Detecting a Dodgy Diamond
Interview with
Meera - Diamonds are forever. A popular slogan and a James Bond advert but it's true. Some diamonds were made as long as four billion years ago and it's partly this resilience along with their incredible beauty that makes them so valuable in society. With so many fake diamonds or synthetics being made today how can you be sure that the large amount of money you're parting with isn't giving you a dodgy diamond? I set out to investigate and went to the De Beers office in London to meet with diamond research engineer, Philip Martineau, from the Diamond Trading Company. I asked Philip how companies like De Beers distinguished genuine gems from fake, synthetic ones. He showed me the two systems they use: DiamondSure(TM) and DiamondView(TM). These screen and analyse diamonds by their light absorption and fluorescence properties. The first stage of analysis uses DiamondSure(TM) - a rapid screening machine that identifies 98-99% of real diamonds.
 Philip - The idea underlying DiamondSure(TM) is that diamonds have resided deep within the Earth for hundreds of millions of years at high temperatures. In that time nitrogen impurities which are found in the vast majority of natural diamonds have aggregated together into groups of atoms which are more stable. In the process the way in which the stone absorbs light is changed in a way that we can detect and a way that's very hard to mimic in an artificial process on any practical timescale.
Philip - The idea underlying DiamondSure(TM) is that diamonds have resided deep within the Earth for hundreds of millions of years at high temperatures. In that time nitrogen impurities which are found in the vast majority of natural diamonds have aggregated together into groups of atoms which are more stable. In the process the way in which the stone absorbs light is changed in a way that we can detect and a way that's very hard to mimic in an artificial process on any practical timescale.
Meera - So DiamondSure(TM) rapidly screens diamonds using the patterns produced from light absorption, particularly the absorption resulting from nitrogen impurities present in the stone. Samples that don't pass this stage are referred to machine called DiamondView(TM).
Philip - In this instrument the stones are illuminated with ultraviolet radiation and the user can then collect the image with the resultant surface fluorescence. By studying that image you can then determine of a stone is a natural diamond or an artificial product of a synthesis process.
Meera - The surface fluorescence from the UV hitting the stone can be viewed on a linked computer monitor. The sample can also be rotated for a full 3-dimensional analysis. Philip had two samples with him when we met - one real and one fake. We put them to the test to find out if these machines are as accurate as they should be.
Philip - Yes, I've got two diamonds here. I'll put the first into the centre of the sample compartment. It says, "measuring, analysing." It gives a result: refer for further tests.
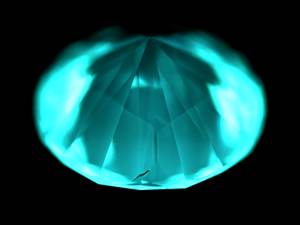 Meera - So this one's not a real one?
Meera - So this one's not a real one?
Philip - we need to look at it in more detail. We'll put that to one side. Put the second on, press the test button: pass. That's a natural diamond. It doesn't need to be looked at in more detail. I'll take this across to the second instrument, DiamondView(TM). We switch on the UV radiation. You can see immediately we have different luminescence coming from different regions of the stone. We can see immediately that this is a synthetic. Because the synthetics are grown in a very different chemical environment from natural diamonds this is a high-pressure, high-temperature synthetic. It's been grown in a metallic environment. That gives rise to different shapes as the samples grow and therefore different tell-tale signals when you look at the sample after growth having polished the surface back. If we switch off the UV illumination you can see that it continues to glow after that. That phosphorescence is a very indicative thing as well.
Meera - Could we see a real one?
Philip - Yes ok. If I just switch on the UV radiation again you can see a sort of tree ring pattern here. The analogy we often use here is if you cut across a tree trunk you can see rings. Those rings correspond to a different stage in the growth history of a tree. In exactly the same way for this natural diamond here you have a central region where the diamond started to form spontaneously. As you go farther out you reach a ring where the luminescence intensity goes up significantly. We know that's because the environment in which the diamond was forming has changed. It may have moved about in the upper mantle or it may have been that it came into contact with a different sort of environment because of other material moving into that region. The important thing is that there's a series of those rings and they relate to the different stages in the diamond's growth. That's very indicative of a natural diamond formation.
 Meera - The complex growth pattern of a diamond as it formed and moved around the Earth's mantle can be seen using UV lasers. Synthetic diamond producers attempt to mimic these conditions associated with diamond formation for technological purposes as well as jewellery imitation. How do they do this?
Meera - The complex growth pattern of a diamond as it formed and moved around the Earth's mantle can be seen using UV lasers. Synthetic diamond producers attempt to mimic these conditions associated with diamond formation for technological purposes as well as jewellery imitation. How do they do this?
Philip - They're made in a very different chemical environment from natural diamonds. There are two basic techniques: high pressure-high temperature synthesis where they're formed from carbon dissolved in metal solvent catalysts. The metals generally used are some combination or other of iron, cobalt and nickel. The second method is chemical vapour deposition. There the synthetic material is produced from carbon containing gases such as methane, usually diluted in hydrogen. There the synthesis takes place at low pressures, usually at about a tenth of atmospheric pressure. Again, a very different environment and it's those differences in the environment which lead the differences which enable us to identify the material and distinguish it clearly from natural diamond.
- Previous Sparkling Science
- Next Diamonds in Industry










Comments
Add a comment