eLife Episode 15: Flu, Cannabis and HIV
In this episode of the eLife podcast we hear about influenza pandemics, eating too much, cannabis and the brain, HIV cure research, and the evolution of sea squirts...
In this episode
00:39 - Preparing for pandemics
Preparing for pandemics
with Colin Russell, Cambridge University
Researchers are exploring new ways to assess the risks posed to humans by non-human influenza viruses. Colin Russell from the University of Cambridge spoke to Chris Smith about how to predict pandemics...
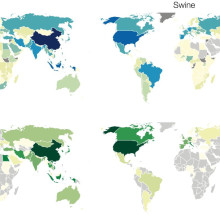
Colin - In the last 100 years, we've had five influenza pandemics in humans. Four of those pandemics started with viruses that came directly from animals and one of them happened probably as a product of an unintentional laboratory release. There's a tremendous diversity of influenza viruses that are circulating in animals right now. In chickens, in pigs, horses, basically every animal in which we've ever looked hard enough to find an influenza virus we find influenza viruses. We're almost certainly going to have another influenza pandemic within the next 10, 20, 30 years. But the question is which virus is going to cause that? It's important that we figure out which virus is going to cause it because our only good preparations right now to combat the next pandemic are the development of vaccines and the development of anti-viral drugs. And it's particularly the development of vaccines that requires very specific knowledge about the virus that's going to cause the pandemic.
Chris - So it's a bit catch 22 because until we know what's coming, it's very hard to defend against it but we won't know what's coming until it gets here.
Colin - Well that's really the difficulty and so right now, we operate under a very simple model whereby viruses that we observe to cause infections in humans are more likely to cause a pandemic than those viruses that we have not observed to infect humans. However in 2009, we got a pandemic of an H1N1 virus from pigs in Mexico.
Chris - No one saw it coming.
Colin - We didn't see it coming and we want to avoid the repeat of the same situation where we think we know what we should be paying attention to but in fact, we get blind-sided by something else entirely.
Chris - So using the power of the connected world we now live in and our better understanding of influenza and how it spreads and circulates, is there a better way to predict what might be around the corner? What are you proposing in this paper?
Colin - Well what we propose is actually sort of a road map of things that we need to pursue in order to do a better job of assessing risk. We need further experimental work in the laboratory. What are the attributes that a virus needs to acquire in order to be able to transmit efficiently from humans to humans? Once we have a better experimental handle on figuring out what these attributes are, it would be really good if we didn't have to rely on wet laboratory experiments. They're very time consuming and they're very expensive. And so, if we had a new virus that emerged today and then we to go into the lab and do a bunch of experiments on it, that would take a lot time and a lot of money when we might actually be better spending that time actually responding to containing that virus because we're better able to assess that as a threat.
Chris - What are you proposing instead then? That we do this in a computer?
Colin - Basically. So ideally we'll eventually get to a place where we can just get the genetic sequence of that virus and then from that genetic sequence be able to infer all of the risk characteristics of that virus.
Chris - How?
Colin - That's where the tricky part comes in because right now we're a long way from being able to do that. We need a combination of more experimental work and we need a combination of development of new computational methods that we can use to infer those characteristics from genetic sequences.
Chris - Is that because we're just looking at very large numbers of viruses and marrying up what those viruses did, what the outcomes were, what sorts of pathogenicity, how will they make people in other words were associated with them and then say, well if we look at the new viruses just emerged does it have or share any of those particular traits we've seen before and marry traits together to make predictions.
Colin - Exactly. It's those individual traits that you just started to refer to there where things like the pathogenicity of the virus. If we can link that pathogenicity back to specific genetic mutations, then we can make inferences about newly emerging viruses. But one of the real difficulties that we face right now is that we don't have a good handle on if mutations in one virus cause the same effect if we saw those same mutations in a different virus.
Chris - I can see how that will work very well for viruses that we know exist and that we have some experience of dealing with but in just the last few years we've discovered that bats may be carrying a new strain of flu no one had ever heard of. So what about viruses that are unknown?
Colin - And they're of course the trickiest ones. And so in addition to this experimental work and the computational tool development to make better inferences from genetic data, we also need better surveillance in animals and we need better surveillance in humans, too. We actually need a more systematic analysis of where we should actually be looking for these viruses to emerge. Most animal influenza surveillance is done in a very ad hoc way. We perceive there to be a problem and so we go and do surveillance rather than figuring out where these viruses are most likely to be transmitting and where they have the greatest potential to transmit to humans. And so to that end we could imagine doing an analysis whereby we look at the distribution of livestock populations figuring out where the greatest density of those populations are and where humans are most likely to be exposed to those populations. We also need better surveillance in humans because right now we have good surveillance here in the UK but for all of the places where we do have good surveillance there's a whole lot of places where we have effectively no surveillance at all.
Chris - No, don't tell me. I bet those are the hot spots.
Colin - Well that's the difficulty right? Because it's in those places where humans have most contact with animals that these viruses are most likely to emerge and its places where humans have most contact with animals that tend to be the poorest and have the least public health infrastructure and thus the least ability to actually detect when these events are actually happening.
Chris - Self-fulfilling prophecy!
Colin - In some ways it's difficult to imagine how it would be any other way in terms of the ability to put emphasis on the detection of emerging viruses when you're at the same time having to deal with a bunch of diseases that don't affect more developed countries...
Food for thought
with William Olds, University of Yale
When we eat a lot, our brain tells us we feel full so we stop eating. But how is this message getting to the brain? William Olds from the University of Yale looked at flies to find the answer, and his finding could help combat obesity...
message getting to the brain? William Olds from the University of Yale looked at flies to find the answer, and his finding could help combat obesity...
William - I was really interested in metabolism and obesity. And so when I started this, I was just looking at the metabolism of flies. You have all this wiring from the brain to the digestive system and it's already been established that if you cut any of these wires that connect the gut to the brain, you're gonna' eat more and you're gonna have issues with your feeding control.
Chris - And that's even in flies so you interrupt the nerve connections between a fly brain and its gut, and flies gain weight.
William - Yes. That is correct!
Chris - Gosh! Do you know why?
William - We're still trying to figure that question out. A hypothesis is that these nerve cells and this communication between the gut and the brain is integral to telling us, "Hey! Stop eating!" To test that, we first used some tricks that you can do on flies that allow you to turn on or turn off nerve cells when you want, where you want. So what we did was we inactivated these neurons and activated these neurons and we just looked at their metabolism, so sugar levels in their blood, and their food intake. We found some nerve cells that connect to the posterior part of the fly's gut so the analogous region would be the colon in humans and we found that these neurons that are tightly wound around this part of the gut have this profound effect on food intake. If you turn them off, the food intake is three to four fold higher than controls and if you turn them on, it's about one-half to one-quarter.
Chris - When you say turn them on, do you mean that you make the cells more active?
William - Yes. That is correct.
Chris - So there's some kind of loop going on here where these cells are informing the brain of something and if you suppress them, they are sort of releasing the brain and the brain just says, "Eat!"
William - Precisely!
Chris - So what do you they are actually doing in the fly? Are they there to say to the fly, "You're full! Stop eating!"
William - That's the current hypothesis I'm working with. In America, we have thanksgiving and the whole point is to eat as much as you want, right, until you get that feeling when you're full. Everybody's experienced that feeling where you just have five or six hamburgers and you feel you just can't eat another bite. You have this feeling of like stretchiness.
Chris - Five or six hamburgers, Will?
William - Yeah!
Chris - Only five or six?
William - Well you know, this is America. We have a terrible obesity problem.
Chris - But your conclusion would therefore be that what these nerve cells might be stretch receptors; they're detecting how full or distended the guts are, and perhaps they're feeding back on the brain saying "I'm stretching myself to the limit. Stop eating."
William - Precisely.
Chris - Are these nerve cells exclusive to the gut or will you find them doing jobs elsewhere in the animal? I'm thinking therapeutically if qw wanted and we are able to find these cells existing in mammals and we want to target them, for instance, as an anti-obesity treatment. It would be much easier if they were exclusively and selectively present in the intestines and nowhere else in the body.
William - Yeah, that's a great question. So these are the homologues, the evolutionary conserved versions of these are expressed in multiple regions in mammals. So we have versions of these in your kidneys, as well as your lungs, they don't all necessarily play this stretch function that you see in flies but the nice thing about them being expressed in this intestinal region is that the amount of drug they have to put in is much lower than something in the central nervous system, the brain for instance. So you have less of a chance of off-target effects. This is a very attractive drug target as a result.
Chris - To a certain extent, have we not been aware for many years that the gut does appear to be sensitive to stretch. I mean it, a - is logical and b - we have got evidence from various lines and various routes that actually if you stretch the gut or distend the gut, signals that appear to be linked to satiety come out. So what does your study add that we didn't really know already?
William - What we know right now is that the stretch is important. You can go back to Homer in the Odyssey. There are multiple passages where they say they go onto their stomachs, cannot be stretched anymore. So it's obviously clearly saying that we've known for a long time but the question is, who are the players? What is exactly going on? How are these nerve cells able to detect that the stretch is going on. We know they are but what is giving them the capability to do that? So what my study shows is that these particular stretch receptors are likely the key player in helping the neuron find out when we've eaten too much and that the gut the is stretched and you need to stop eating.
12:16 - Missed connections
Missed connections
with Zsolt Lenkei, CNRS
Cannabis-detecting receptors are in both adult and embryonic brains. But what is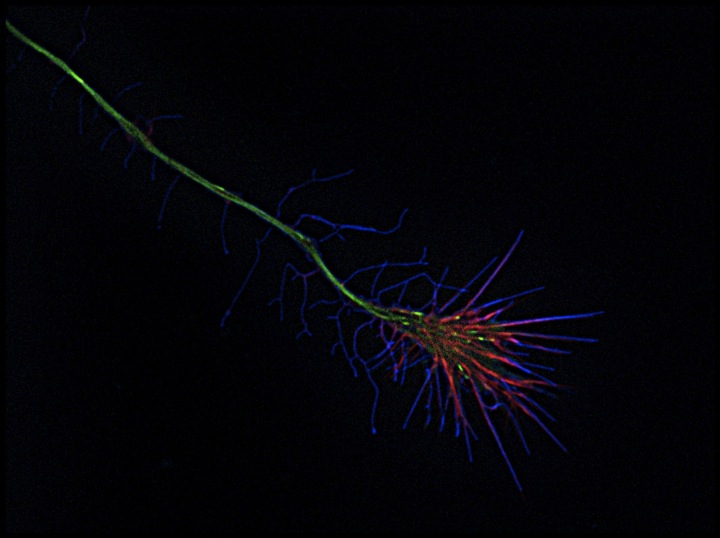 their purpose? Zsolt Lenkei has an intriguing theory, and this could tell us why cannabis users can be at an increased risk of developing schizophrenia.
their purpose? Zsolt Lenkei has an intriguing theory, and this could tell us why cannabis users can be at an increased risk of developing schizophrenia.
Zsolt - These receptors are sensitive to neurotransmitters and they are also sensitive to cannabis and we were mapping the localization of these proteins and we were very surprised that we found a lot of these receptors in very young developing neurons. And this was surprising because these receptors are mostly known as the regulator of synaptic transmission between neuronal cells.
Chris - Why were you surprised that there should be so much of the activity of these receptors in a developing brain because after all, it's producing lots and lots of nerve cells and it's producing lots and lots of connections between nerve cells as those cells develop the brain.
Zsolt - We we're surprised because the expression of this receptor, came very early. When neurons just started to be defined as neuronal cells, the did not have any axons or dendrites. The expression of these receptors was very high during the growth of these neurites during the axons that grow to contact either brain regions. And very surprisingly when these regions were reached and synapses started to form, these receptors just started to disappear from neurons.
Chris - Do you think that that might be because when you've got a developing brain and you want cells to grow a bit, if you let them connect too soon they may make lots of aberrant local connections and not grow to their target before they start connecting to it. Could it be that that's also part of the issue.
Zsolt - Absolutely. Correct connections are very highly regulated in the brain. In order to have the right number of connections, cannabinoid receptors are working as a brake. We started to think that maybe in developing neurons, they can also have a role at braking growth.
Chris - So how can one find out whether or not these endocannobinoid receptors are stopping nerve cells connecting to each other albeit prematurely?
Zsolt - So in the beginning, we were growing neurons in dishes and we treated these neurons with pharmacological ligands, molecules that would either stimulate or block the activation of this receptor and we have found that molecules that activate the receptor will lead to neurons that grow shorter axons and less dendrites. While blocking this receptor led to neurons that had longer or more developed neurites. Then we wanted to have a closer look at the mechanism of these negative regulator effect so we set up endomicroscopy. So we have young neurons from the embryonic rat brain in a dish. We transformed genetically these neurons so fluorescent molecules be label microtubules and also that cytoskeleton. We put these neurons under a microscope where we filmed 20 or 30 neurons typically one or two hours and we were very surprised because as soon as we put molecules that stimulate the activation of the receptor growth cones of the growing axons responded with a very strong contraction.
Chris - So on the surfaces of these growth cones which are the structures that the developing nerve are putting out to grow and find a target ultimately to connect to it. There are receptors for these endocannabinoids. If you stimulate those receptors this makes the growth cone collapse and retract. Do you understand what's going on in the cell to force that retraction?
Zsolt - Simply by looking at the contracting growth cone we have seen that F-actin which is an important cytoskeletal polymer, is redistributed exactly at the places where the contraction takes place.
Chris - So putting all this together, what we've got is nerve cells from very early on in development are expressing a receptor for the brain's own cannabis-like chemicals. This receptor is connected to systems that can rearrange the cells skeleton and this leads to the forcible retraction of these growth cones. Just extrapolating this we know that there's an association between cannabis use and psychosis. Do you think that there could be a connection here? Excuse the pun.
Zsolt - The brain is very sensitive to cannabis in the adolescent period. In the adolescent brain, there is a final large-scale remodelling of connections mostly in regions which are important for cognitive functions. And interestingly in brain areas which are remodelling in the late adolescent period, you find a lot of cannabis receptors. So overstimulation by heavy cannabis use may lead to large-scale remodelling. These individuals will have less connections and psychosis is known as connectivity disease so these molecular mechanisms that we described can explain why psychosis will develop in these individuals.
18:22 - HIV's hidden reservoir
HIV's hidden reservoir
with John Frater, University of Oxford
HIV affects thousands of people, and treatment is very expensive. John Frater 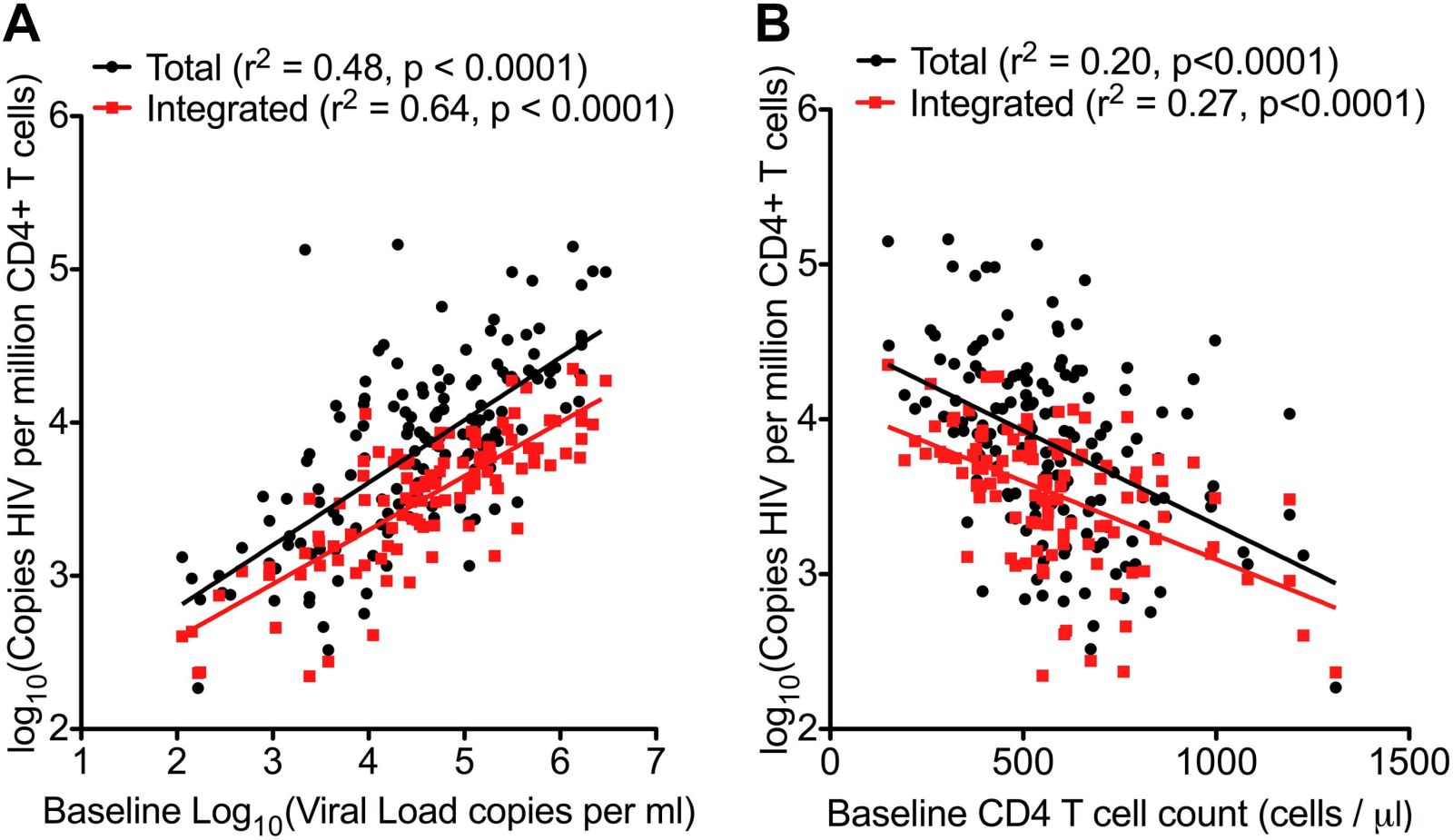 from Oxford may have found a way to spot patients who could periodically stop their treatment without any ill effect.
from Oxford may have found a way to spot patients who could periodically stop their treatment without any ill effect.
John - So we've been interested in trying to find out if it might be possible to cure HIV infection and one of the big barriers to that has always been that if a patient starts therapy but then stops therapy, the virus comes back. And it's always been assumed that the virus will come back immediately and what we found out in a cohort of patients who are quite unusual was that when the therapy was stopped the virus took a while to come back. And in some cases up to a year for a virus that actually can replicate very rapidly and normally can re-emerge in the body within days of drugs being stopped. So the questions we wanted to ask were how long does it actually take for the virus to come back. Why are these patients different and is it possible that we could predict whether some patients might be in a situation where they could stop therapy for a bit. If you'd like go into a period of remission if you wanted to think of HIV like a cancer. You could have a remission from your HIV so you could come off therapy and have a period of drug freedom if we had to go back on again.
Chris - How did you explore these patients? Do you think they were a very unusual sub-set or do you think that they're representative of the broad population of people who have HIV and we just need to know how to spot people like this in that population.
John - What is interesting about these patients is they're all diagnosed very early. The history of most HIV infection and the majority of patients that we see in clinic is that they're diagnosed often a few years after they are infected. During which time the virus has taken hold, it's spread through the body and it's sort of laid itself down in a number of different places. With these patients, they're all diagnosed and started on treatment within around six months and actually most of them were started on treatment within a few weeks of being infected. Because of that short time involved it looks as though something unusual happened and the virus didn't take hold as well as it might had done otherwise. So when therapy was started, the effect was much greater on sort of the total viral burden if you like. And this meant we think that when therapy was eventually stopped, most of the patients did rebound reasonably quickly but in a few of those patients there was this long delay. And we think it was that very early therapy which is unusual which gave them that advantage and this would then argue for screening for patients early to find out if someone has been infected and get them on therapy very quickly because that might make a difference to their sort of longer term outcomes.
Chris - But obviously to screen for something you've got to know what you're looking for. There's got to be some kind of marker. So do you have any feelings to how you could identify people who fit into that category?
John - Yes. So the first thing is to identify patients with primary infections. Once they've been diagnosed and started on therapy, the question is then - is there another measure we can use to say you might be someone who could have a period of remission. This is not yet within any sort of clinical algorithm or protocol if you like and everyone has to stay on therapy but we're thinking about the future. We're looking for sort of tests or bio-markers, that's another name for them, which can help us predict patients and this work that we've done recently: There was one bio-marker which is a measure of how much viral DNA is hiding within the blood cells and if you actually quantify that with a very sort of very precise assay that marker was a predictor of when the virus came back which was a surprise. We always knew that mark was a predictor of how your disease might progress if you became unwell but we didn't know that it would predict actually whether the virus would wake up or not and that was also the difference that we were able to discover.
Chris - In other words, the interpretation of what you find in the more of that DNA there means that more cells are infected and that person's what?, More likely to have a rapid reinstatement of their virus if you stop treatment?
John - Yes, and what we found was that the patients who had the most cells containing viral DNA were the ones who rebounded most quickly. On the other side of the coin, the patients who had only a little bit of DNA hidden within their cells behaved slightly differently. Now some of them also rebounded quite quickly but a proportion of those ones with low DNA had this very sort of protracted duration of being off therapy without the virus coming back.
Chris - Why do you think they have a low risk of the virus coming back? Do you think that just reflects the fact they don't have very much virus lurking in them in the first place and therefore if there's a chance that it's gonna reactivate if you've got less virus there already, you've got fewer rolls of the dice. So it's less likely to happen and it takes longer before it does.
John - Yeah. It may be something that simple. I mean I think one of the caveats to what we've done is that we have taken blood samples and we've only looked in the white blood cells for DNA using a reasonably simple assay. But what we didn't do, we didn't look in other places where the virus can hide - the lymph node, the gut, bone marrow, even in the brain. And so when the virus comes back it could be coming back from any of those places. Our measures are sort of a marker of what might be going on elsewhere in the body but I think you're right I think essentially it comes down to sort of the burden of disease that you have on board and if you can reduce that down to a very low level then the odds or chance of the virus coming back are statistically less.
23:54 - Regulation rules
Regulation rules
with Lionel Christiaen, New York University
If two animals look the same, you might expect that they have similar genes being regulated the same way, however this is not the case, according to Lionel Christiaen of New York University, who has been looking at different species of sea squirts...
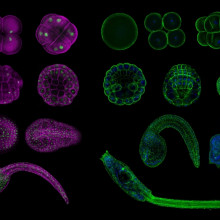
Lionel - My lab studies the development of marine organisms called sea squirts and the reason why we study these animals is because they have very few cells that they use to make their heart. So we can study these by looking at every single cell. And we study the genes which are activated in the cells and eventually tell the cells what to do. So in this particular study, we were wondering about how the mechanisms that control the development of the heart are conserved between very distantly related species. Because their embryos are almost identical to, every cell is organized and different tissues organized in very similar ways. But these animals have diverged more than 500 million years ago but they look identical as embryos. And so we were wondering what are the underlying genetic mechanisms that allow this conservation to have the same organization even though the genetic material is completely different.
Chris - So are they using the same genes and controlling them differently or are they using the same control mechanisms but different genes in order to arrive at this very similar outcome?
Lionel - Okay. So this is a very good question. We found that to make the heart, they used the same genes at about the same time in the same cells. Okay? So the gene activation patterns remain the same in spite of this great evolutionary distance. What we found is that the mechanisms that control these activities are now the ones that have changed a lot.
Chris - Does that mean that if you were to take the control mechanism from one species and put them into the other species, they just wouldn't work despite the fact that the genes are pretty much the same?
Lionel - Yes, exactly. In other words one species cannot read the genetic code of the other species and vice-versa even though their own genetic code has evolved to maintain the output the same.
Chris - Why do think this has happened?
Lionel - Our favourite hypothesis is that every gene is activated by a small group, maybe about five, of proteins called regulators and each regulator can activate many different genes. Not all the genes but many different genes and we think that the cells, they may contain up to 50 regulators. So one way to change in evolution is that if the same gene, instead of being activated by the same five regulators every time, draws five different regulators from the pool of 50 which are available such that the gene would still be activated as time goes by and species change but the exact five regulators that control it may change.
Chris - Why would that be a beneficial strategy for the organism to adopt?
Lionel - I can only speculate but I would argue that this mechanism would buffer against dramatic changes that would end up being lethal. It is not clear that this would be an advantage. What is clear is that it happens and so you may take it from the angle of it happened because it can happen because the cells have the potential to change the underlying mechanism and yet keep the outcome the same.
Chris - So what do you think are the implications of this particular finding are because now, obviously, we have a lot more insights thanks to your work into how this particular sub-group of organisms develop and how evolutionary time has affected the way they control their genes. But what are the broader implications here?
Lionel - One of them is what do model systems mean because for example here we have two species. If we look at the way they develop and gene activity, they look the same. And so we could have focused on describing the mechanisms to regulate gene activity in one species and eventually assume that those mechanisms were going to be the same in the other species because the input and the output are very much the same. But we found this rearrangement of the underlying mechanisms which now suggests that mother species made for biomedical research for example may point to a direction and some of the mechanisms are likely to be conserved in humans for example or in other modern organisms. But then the underlying relationships between the parts may have been rearranged a little bit.










Comments
Add a comment