a good diet. So it is a question of balancing the risk and the benefits. But once you're getting to your 50s and certainly your 60s, if you have one or two vascular risk factors, then usually, you're into that group where the benefit will outweigh the risks, certainly for preventing heart attacks and strokes. And then when you add on the reduction in deaths due to fatal cancers, it does push things in that direction for a lot of people.
Chris - And what about if someone's on an aspirin-like drug which isn't aspirin, and I'm thinking things like ibuprofen, if they've got some aches and pains. It works very similarly to aspirin, doesn't it? Does it also confer the same risk benefits in all of these other things that you've outlined?
Peter - It's a good question. It doesn't seem to so well at least for prevention of heart attacks and strokes. But it may well have, the drugs like ibuprofen and other non-steroidal anti-inflammatory drugs, may have similar benefits for cancer. We don't have hard data from randomised trials but the epidemiology suggests the effects may be similar for cancer.
Can one become addicted to Aspirin?
We posed this question to Professor Peter Rothwell from the University of Oxford...
Peter - Good question and the answer is No.
Is Aspirin effective in non-human animals?
We posed this question to Professor Peter Rothwell from the University of Oxford...
Peter - You can. A lot of research has been done using Aspirin in animals and in fact, the first observations about prevention and treatment of cancer were in animal models.
Is it true Aspirin is poisonous to cats?
We posed this question to Professor Peter Rothwell from the University of Oxford...
Peter - I'm not sure about cats actually. I wouldn't feel confident about saying anything about that. Chris - I know that dogs can take Aspirin because I use to give my dog Aspirin and buprofen, and I've actually spoken to a vet who informs me that these drugs do work quite well in them as anti-inflammatories because my dog had a bit of rheumatism. I don't know about cats though, I have to admit.
Why do willow trees make an Aspirin precursor?
We posed this question to Professor Peter Rothwell from the University of Oxford...
Peter - That's a very good question. It looks very much as though a lot of different plants synthesise salicylic acid. It's very effective in warding off infections and treating particularly fungal infections in plants. So it looks as though it's synthesised for that purpose. And in fact, one of the downsides of modern food production is that we get a lot less salicylic acid from our diet because all of our food now is pristine with no infection.
When shouldn't Aspirin be used?
We posed this question to Professor Peter Rothwell from the University of Oxford...
Peter - Well, anyone with a history of bleeding from the stomach or from the gut should certainly talk to their doctor first. There are other treatments that can be given to help that and allow them to take Aspirin. Anybody with asthma should certainly discuss it with their doctor. Some patients with asthma are allergic to Aspirin, so those are the two main areas.
Can you take aspirin with alcohol?
We posed this question to Professor Peter Rothwell from the University of Oxford...
Peter - You can take a moderate amount of alcohol with Aspirin. There's no major problem there. If you drink a lot then it will increase the risk of stomach problems.
How does Aspirin interact with food or other drugs?
We posed this question to Professor Peter Rothwell from the University of Oxford...
Peter - [There are] not many interactions that could be dangerous. There are some substances that affect the absorption of Aspirin, but in fact, it isn't a problem for the vast majority of prescription drugs.
Does Aspirin alter blood pressure?
We posed this question to Professor Peter Rothwell from the University of Oxford...
Peter - A very small effect. If you take high doses of Aspirin, it can increase blood pressure slightly, but it's not a problem in clinical practice.
Do regular small doses of Aspirin affect red blood cells?
We posed this question to Professor Peter Rothwell from the University of Oxford...
Peter - If you're one of the few people who gets bleeding from your stomach on Aspirin then that can occur very slowly, without you realising it and so, a very small proportion of patients do get anaemia, but in most patients, there's no effect on the blood count.
Would Aspirin be approved today?
We posed this question to Professor Peter Rothwell from the University of Oxford... Peter - No. Chris - Why? Peter - It's probably got too many side effects. Even though it's an effective drug, the drug companies would worry that they'd be sued because of the risk of bleeding, and that it wouldn't be commercially viable because the lawsuits would offset their profits.

51:48 - How do pain medications target pain?
How do pain medications target pain?
We put this to Tim Warner from Barts and the London School of Medicine and Dentistry...
Tim - The explanation for this lies in what causes the pain and how we experience it. If we imagine, for instance, pain coming from something like a damaged tissue - you could think of something like an arthritic knee for instance. In that knee, there's a local generation of factors that sensitise the local pain nerve endings. So that local sensitisation depends upon what's happening locally in the knee and then to the nervous pathways, this is then taken as a signal to the brain where we perceive the pain. And so, that part of our perception depends on what's happening in the central nervous system. So we have that as an idea in our heads. We can think about how the pain-relieving drugs work. The non-steroidal drugs that we use for muscle, joint aches and pains, and other drugs like ibuprofen, they stop the formation of the sensitising factors - in this example, the knee, and only the arthritic knee is making the factors. It's only there that the ibuprofen-type drugs act, and so, we feel less pain in our inflamed knee. And at the same time, the other knee doesn't go numb because it isn't making the sensitising factors. So there's nothing there for ibuprofen to inhibit. If you had a more intense pain, say an operation on your knee, so you might use something stronger such as a morphine type drug, and those ones do work within the central nervous system. So those drugs are going to cut down the signals in the brain, coming from the nerve collections in the knee, and so, they cut down the sensation of pain by a central effect in the brain, and not by acting locally in the knee like ibuprofen. But because they act centrally, they have a tendency to also generally dampen down pain pathways. So to some extent, you may have a feeling of numbness somewhere else.










Comments
Add a comment