An antibacterial protein secreted in the small intestine creates a tiny "no man's land" between the wall of the intestine and the bacteria that live inside the gut. Breakdown of this physical buffer could lead to Inflammatory Bowel Disease and other chronic problems.
The mammalian intestine is full of bacteria, around 100 trillion of them. We know that many of them are key to keeping us healthy, performing important metabolic roles that our own cells can't. But we don't fully understand how the immune system tolerates the presence of so many foreign cells.
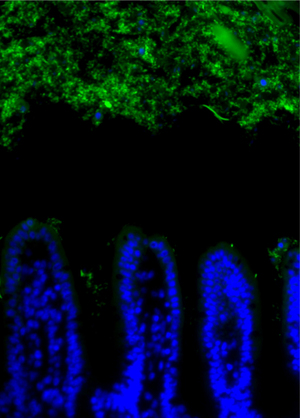 One way is to keep the bacteria physically isolated from the epithelial cells lining the digestive system, creating a "buffer zone" that stops the immune system from reacting. Different components of the digestive tract have different strategies. The colon, for example, deploys a dense mucus layer on the surface, which is too compact to allow bacteria through and creates a gap of around 50 micrometres between bacteria and the epithelium. This technique cannot work in the small intestine, however, as the dense mucus would block the nutrients that are absorbed in the small intestine.
One way is to keep the bacteria physically isolated from the epithelial cells lining the digestive system, creating a "buffer zone" that stops the immune system from reacting. Different components of the digestive tract have different strategies. The colon, for example, deploys a dense mucus layer on the surface, which is too compact to allow bacteria through and creates a gap of around 50 micrometres between bacteria and the epithelium. This technique cannot work in the small intestine, however, as the dense mucus would block the nutrients that are absorbed in the small intestine.
Writing in Science, Lora Hooper and colleagues at the University of Texas Southwestern Medical Center at Dallas took sections of mouse intestine and used a technique called FISH (fluorescence in situ hybridization) to see where the bacteria were found. In the small intestine, they saw the same 50 micrometre gap as seen in the colon. However, if the mice lacked a certain gene, involved in the innate immune response to bacteria, this gap vanished, despite overall levels of bacteria being the same.
The gene responsible controls the expression of several key antimicrobial proteins, including RegIIIγ. By knocking RegIIIγ out in otherwise healthy mice, the researchers were able to show that this protein is indeed responsible for maintaining the gap between gut and bugs. In its absence the adaptive immune system starts to respond and it's this response that could lead to inflammatory bowel disease.
There is still a lot to understand about the roles of the immune system and epithelial cells in tolerating gut bacteria, and achieving the balance required to develop, and maintain, a healthy commensal community. RegIIIγ only controls certain groups of bacteria, so the hunt is on now to find the other proteins involved making bacteria keep their distance.










Comments
Add a comment