This week, we find out how to get useful gas from useless coal, and make money from waste carbon dioxide! Underground coal gasification could allow us to access huge amounts of energy in inaccessible coal seams. We find out how it works as well as exploring a new method for capturing waste carbon and turning it into useful chemicals. In the news, dinosaurs inspire new designs for aircraft, spotting a star being ripped apart by a black hole, and the South African bid for the world's biggest radio telescope. Plus, Diana asks what the point is of "junk" DNA?
In this episode

01:43 - Spotting A Black Hole Swallowing a Star
Spotting A Black Hole Swallowing a Star
A unique astronomical event - an incredibly bright and long lived burst of gamma rays - was probably the result of a black hole destroying a star, according to research published in the journal Science this week.
On the 28th of March this year, NASA's swift satellite observed a bright flash of gamma rays emanating from a distant galaxy, around 3.8 billion light years away. Gamma Ray Bursts like this are associated with some of the most energetic and destructive events in our universe, such as the supernova and collapse of a massive star into a black hole or neutron star.
 This flash, now known by the catchy name of SW 1644+57, was unusual. Normal gamma rays bursts are intense, but short lived, and followed by an "after-glow" of lower intensity emission. The event in March continued for weeks, outlasting any previous burst, and rather than fading away quietly, it was followed by a number of less intense bursts of radiation - almost like an earthquake and its aftershocks.
This flash, now known by the catchy name of SW 1644+57, was unusual. Normal gamma rays bursts are intense, but short lived, and followed by an "after-glow" of lower intensity emission. The event in March continued for weeks, outlasting any previous burst, and rather than fading away quietly, it was followed by a number of less intense bursts of radiation - almost like an earthquake and its aftershocks.
To determine exactly what caused this unique event, it was vital to determine whereabouts in the galaxy it had occurred. Andrew Levan and colleagues at Warwick University used subsequent observations taken by the Hubble Space Telescope and Chandra x-ray observatory to confirm that the event had taken place at the heart of the galaxy.
This left only one logical conclusion as to the cause of the event - they had witnessed a star about the size of our Sun being torn apart by a massive black hole. The enormous gravitational pull of a black hole at least a million times the mass of the Sun would cause the star to initially distort into a banana shape, before being ripped into a ring of material, raining down onto the black hole.
Spinning black holes tend to radiate this energy in two highly columnated beams, one from each pole, and this time, the Earth happened to fall right in the firing line. Dr Levan now hopes to look at historical data to find evidence of off-axis events, where the column of light is not aimed at the Earth, to find out how rare these extraordinary events really are.

04:41 - Comet Hartley 2
Comet Hartley 2
Comets are small astronomical bodies that mostly travel from the far reaches of the solar system inwards. As they move closer to the sun they heat up, evaporating volatiles like carbon-dioxide and water to produce their characteristic tail.
They are quite difficult to study as although their tails can be enormous, the actual comet is relatively small. They are only a few kilometers across and surrounded by a cloud of tail forming particles when they are close to us. There have been a couple of missions to comets. The most recent was the NASA deep impact mission which was a bonus. After visiting the comet Tempel 1 in 2005 they visited the relatively small and unpredictable comet Hartley 2 in 2010. This comet is slightly unusual and spins peculiarly, occasionally showing hiccups in its tail. A team from the University of Maryland have just finished analysing the data from this flyby.
They have found that the comet looks like a giant 1km long dumbbell with jets of material flying out of the ends. At the moment slightly more material is flying out from the small end than the large one. The amount of water compared to carbon dioxide also appears to be lower at the small end than the large one. This might indicate that a comet has a varied structure in its original makeup.They also saw a very pristine white ring around the middle of the comet which indicates that some of the material flying out might be re-depositing around the middle.
So comets might be a more active and varied type of object than was previously thought. Hartley 2 will certainly continue to be studied from ground based telescopes to see if more can be learnt about its strange behaviour.
14:05 - Porous Molecules By Design
Porous Molecules By Design
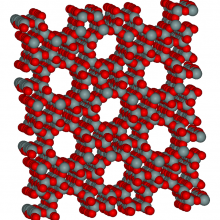
Porous molecules, useful in a huge range of applications including separating chemicals and catalysing reactions, may be designed on demand thanks to a new method published in the journal Nature this week.
Molecules with well defined porosity and well understood activity, such as zeolites and metal-organic frameworks (MOFs), play important roles in many chemical processes. Touted as a safe solution to hydrogen storage due to their ability to adsorb enormous amounts of gas on their surfaces, they're also useful in water purification and as molecular traps. But their production often distributes functionality fairly randomly between the pores, placing an upper limit on their use.
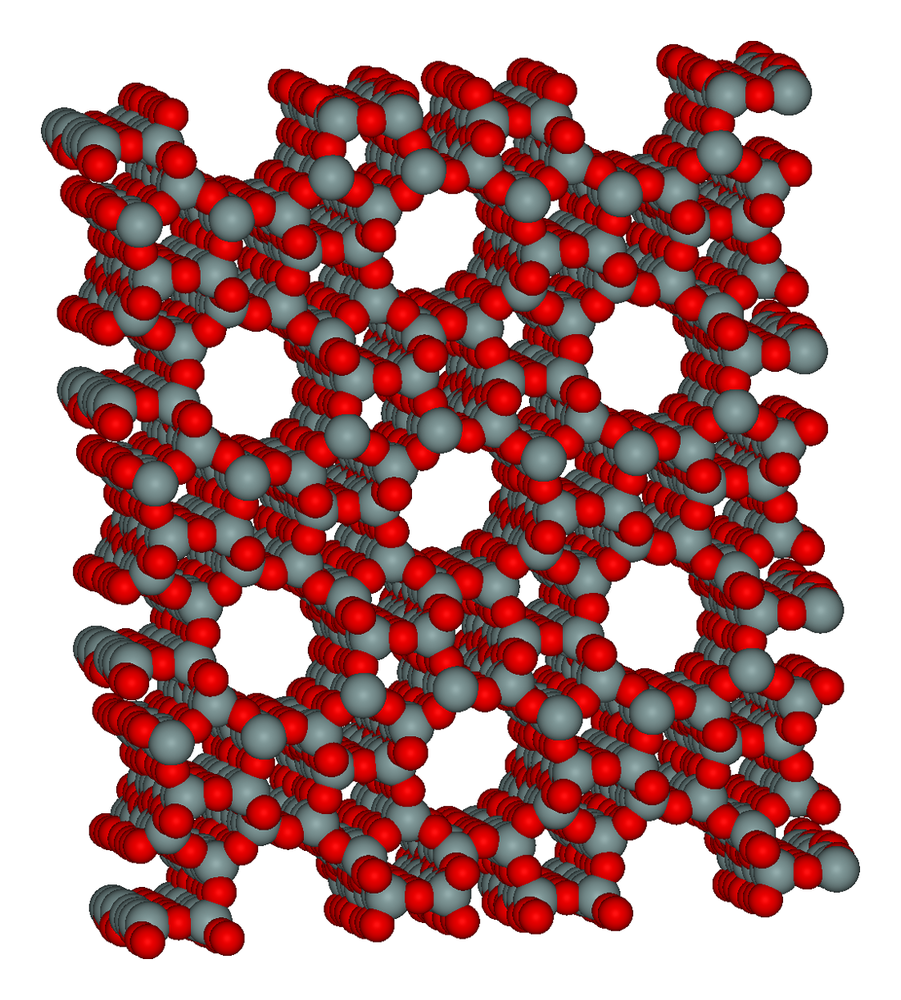 Professor Andrew Cooper, at the University of Liverpool, working with groups at University College London and Cambridge University, have developed a method for modelling nanoporous molecular frameworks with atomic precision, then producing them from modular components.
Professor Andrew Cooper, at the University of Liverpool, working with groups at University College London and Cambridge University, have developed a method for modelling nanoporous molecular frameworks with atomic precision, then producing them from modular components.
Cooper and colleagues use a "lock and key" assembly technique, mixing porous organic cage molecules and allowing them to self assemble. They can accurately model and predict how these molecular modules will mix, meaning specific functionality can be computer designed.
One of the advantages of this technique is that the molecules are soluble. This means that once the constituent pore modules are synthesised, researchers need only mix the modules in solution, allow them to self assemble, and then remove the solvent.
The researchers have put their method to the test, and produced four bespoke molecular cages, and confirmed their structure experimentally. This, they say:
"Opens the way for in silico prediction of structure and properties for new candidate porous materials based solely on two-dimensional chemical sketches, thus allowing 'design by computational selection'."

16:52 - Pterosaurs inspired aircraft
Pterosaurs inspired aircraft
Although for most airliners being able to change direction very quickly isn't hugely important, as unmanned aerial vehicles are getting used more and more, especially in complex urban environments, being able to turn sharply can make the difference between carrying on its mission or ending up splattered against a wall.
A very productive seam of ideas for engineers has been bio-mimetics, copying ideas from living things. This has given us ideas ranging from velcro to catseyes in the road, but Brian Roberts and Rick Lind from the University of Florida and colleagues have been taking this strategy one step further. They have been taking ideas from dead creatures, very dead creatures that haven't lived for 65 million years, pterosaurs to be precise.
These were a form of flying reptile which survived for over 150 million years and included the largest ever flying animal with a wing span of 40 feet. One striking feature of many of these pterosaurs is that they have large head crests. It has been suggested that these were just for display but they may have had an aerodynamic purpose, acting like a rudder.
So the group wondered if putting your rudder at the front of a plane might be useful. They have done lots of modeling and found that for the same size rudder at the front of the plane, it should give the plane a 15% smaller turning circle. The problem is that you also loose stability so they are looking into somehow morphing or moving the rudder around to get the best of both worlds.
So in the future we might see pterosaur inspired planes flying around our cities. Who knows what else ancient animals and plants might inspire.

20:13 - Planet Earth Online - Carbon Dating
Planet Earth Online - Carbon Dating
with Tom Higham, Oxford Radiocarbon Accelerator Unit
Dave - In the late 1940s a Chemist called Willard Libby, who originally worked on uranium during the Manhattan project, developed a method of dating material with a biological origin. As it's radioactive, the levels of carbon-14 in the material decreases as it ages but the amount of the stable carbon-12 doesn't change. Therefore by looking at the ratio of carbon-12 and radioactive carbon-14 we can date the material. Radiocarbon dating is an extremely accurate and useful tool to date an archaeological find that contains any previously living material. Planet Earth podcast presenter Sue Nelson met Tom Higham from the Oxford Radiocarbon Accelerator Unit to find out how it works.
Tom - This machine's job is basically to take tiny amounts of graphite, which contain carbon and therefore the carbon 14, derived from the archaeological bone or charcoal or whatever it happens to be. It then separates just the carbon-14 from all the other interfering particles, rather like a large sieve. The way it does that is by particle acceleration, by beam bending the particles so that they can be separated by magnetic forces. The problem that we have though is that only a tiny amount of that carbon is carbon-14. One particle in a billion, billion is carbon-14. That's why we need such a large machine, to be able to separate out all the interfering particles that aren't carbon 14.
Sue - So if you had for example a bone that you wanted to analyse, where would it actually go?
 Tom - The first stage is get the bone and to take about half a teaspoon of powder from the bone to extract the collagen. Stage two is then to combust the collagen, purify the gas and convert the carbon dioxide into graphite. Then the graphite is loaded into one of those small aluminium holders there.
Tom - The first stage is get the bone and to take about half a teaspoon of powder from the bone to extract the collagen. Stage two is then to combust the collagen, purify the gas and convert the carbon dioxide into graphite. Then the graphite is loaded into one of those small aluminium holders there.
Sue - This almost looks like a gun canister with bullets.
Tom - Yes and then you see this little hole on the top? It's about a millimetre in circumference. That contains the graphite from the bone and it's pressed very firmly onto the top of this target. It's then put into this large wheel of samples here which number about 50 or 60. Once the accelerator is loaded with this wheel, a caesium beam is projected onto the surface of the graphite. That process, called sputtering, gives a charge to the particles. That is really, really important because if we don't give the particles a charge, it means we can't bend them, we can't move their trajectory and separate the particles from other particles of a different mass.
Sue - Let's go into the quieter area now...
Tom - This is the control room where the measurements are monitored and the machine is started and stopped and also where the dates are calculated. In the old days it used to take a day or so to calculate a batch of radiocarbon dates. Now of course we have computers that do it in the space of a blink of an eye.
Sue - How do you actually tell the age of something from the amount of radioactive carbon in it?
Tom - The dating process is built around the half-life of radiocarbon. The half-life simply means the amount of time it takes for half of the radioactive carbon that is present per gram of material in a living organism to decay after death and disappear. We know that the half-life of radiocarbon is about five and a half thousand years. That means that every 5,500 years the amount of radiocarbon declines by half. So after ten half-lives you're back to around 55,000 years. Then there should be no more carbon-14 left, so that marks the limit of the dating technique. The great thing about radiocarbon dating is that carbon is ubiquitous in the biosphere. There are many different types of materials that we can date - whether they be bones, charcoal, wood to things like molluscs and fish, fish bones, pollen. Since the 1950s there's been a doubling of the amount of radiocarbon in the world because of the nuclear testing that took place in the 1940s, 50s and 60s. So, if we measure something that's modern, modern being since 1950, we can get a very high level of radioactive carbon that we know therefore must equate with a date in the modern period.
Sue - Modern particle accelerators and pre-treating samples to remove contaminants have improved the accuracy of radiocarbon dating. In some cases, artefacts need to be re-tested. Tom's unit helped produce recent research which suggested that Neanderthals probably died out ten thousand years earlier than previously thought.
Tom - Unfortunately in many cases, when we do this work, we find that the previous dates are underestimating the real age. There's a huge amount of work to be done still in applying these new techniques to find the real chronological picture.
Sue - So you could actually put a lot of noses out of joint?
Tom - Yeah, that's right. I think it's fair to say there are lot of people with a lot of vested interests in this. They've written a lot over the last few decades and they've been involved in bitter arguments that, in some cases, are over data that we now know are probably not reliable. So yes, there is a tendency to do that.
Dave - That was Tom Higham from the Oxford Radiocarbon Accelerator Unit talking to Sue Nelson.

25:40 - Underground Coal Gasification
Underground Coal Gasification
with Dermot Roddy, University of Newcastle
Dave - Underground coal gasification is a way to get useful fuel from otherwise inaccessible coal seams that are simply un-economical to mine. To explain more we're joined by Professor Dermot Roddy from the University of Newcastle. Now Dermot, what actually is underground coal gasification?
Dermot - The easiest way of thinking about it is to remember that for the last 30 years we've been used to bringing natural gas into our homes from the North Sea. Before that the gas came from the local gas works. There you would gasify coal to turn it into a mixture of hydrogen and carbon monoxide and that's what we brought into your homes. With underground coal gasification the difference is that you don't have to mine the coal before you gasify it. So you're injecting oxygen and steam into the coal while it's still in the coal seam.
Dave - So, if you're injecting oxygen and steam, there's some chemical reaction going on underground to turn it into a gas?
Dermot - That's right. It's the same as what would have happened in a gas works. The idea is that you drill down into the ground maybe 800 metres or more and then you can deviate the drill, turn it through an angle, and follow that coal seam as far as you want before drilling a larger hole down to meet it. When you inject oxygen and steam into that maybe 8cm diameter hole, you start to gasify the coal. You bring that gas, that synthesis gas, up to the surface.
Dave - So what do you use this synthesis or syn-gas for?
Dermot - Some people would use it for generating electricity because you can use it much the same way as you would use natural gas in a combine cycled gas turbine. Alternatively you can use it, as it's been done for many years in South Africa, for synthesising fuels like your petrol and diesel and so on. Or if you want, and we tend to do this up here in the Northeast, you can use synthesis gas to make chemicals, polymers, and so on.
Dave - Okay, so it's obviously a useful thing. I guess you can get to coal seams you couldn't do otherwise because they're too small or in too awkward places?
Dermot - That's right. We're particularly interested in deep seams because you want to go down well below the top of the water table, down to the point where you find permanently unusable water, so you're deliberately going very deep underground. In the UK for example it was very unusual to go down below say 250 metres. Here we're saying we want to go down at least 800 metres and you can certainly get into very thin coal seams. What that means a practical terms is that whereas in the past a respectable coal mine made upwards of just 1 or 2 million tons of coal per year, the UK has got about 50,000 million tons of coal that nobody has any intention of ever mining. So that's the size of the venture. It's not just the odd little coal seam here or there that you might want to do this with. You're talking about a thousand years worth of electricity generation, or something like that, from coal that today people wouldn't think of mining.
Dave - So if you're pumping steam down these pipes, I guess this reaction has got to be quite hot to happen. Does this mean there's an energy penalty involved in converting the coal from a solid into the gas?
 Dermot - One way of looking at it is if you didn't do this, all of the energy would remain underground and you will never get to use it. You want to bring as much of the energy that's in the coal up to the surface. If you just burn coal you release all of the energy. The whole point in gas works or in underground coal gasification is that you partially combust the material so that actually, most of the energy is still in the gas when you bring it to the surface. That's the whole point because then you're saying, "This gas is a source of energy. I haven't actually used up very much of the energy in this partial combustion reaction that turns coal into hydrogen and carbon monoxide".
Dermot - One way of looking at it is if you didn't do this, all of the energy would remain underground and you will never get to use it. You want to bring as much of the energy that's in the coal up to the surface. If you just burn coal you release all of the energy. The whole point in gas works or in underground coal gasification is that you partially combust the material so that actually, most of the energy is still in the gas when you bring it to the surface. That's the whole point because then you're saying, "This gas is a source of energy. I haven't actually used up very much of the energy in this partial combustion reaction that turns coal into hydrogen and carbon monoxide".
Dave - I guess just mining conventional coal is actually quite an energy intensive process. How does this compare with the conventional mine?
Dermot - There's very little experience of actually mining at these kinds of depths. I think the other thing you find with mining at these kinds of depths is that it's incredibly dangerous. You look at the death rates that people are accepting for deep seam mining, in places like Russia and China, and you really wouldn't be able to do that in Europe. I think from an energy point of view, you expect that about 80% or 85% of the energy that's in the coal now will end up coming to the surface. The rest you lose.
Dave - Okay, so this is actually quite a useful technology.
Dermot - Absolutely, yes.
Dave - Are there any other environmental impacts? Other than just the fact I guess that you're burning coal, which is not ideal in terms of carbon dioxide.
Dermot - From my point of view, I would say, if you want to do this in the UK or in Europe you absolutely must have a carbon management strategy or nobody is going to allow you to do it - nor should they actually in my opinion! When people talk about carbon capture and storage, that's a technology that should be used in all coal fired power generation. My view would be that the exact same applies with underground coal gasification. So it should only really be used in conjunction with some kind of a carbon management plan. You have to capture the carbon dioxide. With integrated gasification combined cycle technology you capture the carbon dioxide first, before you put the hydrogen rich gas through your gas turbines. Then once you've captured it you do something with it. You can put it in the pipeline and people write extensively about that. We're working on technology that would put the carbon dioxide back into the void you've just created in the coal seam. There are other people who say they've got clever ways of using carbon dioxide as a chemical feedstock, but in the UK context or European context underground coal gasification is a complete non-starter unless you've actually got a carbon management plan.
Dave - Brilliant! Thank you very much, Dermot Roddy from the Sir Joseph Swan Institute for Energy at Newcastle University.

32:12 - SkyMine - Capturing Carbon for Profit
SkyMine - Capturing Carbon for Profit
with Joe Jones, Skyonic, Texas
Ben - We are joined by Joe Jones who is from Skyonic in Texas. They've developed what they call a 'SkyMine', a system that not only extracts CO2 but actually turns it into something that they can sell. Thank you ever so much for joining us Joe.
Joe - It's a pleasure.
Ben - How does the SkyMine actually work?
 Joe - Skyonic Corporation has been working for 6 years on a SkyMine process to capture carbon dioxide from fluid gas and convert it into solid carbonate and bicarbonate minerals. We do that by exploiting an ionic chemical property of carbon dioxide. We add some acid gas that forms weak carbonic acid when it's dissolved in water. We combine that weak acid with a strong base to form a neutral salt, just like you may have done in your high school chemistry laboratories. We can make sodium carbonate, which is the washing soda that's used in detergents, sodium bicarbonate, the edible salt that can soothe your stomach, or we can make calcium or magnesium carbonates that form limestone or dolomites. Also, we can precipitate calcium carbonate which makes up 40% of white paper among other things.
Joe - Skyonic Corporation has been working for 6 years on a SkyMine process to capture carbon dioxide from fluid gas and convert it into solid carbonate and bicarbonate minerals. We do that by exploiting an ionic chemical property of carbon dioxide. We add some acid gas that forms weak carbonic acid when it's dissolved in water. We combine that weak acid with a strong base to form a neutral salt, just like you may have done in your high school chemistry laboratories. We can make sodium carbonate, which is the washing soda that's used in detergents, sodium bicarbonate, the edible salt that can soothe your stomach, or we can make calcium or magnesium carbonates that form limestone or dolomites. Also, we can precipitate calcium carbonate which makes up 40% of white paper among other things.
Ben - So with the limestone versions and the precipitates and so on, that's a very good way to capture carbon that you can then bury in the ground again. A good way for storage. But with the other products, there's a market for those. You can actually sell the result of recycling this waste.
Joe - Indeed. In the sodium carbonate and bicarbonate's market, there's a little over $10 billion of chemical product that is currently either mined from archaeo-carbon, which is releasing ancient sequestered CO2, or is made by synthesis techniques that use a great deal more energy. Thereby they generate more carbon footprint as well.
Ben - Where does the base that you react it with come from? Does that have to be mined? Is it an expensive process to produce it in the first place?
Joe - Well sadly there are no base mines that are available so you have synthesise it from salt and water itself. If you take saltwater and energy, either in the form of electricity for electrolysis or using heat energy, you can force the neutral salt to split itself into hydrochloric acid and a very usable base.
Ben - So there's obviously an energy cost in order to do the electrolysis in the first place, but I think there is an energy cost for all of the different carbon capture mechanisms we have. At the absolute least, you need to increase the output of your power station by 20%, and I think some of them, as far as 90% - You're almost doubling the amount of energy you need to produce in the first place, just to capture back the carbon! How efficient is the SkyMine? How much extra energy do you need?
Joe - The SkyMine does have a positive engineering leverage so that it does sequester more carbon dioxide than its operation generates. This is an important thing for a carbon capture system to do. Currently carbon capture and sequestration, as you mentioned, quotes in the 20% for the CC portion of carbon capture and sequestration. However for the sequestration part they can get it to be over 45%. Our first plant that is being built in San Antonio Texas is designed to achieve a 40% energy penalty, just under the competing CCS-amine technology. With fundamental improvements, more research that we have underway, we have demonstrated in the laboratory that we are able to drive that below 20%.
Ben - With the amine technology you're actually left with some quite nasty toxic chemicals and you've got to obviously purify the amines in the first place. I guess one of the real advantages to this is that the products you're left with, not only is there a market for them, but also they're quite benign.
Joe - Benign indeed. Some 4 billion years ago a tremendous amount of limestone and other minerals were created from what was at that time, a predominantly carbon dioxide atmosphere. As you likewise mentioned, that mineral sequestration is going on around us everywhere. Here in Travis County Texas, there are some 450 pentilion tons of limestone that were once part of the atmosphere of the earth.
Ben - Pentilion - that's unimaginable!
Joe - It's a word you don't get to use in normal conversation.
Ben - That brings me on to - how scalable is this? Is this something where you could put it on any coal fired power plant in the world at the moment? Or are we still looking at a fairly small scale?
Joe - The plant that we're building in San Antonio, the Capitol SkyMine plant, will be manufacturing 75,000 metric tons of CO2. Scale is important because in the United States, we emit some 2.6 billion metric tons a year of carbon dioxide. In theory though, the plants can be scaled effectively infinitely. There's far more salt, water and room for minerals than there is carbon in the earth to burn.
Ben - One of the complaints about green technologies, about technologies that capture carbon, is that it's not economically viable. Obviously, you've found an excellent way around that. But in the future let's say we have a SkyMine on every power plant and we're producing large quantities of these products, what happens if the market becomes completely flooded?
Joe - Well, given the scale of the carbon emissions that we have, that would indeed occur. At that point you would then be able to operate the plant simply for the purpose of sequestering CO2 and lay aside the minerals. Or in the case of the example from your earlier interview, we could put the minerals back down inside a stripped coal seam for the purpose of sequestering it there. But at that point you then cross into cost. Right now, the US Department of Energy estimates that amine sequestration costs between $100 and $300 per ton to capture that way. We estimate that we can arrive at a $25 to $50 range for that incremental cost past beyond markets.
Ben - That's the remarkable difference and this sounds like fantastic technology. What sort of time scale are we looking at before we'll see this turning up in different power plants?
Joe - Our first large scale commercial plant, we've been running pilot plants here in Texas for the last 6 years, will go online in 2013. Thereafter it can be replicated worldwide. About a 2-year construction time, 25-year plant life and we can be making a dent in carbon certainly inside the next 10 years.
Ben - Well that's very good news. I'm glad that we have these technologies to be optimistic about. Thank you ever so much for joining us. That's Joe Jones. He's President and CEO of Skyonic over in Texas.

39:49 - Naked Engineering - Steam Engines
Naked Engineering - Steam Engines
with David Gates, Cambridge Museum of Technology
Dave - Earlier this week, I met Meera at Cambridge's Museum of Technology to explore the engineering of an iconic bit of coal fired power, the steam engine...
Meera - This week Dave and I are aboard a Foden steam wagon, chugging around the Museum of Technology here in Cambridge. It is a scaled down model of a wagon and this site is the old sewage pumping station in Cambridge. Now Dave, why have you brought me here?
Dave - Well steam engines historically are one of the most important pieces of technology. They took us from an economy which was entirely based on animals and stuff you'd grow off the ground to basically a modern world. Even today they produce over 70% of the energy which we use in this country.
Meera - Joining us this week aboard the wagon, or in fact driving it, is the main engine driver here at the Museum of Technology, David Gates. Now David, how exactly are we moving along? How does a steam engine work?
David - Well you take your fuel source. In this case it's coal which is burnt and heats the water to create steam. The steam then operates the pistons to create the motion to move the engine.
Dave - Because water is going from a liquid to a gas it expands hugely at about 100 degrees Celsius. It's roughly 1500 times greater at high temperatures, or even more, and that huge expansion can be used to drive a mechanism. So from a physicist's point of view you're just causing water to boil, getting a large amount of expansion, then you somehow connect that to the wheels. The actual engineering practicalities of that are a lot more complicated though.
Meera - Yeah. I imagine there are many steps involved, David.
David - There are indeed, yes. In fact, we have a model here which shows those various steps.
Meera - So we've come inside to the model now and in fact, this represents a Hedley steam engine which is located just behind us. It's a cross section so we can see what's really going on inside. There's a cylinder towards the back of the steam engine into which the steam enters. There's also a tube coming out and a valve on top. So what's really happening here to control the movement of steam into this?
David - Well your valve on top allows the steam to go into one end of the cylinder which will then push the piston inside until it gets to the end. Then the valve on top would change direction, this will allow steam to go into the other end of the cylinder and push the piston in the opposite direction.
Dave - So there's more pressure on the side of the piston where the steam is coming in than on the other side. That means there's an overall force in the piston which pushes it along. Then the piston is attached by a series of linkages to the wheel which is actually doing the work.
Meera - Okay. Well we can actually turn this model on then to see the piston movement in action.
Dave - The piston is then attached to a long rod which is then connected to a crank, allowing it to turn the wheel. This wheel must be somehow attached back to the valve so that it can be opening and closing at the right time.
David - Yes. There is basically a cam, off-centre, which changes the direction of the valve.
Meera - It's all a very smooth motion and the wheel is just continuously turning here.
David - That's correct, yes. You have to have rather a large fly wheel in a simple engine like this one just to create the motion required to keep up the momentum.
Dave - I guess the other problem you've got is that you can get steam into the cylinder, but where does it go once it's in there?
David - With this particular engine, when the steam comes out of the cylinder it just passes to atmosphere and that is a complete waste. Later engines used the steam more than once.
Meera - So we've now entered a very large room with two extremely impressive engines here. These are the main sewage pumping engines on the site and firstly, from the cylinder down to where the fly wheel is, it's about 11 metres in length. This engine, and the fly wheel itself, is about 7-foot high. It's very grand, David.
David - These engines were built in 1894 by Hathorn Davey of Leeds. They have two cylinders on these.
Meera - This is where the whole fact that steam is being used twice really comes in.
David - Yes. So when steam leaves the small high pressure cylinder there's a lot of useful energy in it, so it's used a second time in the low pressure cylinder.
Dave - And so, because the steam has already expanded maybe by a factor of two or three in the small cylinder, it's going to be a lot bigger. It's going to take a lot more space and you need a bigger cylinder to let it expand again, a second time, and get some more useful work out of it.
Meera - What's the benefit of letting it expand again?
Dave - Well essentially, you've still got steam under quite at a pressure coming out of the first cylinder. You can't let it expand all the way to atmospheric pressure, the cylinder would be immense and it would be very expensive to build the engine. So you can use a second cylinder to let it expand again and get more of the energy out of it. This means you get more work for the same amount of fuel.
Meera - So these two processes of expansion are all still basically working though to push the piston, David.
David - That's right. Yes, you have a piston in each cylinder but they're connected to the same rod which operates the pumps.
Meera - And another way to get even more energy out of the steam is to use a condenser, which this engine also uses.
David - That's right. When the steam leaves the low pressure cylinder there's still a lot of useful energy there, so it's passed into a condenser where the steam is then turned into water to be used for feeding the boiler. It also creates a vacuum, so it's actually sucking the piston along as well as it being pushed. This makes it more efficient.
Dave - So again, you're getting more pressure difference across the piston so you're getting more work out. Again you get more energy for free essentially.
Meera - And what about the amount of say, energy being consumed? The amount of coal being burned and so on?
David - Well with the way we operate these engines at present, we will burn round about 3 quarters of a ton of coke per day.
Dave - So that's equivalent to hundreds of litres of petrol you're burning, just to run an 80-horsepower engine for maybe 3 hours in the day. So, the efficiencies are nothing like even a modern car.
David - Steam engines, really one of the reasons they went out of use, was low efficiency.
Meera - They really did change industry a great deal though. We've seen it in motion for example in a wagon, it's used here for pumping in the sewage plant. As they developed, they had more and more applications.
David - Indeed, yes. It really was the steam engine that turned the world around. With the spinning mills as well you could create virtually anything you wanted.
Dave - And in fact, even today, most of the electricity you use at home is generated using steam engines. Not reciprocating engines like this but a steam turbine. They basically work by blowing steam pass a load of vanes. It's essentially very specifically designed windmills. They spin incredibly fast and you get a huge power density from your steam engine, allowing coal power stations, nuclear power stations, oil power stations all to produce lots of steam and generate electricity from it. You use this electricity every time you turn on a light switch.
David - So essentially, although people think steam is old technology, it still powers the modern world that we live in.
Dave - That was David Gates, the main engine driver at Cambridge Museum of Technology.
Why are synfuels cleaner than traditional fuels?
Dermot - They're cleaner because your starting point is essentially pure hydrogen and carbon monoxide, so you've got no sulphur in there. As long as you're blowing your gasification with oxygen rather than air you've got no nitrogen in there to give you oxides of nitrogen, so you really ought to be able to synthesise a designer fuel, which is effectively what you do. It can be a diesel type fuel, a petrol type fuel, an equivalent of jet kerosene for aircrafts... Ultimately the car companies would like to make an engine that's a combination of a spark ignition engine and a compression ignition engine. They'll specify exactly what properties they want the fuel to have and we can make that.
Ben - So by spark ignition you mean - petrol engines are spark ignition, but the compression...
Dermot - Yes, diesel engines are compression engines and they would like to get away from that situation. We have to make two types of engine for two types of vehicle and just make one fuel that has the advantages of both. They'll specify exactly how they want that fuel to perform and we synthesise that.
Can we make recycled oil from carbon dioxide?
Joe - Well, taking CO2 and reconverting it into fuel, CO2 itself is completely spent fuel so one has to add energy to go back up the thermodynamic curve from carbon dioxide to usable fuel. The most efficient chemistry for doing so is photosynthesis. It is an intricate process that adds energy from sunlight. Nothing else that man has done on a pathway has approached the efficiency of photosynthesis. At Skyonic, we've been working with the University of Texas on bio-algal fuels made from bio-algae. We've been feeding it with bicarbonate of soda manufactured from our SkyMine process and they've reported very good results. 90% incorporation of the CO2 and a tripling of the growth rate because it actually creates an algae bloom. It also throws some of the biologic triggers inside the algae that make it produce oil preponderantly compared to plant matter.
Ben - So we can't mimic nature yet, but we can of course get nature to do that work for us.
Joe - We can hitch a ride on nature at this point and it's probably the most promising means for making fuel directly from CO2.
How can coal be converted to petrol?
Dermot - Well the South Africans have been doing this for a very long time because of the years of sanctions. The well-known technology is called Fisher-Tropsch technology and that can be used to synthesise hydrocarbon chains. Traditionally, the process was used for synthesising diesel but you can synthesise a naptha-type material if you want to or a kerosene type material. It's just a question of optimising the reaction conditions and choosing the right catalyst. People are starting to develop a range of fuels now from synthesis gas.
What are the harmful impacts of underground coal gassification?
Dermot - It's very important to do it at depths where all of the water has been formally classified as permanently unusable. These are very, very salty water deposits at depths of a kilometre or thereabouts. Nobody would ever extract those kind of waters, the very fact that they are heavily salty means they're poisonous anyway. You have to be operating at the kind of depths where nobody would ever bring that water to the surface as a water supply. Then no matter what happens, you're not going to cause contamination that causes any problems.

Catalytic converters for coal power stations?
Joe - Well, catalysts generally don't change chemistry. They only drive it at accelerated speeds to the same end. When a power plant operates perfectly it does achieve complete combustion. So, a catalytic converter may be good for making carbon monoxide into truly carbon dioxide. It's still carbon dioxide at the end of it so you can't clean up with that fashion. You can only change it from a gaseous form into a mineralised form or into some other form. You have to change state.
52:17 - What is the purpose of non-coding DNA?
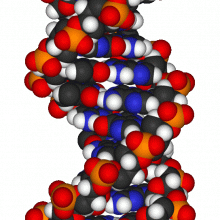
What is the purpose of non-coding DNA?
We put this to Julian Huppert, Member of Parliament for Cambridge and lapsed academic, formerly working on genomics.... Julian - Well, there are various ways of thinking about it and we're still trying to understand some of it. In some ways, the coding sections are the recipes for the proteins that we have. The genes and the non-coding DNA are very important in saying how much of this should be there, when is it turned on, when is it turned off. All of those controls are hidden in the non-coding sections of DNA. There are some other bits which are old things which we don't really use such as genes which were useful a few million years ago, we still have bits lying around. Also there are some bits which really are junk. They are viruses that have crept into our genome over time.
What I used to work on was something called the G-quadruplex. DNA of the right sequence can form four stranded knots. These can act as off-switches which stop the gene from being active. It started off as an interesting theoretical curiosity. We actually found that almost half of all human genes seem to have these switches in a way that could be playing a role in turning them on and off. In particular, most cancer genes had these little structures which form little knots at the beginning of the gene, marking it as off.
Diana - So-called junk DNA can be acting in other ways as on/off switches, packaging for coding DNA or instructions on how that DNA is unpacked. Imat faal said on the forum that DNA can have another use altogether, finger printing for identification.
- Previous Dinosaur Inspired Aircraft
- Next Steam Engines










Comments
Add a comment