Diamonds in Industry
Interview with
Chris - Diamonds also have some technological applications as well. Chris Wort is from Element 6 which is a diamond manufacture and technology company. He's here to tell us a bit more about some of these applications. Hello Chris.
Chris W - Good evening, Chris.
Chris - Thank you for joining us. What sorts of things can you do with diamonds apart from putting them on your fingers?
Chris W - That's a very good question. Diamond is really quite an exceptional material. It has properties other than its beautiful brilliance that's used for the gem side of it. It's very very hard and people know that diamond will scratch glass but it will scratch absolutely everything else. They were the traditional abrasive type of applications of cutting and polishing. It's used for rock drilling, for oil prospecting where you're basically just using the hardness of diamond. In addition, diamond also has very high thermal conductivity that can be used for thermal management, for example, of electronic devices.
Chris - So in other words you could use it for heat sinks or something? You could draw heat away from things with it?
 Chris W - Well, it's really heat spreaders so it's exceptionally good at removing heat from hotspots and for example silicon devices. It's also optically transparent so you can use it for laser windows.
Chris W - Well, it's really heat spreaders so it's exceptionally good at removing heat from hotspots and for example silicon devices. It's also optically transparent so you can use it for laser windows.
Chris - How does that work then? Tell us about the lasers.
Chris W - You can synthesis the diamond as a fully crystalline plate and when you get the conditions correct this plate is perfectly transparent to certainly infra-red radiation. If you can polish it flat and parallel you can then use it as a laser exit window. Because it has a very high thermal conductivity and a very small thermal expansion coefficient you really get very little deviation of the beam.
Chris - In other words, when you make a laser beam out of a diamond it doesn't change its shape very much when it gets hot and also because it's very, very good at conducting wavelengths of all types of light then it doesn't hold anything back. Everything just goes straight through.
Chris W - Yes, that's pretty much it. You get a much better beam quality through a diamond window than any other laser windows.
Chris - The thing is that diamonds are obviously not trivial to find and not found in big enough quantities to make lasers so how are you getting round that problem?
 Chris W - In this particular case the diamond is synthesised by a technique called chemical vapour deposition. That's where you use carbon in its gaseous form. You excite it to a relatively high temperature of a few thousand centigrade and if you get the conditions right the carbon effectively condenses on a substrate. It forms as a diamond layer.
Chris W - In this particular case the diamond is synthesised by a technique called chemical vapour deposition. That's where you use carbon in its gaseous form. You excite it to a relatively high temperature of a few thousand centigrade and if you get the conditions right the carbon effectively condenses on a substrate. It forms as a diamond layer.
Chris - How big are diamonds you can make with this technique? Are you talking a pinhead or can you literally make sheets of diamond like that?
Chris W - No. You can certainly make six inch discs of polycrystalline diamond plates and you can make it millimetres thick. Equally you can coat surfaces with diamonds; use its very hard wear-resistant properties, if you like. It's basically many inches in diameter and many millimetres thick.
Chris - What is it about the chemistry of diamonds that gives them these amazing properties?
Chris W - Well, basically it's a very small atom and very tightly covalently bonded in a cubic lattice.
Chris - What does that mean to the average person in the street?
Chris W - It's very hard and very tightly bonded and very stiff.
Chris - If you were to sort of zoom in with a very powerful microscope and look inside a diamond at the arrangement of the atoms - just very simply - how are they organised?
Chris W - They're in a tetrahedral arrangement so each carbon is attached to four others. It's very stiff and because the carbon-to-carbon bond is short it's a very tight bond. Unlike other materials we've been discussing on this programme diamond is just one element. It is just carbon but it is carbon arranged in a particularly stiff, lattice structure.
Chris - It's interesting because I had a barbecue the other day and I burned another form of carbon, graphite, charcoal and that's certainly not very hard. Why is it so different between diamond and charcoal?
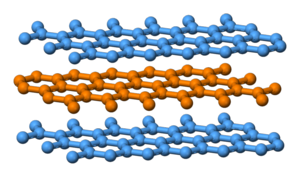 Chris W - It's a different form of carbon. In graphite and charcoals you don't have this tetrahedral bonding. It's actually in hexagonal plates. Although they're quite strong in one direction they're very weakly bonded in the other direction. In actual fact graphite can be used as a very good lubricant for that purpose.
Chris W - It's a different form of carbon. In graphite and charcoals you don't have this tetrahedral bonding. It's actually in hexagonal plates. Although they're quite strong in one direction they're very weakly bonded in the other direction. In actual fact graphite can be used as a very good lubricant for that purpose.
Chris - If we took a lump of diamond and heated it up on a barbecue could you burn it?
Chris W - Oh yes, it will burn. It is, after all, coal. It will burn like coal. If you get it hot enough it starts to oxidise and form carbon dioxide.
Chris - It'd be a pretty expensive experiment to do I suppose. So what other things are you doing at Element 6? What other applications? Some of the things on your websites suggest things like making speakers for hi-fis.
Chris W - It's actually tweeters. This is again using chemical vapour deposition to lay down a very thin layer of diamond in not quite a flat plate but it's a very shallow dome. Because diamond is very stiff and it is relatively low-density you can make a small piston that will displace air for the tweeter in a hi-fi system.
Chris - What sorts of sound frequencies will it produce when you do that?
Chris W - Well, it'll go up to substantially higher frequencies than, for example, a conventional aluminium tweeter just because of its rigidity and its low mass. It has what we call a break-up frequency in excess of 70kHz which is well-beyond the audible range of humans.
Chris - That's ok if you're a bat, isn't it? What about for a human?
Chris W - Well, for humans in actual fact the break-up frequency is important because you get harmonics that occur at lower frequencies. It's the harmonics that the human ear can detect at the low 20kHz that make a clattery tinny sound. If you have a higher break up frequency you actually have a more perfect sound in the whole of the audible range.
Chris - Lastly, one other cutting edge piece of technology you're developing is things to make scalpels even sharper.
Chris W - Yes, traditionally ophthalmic surgery knives have been made from natural diamonds. We're also able now to make a crystalline diamond which will give an almost anatomically sharp edge. It's extremely good for precision surgery.
- Previous Detecting a Dodgy Diamond
- Next Sustainable Solar Solutions?










Comments
Add a comment