Better brain cell transplants
A 3D brain cell culture matrix that works as a brain implant has been announced by US researchers.
Unlike other parts of the body, 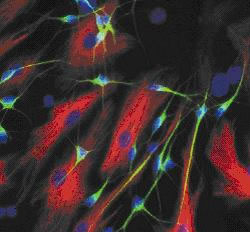 damage to the brain or spinal cord is not spontaneously repaired, leading to lifelong disability.
damage to the brain or spinal cord is not spontaneously repaired, leading to lifelong disability.
Neurodegenerative diseases, which are caused by the loss of cells from the brain, are also irreversible.
For these reasons, research into therapeutic interventions for these conditions is focusing heavily on ways to replace lost nerve cells.
Over the past two decades, scientists have achieved some success with the transplantation of new nerve cells into certain parts the brain, with Parkinson's Disease being one condition that appears to respond to this approach.
The transplanted nerve cells appear to be able to wire themselves into the host nervous system and help to reduce the severity of the patient's symptoms.
But the success of these strategies, doctors suspect, is being held back because so few of the cells that are transplanted actually survive. Current estimates put the cell survival rates at under 1%.
Now a research team at Rutgers University in New Jersey, US, writing in Nature Communications this week, have developed a three dimensional culture material that can be injected, laden with new nerve cells, into the nervous system.
Cells implanted this way showed a 38-fold greater survival rates compared with traditional grafting techniques.
Seen down a microscope, the new material resembles a fibrous bird's nest woven from a network of thin filaments each about one one-thousandth of a millimetre across.
These fibres are made from the tissue-friendly chemical poly(desaminotyrosyl tyrosine ethyl ester carbonate), pDTEc, using a technique called electrospinning.
Starting with human adult stem cells, the team turned on a genetic signal called NeuroD1 which triggered the cells to specialise and turn into brain cells.
Seeded onto the pDTEc culture matrix, these cells matured into neurones capable of firing electrical nerve impulses.
The team injected small discs of their matrix, replete with a hundred or so cells, into slices of cultured brain tissue.
Over a few days, the new matrix-supported nerve cells put out connecting branches nearly one millimetre long, which was three to four times longer than free-floating cells injected using traditional techniques.
Even more impressive was the result when the cell matrices were injected into the brains of experimental mice and examined three weeks later.
Compared with control animals, which received comparable injections using the techniques currently used in human patients, the animals with cell grafts made using the new matrix material had nearly 40-fold greater cell survival with almost 5% of the injected cells surviving.
The grafted cells also made connections, called synapses, with other neurones, including with the surrounding brain tissue, proving that they had successfully wired themselves into the host brain.
"Similar prototypes," say the researchers, "could be engineered to apply human neuronal cells of varying subtypes for targeted treatment of a wide range of CNS disorders..."





Comments
Add a comment