Protein, Plague and Playing Nicely With Others
In this NewsFlash, why a high protein diet stops you from snacking, the protein "restraining order" for gut bugs, and how Plague hasn't changed in 600 years. Plus, we discover the DNA scalpel technique that can repair single mutations leaving no trace...
In this episode

00:25 - A low protein diet increases snacking
A low protein diet increases snacking
Having a lower percentage of protein in the diet can lead to more snacking behaviour and more intense hunger pangs, a new study has revealed...
The team from the University of Sydney, led by Alison Gosby, wanted to test whether something known as the protein leverage hypothesis, or PLH, which suggests that a biological preference for a high protein diet, combined with a decrease in the proportion of protein in the diet in relation to fat and carbohydrate, can lead to obesity, applies to humans, as it does in other species.
Obesity is a huge problem (no pun intended) - almost a quarter of British adults are now classed as obese. Previous work in the United States has shown that over the last 30-40 years, protein intake has decreased from around 14% of the diet to 12.5% of the diet, and total energy intake has increased. The possible reason for this is that due to a lower intake of protein at mealtimes, people are craving extra protein and seek it from snack foods, which then increase their total daily energy intake.
In the new study, published in PLoS One, participants were fed either a 10% protein, 15% protein or 25% protein diet for three four day periods. Their total energy intake and their perceived hunger levels were monitored over each four day period. Their main meals were regulated to contain the right amount of protein, but also available were 'anytime foods' like cheese scones, yoghurt or muffins.
They found that the participants on the 10% protein diet increased their total energy intake by 12% compared to those on the 15% protein diet. And it was the 'anytime' snack foods that made up 70% of the increased energy intake, rather than just eating more of their provided meal at mealtimes. It also tended to be the savoury snacks that the participants preferred, which the researchers suggest is due to the association of protein with savoury food rather than sweet. There wasn't any significant difference in energy intake between the 15% and 25% protein diets, suggesting that increasing protein in the diet above a certain amount will not necessarily decrease intake of other foods.
Average perceived hunger levels across the day didn't differ between the diets, but on the 4th day, participants on the 10% protein diet reported significantly more hunger 1-2 hours after breakfast than those on the 25% protein diet.
So what do these results mean in the context of the protein leverage hypothesis? Well, the researchers are keen to point out that this is only a small study, and alone it doesn't prove that the PLH is responsible for the increased levels of obesity in society, but they point out that along with other larger studies and the fact that protein intake has indeed decreased over the last 30-40 years, coinciding with the rise in obesity, it does seem to support the hypothesis.
They also mention that if the participants on the 10% protein diet continued to maintain their increased energy intake that they showed over the 4 day trial, without increasing their activity level, they could expect to put on a kilo every month!
03:51 - Protein “Restraining Order” for Gut Bacteria
Protein “Restraining Order” for Gut Bacteria
An antibacterial protein secreted in the small intestine creates a tiny "no man's land" between the wall of the intestine and the bacteria that live inside the gut. Breakdown of this physical buffer could lead to Inflammatory Bowel Disease and other chronic problems.
The mammalian intestine is full of bacteria, around 100 trillion of them. We know that many of them are key to keeping us healthy, performing important metabolic roles that our own cells can't. But we don't fully understand how the immune system tolerates the presence of so many foreign cells.
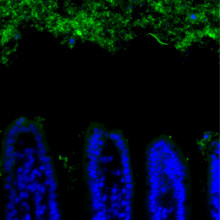
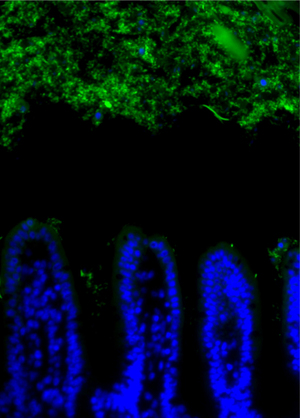 One way is to keep the bacteria physically isolated from the epithelial cells lining the digestive system, creating a "buffer zone" that stops the immune system from reacting. Different components of the digestive tract have different strategies. The colon, for example, deploys a dense mucus layer on the surface, which is too compact to allow bacteria through and creates a gap of around 50 micrometres between bacteria and the epithelium. This technique cannot work in the small intestine, however, as the dense mucus would block the nutrients that are absorbed in the small intestine.
One way is to keep the bacteria physically isolated from the epithelial cells lining the digestive system, creating a "buffer zone" that stops the immune system from reacting. Different components of the digestive tract have different strategies. The colon, for example, deploys a dense mucus layer on the surface, which is too compact to allow bacteria through and creates a gap of around 50 micrometres between bacteria and the epithelium. This technique cannot work in the small intestine, however, as the dense mucus would block the nutrients that are absorbed in the small intestine.
Writing in Science, Lora Hooper and colleagues at the University of Texas Southwestern Medical Center at Dallas took sections of mouse intestine and used a technique called FISH (fluorescence in situ hybridization) to see where the bacteria were found. In the small intestine, they saw the same 50 micrometre gap as seen in the colon. However, if the mice lacked a certain gene, involved in the innate immune response to bacteria, this gap vanished, despite overall levels of bacteria being the same.
The gene responsible controls the expression of several key antimicrobial proteins, including RegIIIγ. By knocking RegIIIγ out in otherwise healthy mice, the researchers were able to show that this protein is indeed responsible for maintaining the gap between gut and bugs. In its absence the adaptive immune system starts to respond and it's this response that could lead to inflammatory bowel disease.
There is still a lot to understand about the roles of the immune system and epithelial cells in tolerating gut bacteria, and achieving the balance required to develop, and maintain, a healthy commensal community. RegIIIγ only controls certain groups of bacteria, so the hunt is on now to find the other proteins involved making bacteria keep their distance.

06:29 - DNA Scalpel Fixes Mutations; Leaves No Scars
DNA Scalpel Fixes Mutations; Leaves No Scars
Professor David Lomas, Cambridge Institute for Medical Research
Ben - A new technique to repair errors in DNA while leaving no trace has been reported in the journal Nature. The researchers have corrected an error that leads to an untreatable liver disease, and this technique could eventually lead to treatments for an extremely wide range of genetic illnesses. Joining us to explain more is Professor David Lomas from the Cambridge Institute for Medical Research. David, thank you so much for joining us.
David - My pleasure. Thank you for the invitation.
Ben - First of all, what is this liver disease? What causes it and what are the symptoms?
David - Alpha 1-antitrypsin is a protein that's produced from the liver, and it bathes all the tissues in the body. The role of the protein is to protect the tissues against enzyme damage. There is a genetic mutation in this protein that's found in 1 in 25 of the population. So 1 in 2,000 are homozygous and it affects 30,000 people as homozygotes in the U.K. So it's common. This mutation causes the protein to misfold and accumulate within hepatocytes. That gives rise to liver disease for which the only treatment is transplantation. Now you imagine the lack of an important circulating protein means there's a lack of protection for the tissues and the lung is most susceptible, and these people will develop volume onset emphysema particularly if they smoke.
Ben - So, it is a liver disease but it also affects other systems?
David - Correct. The primary abnormality is the protein misfolding in the liver, accumulating in the liver and then the secondary effect is on the lung.
Ben - And as you said it's quite common so it's obviously a good target for us to look at. So what have you done? What have you reported in this particular paper?
David - So, what we're trying to do is to think of a different way of producing liver cells that one day may take over from the damaged cells within patients. So we started with my patients and we took a skin biopsy, and we isolated the fibroblasts. We then reprogrammed these fibroblast so they became stem cells. Now, these stem cells still have the genetic mutation, so we corrected the genetic mutation and to give you some idea of the magnitude of the problem, we changed one or two base pairs in 6 billion. We left the rest of them unchanged and then, having made that correction, we have a normal healthy stem cell. We differentiated those to liver cells, which now work beautifully in the test tube, and then we put them in mice. We showed that they were viable in mice and they produced the relevant liver proteins.
Ben - So your technique is sort of a genetic scalpel; you're going in there with extreme precision to cut open the DNA, take out the errors, repair it, and not leave a genetic scar.
David - Absolutely right. Molecular scalpel is a good description.
Ben - What at the moment is stopping us from using this in humans? It seems to be very well received in mice, and I understand you put them into mice with no immune system so you don't get rejection. But quite often, we've seen with stem cell research, like the cells don't really integrate properly, they might form tumours, there are all sorts of problems. So, what's standing in the way now?
David - So there's two issues to highlight. The first is that the cells that we get from reprogramming look like liver cells and behave like liver cells but are an immature version of the liver cell You can consider them as a foetal cell, that's the good way of looking at them. Now, they seem to function well in the mouse. They seem to produce the relevant proteins and they seem to integrate. But we don't know whether long term, they can take over the function of the liver and repopulate and replace damaged liver cells. The second issue is that when you reprogram skin cells into stem cells and then differentiate them into hepatocytes, you collect point mutations in the genome. And from our sequencing, we found about two dozen point mutations. Now, we think looking at the bioformatics that they're okay so they're probably not going to have any effect, but you don't know for sure. The safety signal that we saw in these experiments is that when we put them in the mouse six weeks later, they function normally. There didn't appear to be any malignant potential. But those two issues need to be addressed, particularly the last one, before we can move this through to clinical trials.
Ben - Two dozen mutations doesn't actually sound like that many. I imagine there are far more just going from parent to child?
David - Exactly, but the problem is that we need to understand what they mean and what they do, and it's the context of the mutation that's important. So they may be fine. This may not be a problem in real life. However, we need to think about it very carefully, and that's one thing that we need to address as we develop our clinical studies.
Ben - I guess the reprogramming method is a very artificial situation in which these mutations are forming. So they could form in regions that would otherwise be very well protected.
David - Correct, and it's just important to stress the safety side of these things. I mean it's a nice story and we can go from skin, correct the genetic defect, and come up with liver cells that appear to function beautifully. But if we're going to put them into patients, we need to develop strategies whereby we first of all do no harm, and the cells that we put it are not only viable and healthy but, they won't have that malignant side effect.
Ben - What do you see the future for this sort of technique? If you can pick out one or two bases in an entire genome then surely you can do this for pretty much any genetic disorder.
David - That's correct, you can use this molecular scalpel for any genetic disease and you can correct the genome and leave the rest of genome pristine and that's the central part of this paper. So you can apply it to any genetic technique. It's then getting cells that are relevant and clean, and viable, and healthy that you can use in patients. So we're grappling with that. So having done this, we're trying to think, "how can we put this through in patients in a safe way?" And our next step probably, because the grappling is still ongoing, is to encapsulate the cells in some way in a polymer mix, an alginate, which means that we can put it in the human body in a test environment whereby it's safe. So, if the cells do develop a malignant potential, they won't escape beyond the capsule and then we can retrieve the cells at a later date and show that they function normally and there's no malignant potential; and I think that stepping stone is quite important before we get through to clinical trials.
Ben - And that, of course, would show you how they behave in context with all of the other chemical factors around that would be there in a real situation.
David - Absolutely, and remember of course these have come from the individual that you are about put them back into, so they have the same genome and theoretically, there should be no requirement for immunosuppressive treatments, and there should be no rejection.
Ben - Excellent. Well, that's extremely promising. Well, thank you ever so much for joining us.
David - Thank you.
12:56 - Black Death Bacterium Unchanged in Centuries
Black Death Bacterium Unchanged in Centuries
Researchers have for the first time mapped the genome of Yersinia pestis, the bacterium that caused the Black Death - the plague between 1347 and 1351 that killed up to 30 million people - 50% of the population of Europe at the time.
This bacterium is still around today and continues to cause cases of bubonic plague, the other name for the Black Death, though not with the same virulence or scale that it did in mediaeval times. Why? No one knows for sure.
One possibility is that the more ancient forms of the bacteria carried mutations or genetic changes that could account for their more aggressive behaviour.
To find out whether this was the case, McMaster University scientist Kirsten Bos, together with colleagues in Germany, the US and Canada, looked at bacterial DNA extracted from teeth collected from four victims buried in 1348 in a "plague pit" in what is now East Smithfield in London.
The DNA they recovered from the teeth was in quite poor condition having been broken up by the passage of time into many short fragments. But by making millions of copies of the individual pieces and then using a computer to work out which bits overlapped with each other, they were nonetheless able to reassemble - for the first time - the complete genome of the chromosome of the 14th century strain of the bacterium as well as the sequences of two smaller rings of DNA, known as plasmids, that are also present in the bug's cells.
The big surprise for the scientists, when they compared the sequence of the 14th century Black Death strain with its milder, currently-circulating counterparts, was how few differences there were between them.
The results from the London victims also show that, by the time of the Black Death, the plague bacterium had only recently emerged in the human population - perhaps a century or so before the Black Death occurred; in fact, owing to the close relationship with strains from across the world, the scientists think the spread of the Black Death plague was the first main dissemination of the bacterium globally.
So if the Black Death strain of the bacterium is so similar to the Yersinia pestis isolated from plague sufferers today, why was the outbreak in the middle ages so devastating?
At the moment scientists don't know. It's clear that the bacterium is essentially the same, so something else must account for the increased past virulence. It may be that those who died historically had a genetic vulnerability to the bug, social or economic factors may have played a role. Maybe a second, different infection was co-circulating alongside the plague which boosted its transmission and virulence? Or perhaps climate or even the behaviour of the vector - the fleas on rats - that spread the disease had a role to play. Those are the questions that need to be addressed next.

15:43 - Need help? Ask a Child, Not a Chimp
Need help? Ask a Child, Not a Chimp
Children are more likely to seek assistance in a task than chimpanzees, suggesting that a motivation to work together could be part of what makes us "human". Writing in the journal Current Biology, Michael Tomasello from the Max Planck institute for Evolutionary Anthropology, in Leipzig, Germany, and colleagues Yvonne Rekers and Daniel Haun, wanted to test the idea that humans are more motivated to collaborate than our closest relatives, the Chimpanzees.
 Both children and Chimps are known to collaborate. They recognize when they need help and actively recruit a partner to assist them. Chimps are also known to choose good collaborators over bad collaborators, so clearly possess the cognitive abilities to understand the need for, and the problems with, sharing a task. However, Chimps generally keep to themselves. They live in groups but most of their foraging is done individually, groups of males only rarely work together to hunt. This gave rise to a hypothesis that motivation, not ability, was behind human collaboration.
Both children and Chimps are known to collaborate. They recognize when they need help and actively recruit a partner to assist them. Chimps are also known to choose good collaborators over bad collaborators, so clearly possess the cognitive abilities to understand the need for, and the problems with, sharing a task. However, Chimps generally keep to themselves. They live in groups but most of their foraging is done individually, groups of males only rarely work together to hunt. This gave rise to a hypothesis that motivation, not ability, was behind human collaboration.
To test this, the researchers devised an experiment where the subject, a chimp or a child, could pull a board full of food into their chamber in one of two ways: Either alone; by pulling two ropes simultaneously, or collaboratively; pulling a rope in their chamber while a partner pulled the other. The partners were familiar animals - from the same social group for the Chimps, and the same kindergarten for the children. Both methods resulted in the same amount of food for both test subject and partner, ensuring there was no advantage to one over the other.
Human children chose to collaborate around 78% of the time, significantly more often than did the chimpanzees, only around 58%. This shows that the children chose to collaborate far more than would be expected by chance alone, while the chimps chose randomly.
So why would children prefer to collaborate? One hypothesis is that we have an innate drive for "fairness"; as the partner child was given the same amount of food regardless of whether they cooperated or not, they could be seen to be getting a "free ride" on the other child's efforts. To test this, the researchers devised a second test, where the partner child only received a food reward in secret. Despite this, the children still chose the collaborative board significantly more often than by chance alone.
This shows that children are more motivated to collaborate than Chimps. The authors speculate:
"Collaborative foraging may well have been a key behavioural domain in which humans evolved a suite of new proximate mechanisms, both cognitive and motivational, for collaborating with others in ways that eventually led to the many complexities of modern human societies."
They now propose similar experiments to compare different primate species and explore the evolutionary history of collaboration.
19:26 - Fighting Infections and Mimicking Muscles
Fighting Infections and Mimicking Muscles
Robert Modlin, UCLA; Andrew Schwartz, University of Pittsburgh; Ray Baughman, University of Dallas at Texas; Marc Vendhoen, Babraham Institute
Using Vitamins to Fight TB
 Vitamin D supplements could be used to fight Tuberculosis in at-risk populations.
Vitamin D supplements could be used to fight Tuberculosis in at-risk populations.
Darker skinned communities are known to suffer from deficiencies in Vitamin D when living in Northern climates and as a result, are also more susceptible to infections such as TB. By Comparing groups of African-Americans and Caucasians, UCLA's Robert Modlin discovered the cause of this link to be a change in the immune system's ability to attack the TB bacteria. A change controlled by vitamin D.
Robert - When you go to the doctor and have your Vitamin D measured, that's the precursor form and that gets into the infected cell and if that cell becomes activated, it gets converted to the active form triggering antimicrobial peptides that kill the bacteria. What happens if your precursor level is low, you can't achieve high enough levels to trigger this antimicrobial mechanism and then you can't kill the bacteria. There's probably a correlation with the level of the Vitamin D in your blood and your risk of getting TB.

Brain Computer Interfaces to aid Prosthetics
A brain-computer interface is enabling patients with spinal cord injury to move a prosthetic arm using their thoughts alone.
The technology, currently being trialled at the University of Pittsburgh School of medicine, uses electrodes to collect and decode signals in the brain directing an object, in this case a prosthetic, to move. A feedback loop is with the patient seeing they movement generated by their signals and correcting for any discrepancies.
Andrew Schwartz is leading the trial...
Andrew - There's been a big effort sponsored by the Defence Department of the US Government, and National Institute of Health to develop a very nice prosthetic device. This device has many of the capabilities of our own arm and hand. So we'd like to be able to get a rich enough control signal that we can operate that device. We'd like the subject to be able not only to reach out and grab things but to actually do dexterous tasks that require coordinated finger movements.
Another reason to eat your greens
 Eating your greens really does keep you healthy, by
Eating your greens really does keep you healthy, by
keeping infections at bay.
Working with mice, Marc Veldhoen from the Babraham Institute found that reducing the amounts of cruciferous vegetables such as cabbage and broccoli in the diet over 2 weeks, caused a 70-80 percent decrease in intra-epithelial lymphocytes, or IELs. These are white blood cells found in the skin and the gut which are the first line of defence in the body.
With reduced levels of these cells the mice has fewer anti-microbial proteins and increased susceptibility to injury as these lymphocytes are needed for wound repair in addition to their role as a barrier from the outside world.
Marc - If you have reduced numbers of IELs, your intestine is much more prone to injury. You're much more prone to inflammation when the IEL numbers are reduced. The intra-epithelial lymphocytes express a receptors which is called the aryl hydrocarbon receptor to very high levels and compound found in green vegetables which is called indole-3 carbinol, creates a ligand which has very high affinity for this receptor. It's a very direct effect. If they don't have the component or they don't have the receptor then they don't survive.
Mimicking Muscles with Nanotubes
The twisting movements of muscles can be mimicked using
bundles of  carbon nanotubes.
carbon nanotubes.
The strong, but stiff characteristics of these nanotubes have been manipulated by Ray Baughman and colleagues from the University of Texas at Dallas to create flexible yarns that twist rapidly when dipped into an electrolyte and injected with charge. They then untwist when the charge is removed. This rotation simulates that seen in elephant trunks, squid tentacles and human tongues.
Ray - Right now, people are imagining micron scale robots that do self-repair in the human body but in order to have such microrobots, perhaps in swarms, you need motors and our technology can provide a type of motor. Bacteria in nature use torsion to propel themselves. Our artificial muscles provide this type of torsion.










Comments
Add a comment