Tinnitus, hyperacusis, salamanders, chemical harpoons and the role of ultrasound and song in the mating rituals of mice and flies go under the microcope in this edition of the eLife Podcast.
In this episode
What causes tinnitus?
with Richard Salvi, University of Buffalo
As many as one person in every four is affected by tinnitus. This is a phantom ringing or mechanical sound experience which occurs in the absence  of any real sound input, and often in people with prior damage to their hearing. So, why does it happen? Richard Salvi explained his findings to Chris Smith...
of any real sound input, and often in people with prior damage to their hearing. So, why does it happen? Richard Salvi explained his findings to Chris Smith...
Richard - Well, because tinnitus is often perceived as coming from one of your damaged ears, the original theories were tinnitus originates from abnormal activity in your inner ear that's been damaged. However, studies have been done where the auditory nerve that connects the ear to the brain - when that's severed because of a surgical intervention, the tinnitus persists. And this lead people to believe that tinnitus might actually be generated somewhere in the central nervous system, maybe in the auditory pathways.
Chris - And how have you sought to find out which of those two it is with these experiments?
Richard - In these animal experiments, we have a way of inducing tinnitus with a drug that's commonly used by most people in the world - Aspirin. If you take a really, really high dose of Aspirin, it will induce this phantom sound of tinnitus. So, we induced that in animals. We have behavioural techniques where we can interrogate the animal. We can ask the animal, "Are you hearing silence? Are you hearing a sound?"
Chris - And what is that test? How do you do that?
Richard - We train the animal initially to press the right bar under two sound conditions - when they hear quiet or when they hear an amplitude modulated noise, a noise that would sound like "thhssssk, thhssssk, thhssssk, thhssssk, thhssssk, thhssssk, thhssssk, thhssssk!!", or press the left bar if they hear just a steady sound. "thhhhhhhhhhhhssssk!" After the animals learn that task, then we induce tinnitus by giving them a high dose of Aspirin. And on the quiet trials, if they have tinnitus, they no longer hear quiet and they shift their behaviour from the right bar, to the left bar which is associated with the steady sound. So, they're tricked into believing quiet is steady sound.
Chris - And how does that help us to understand physically and physiologically what tinnitus is?
Richard - Once we know that the animals are perceiving this phantom sound, then we can do electrophysiological studies. We can put electrodes into the different parts of the auditory brain or other non-auditory pathways and see what's happening to the neural activity, how it's changed. What we found is parts of the central auditory pathway became very hyperactive when we played a sound to them, whereas, their inner ears became less active. So, it looked like the brain was compensating for the peripheral hearing loss.
Chris - It's rather like if I've got my radio tuned in and there's a little bit of static or interference in the background, and the conversation is rather quiet, I turn up the radio to hear better. But there's still an inherent hiss there which will become louder, and that's what's happening in these animals.
Richard - That's exactly the analogy that I use to explain this. By turning up the volume control, you hear all the static background in your radio station.
Chris - Does this give us any insights then into how we can manage tinnitus better? Now you understand a bit more and you have some physiological proof as to what appears to be going on in the auditory system. Can we shut this off?
Richard - Well, there's ways of mitigating it. One of the most effective ways is if somebody's completely deaf and has tinnitus, you can put in a prosthetic device called a cochlear implant which electrically stimulates the stump of the auditory nerve. And when you turn the cochlear implant on and put information back into the central auditory nervous system, in about 90% of the people, the tinnitus disappears.
Chris - What about people who are the reverse of deaf? Because there are people who hear sounds and they experience them, perceive them, as distractingly loud.
Richard - You're describing a phenomenon we refer to as hyperacusis. So, if you turn up the central gain mechanism, a really weak signal now that comes in from the periphery and reaches the central nervous system, if you've got your volume control turned up too high, what's gonna happen - sounds will be, sound super loud to you. So, we think this central gain hypothesis is a way of explaining both tinnitus and hyperacusis.
Chris - And if one explores the auditory system when these processes are happening, is it just a discrete zone that's affected or do other brain regions affect the process too? So, for instance, is someone sensitive to sound when they're feeling emotionally sensitive, for example?
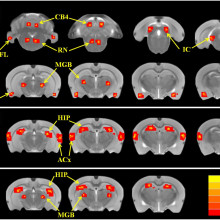
Richard - Yes. In our study, we found not only the auditory parts of the brain became hyperactive, but an area called the amygdala that assigns emotional significance to sound. A second area that became activated was the reticular formation. This is an arousal area that gets you excited. And a third area was the hippocampus. This is an area that's involved in spatial navigation. We think all of this network might play an important role in tinnitus and hyperacusis.
06:26 - How salamanders avoid senescence
How salamanders avoid senescence
with Dr Maximina Yun, UCL
Lose a limb or another part of your body and they'll never grow back. But in some animals, that's not the case. And entirely new arms, legs, and other organs can regrow from scratch. So, why the difference?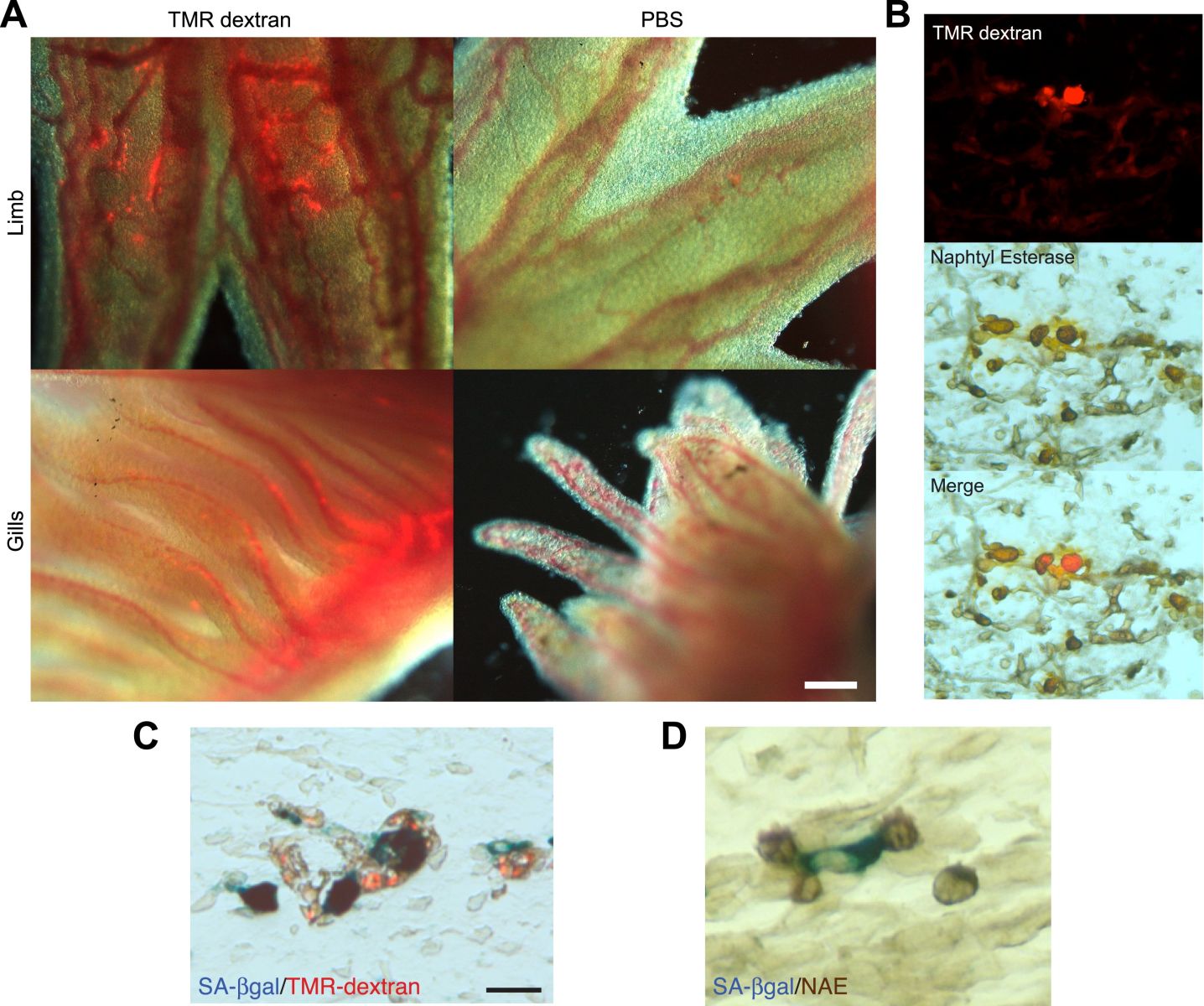 According to UCL's Maximina Yun, it's down to a powerful system operated by macrophages for eliminating senescent or aged cells as she's been showing in salamanders. She spoke with Chris Smith...
According to UCL's Maximina Yun, it's down to a powerful system operated by macrophages for eliminating senescent or aged cells as she's been showing in salamanders. She spoke with Chris Smith...
Maximina - Even from the shoulder level, they can regenerate their full arms, and also they can regenerate entire legs, their tails, parts of their heart, parts of their eyes and brains.
Chris - And if you keep on doing this to a salamander, if you let its leg grow back and then you chop it off again. Does it get less good at doing that regeneration process each time you do it or is there no decrement in regenerative capacity with time like that?
Maximina - You're right. There's absolutely no loss in regenerative abilities. So, every time they regenerate a new limb, this happens absolutely perfectly. And actually, the regenerated limb is identical to the one that was lost.
Chris - So, do we have any idea as to how they're doing that or why they can do that, and why they don't suffer a sort of regeneration fatigue? Because, normally, if you flog a tissue to keep on repairing and restoring itself, the cells do eventually do give up the ghost, don't they? They become very old and they senesce, they switch off, they won't divide anymore.
Maximina - Exactly. When we started this work, we did not have much of an idea of why this happened. However, we think that our work has illuminated some these aspects. So, basically, what we found is that through the course of regeneration, these cells which are called senescence cells, do appear. However, the salamanders have an incredible capacity to eliminate these cells which will otherwise result in the aging of the tissue, so that at the end of regeneration process, there are no cells remaining. They have all been eliminated.
Chris - Aright. So, you've got a system where they regenerate in the same way, let's say, one of our tissues would. They do produce cells which are, for want of a better phrase, tired, because they divided a lot and they become senescent. But then, something happens to those cells. They get removed and it leaves just a pristine playing field again. The cells are all non-senescent fully functional cells again at the end of the regeneration.
Maximina - Exactly. You're total right. And what our research told us as well is that the immune system, and in particular the macrophage, is an essential part of the mechanism by which these cells are eliminated.
Chris - They're actually actively seeking out and destroying these senescent cells, are they?
Maximina - Exactly.
Chris - Do we know what they're looking for? What do these macrophages go after? How do they tell you are a senescent cell, you've divided too many times, you are gonna be destroyed.
Maximina - Well, that's an excellent question, because it's something that we haven't yet addressed, at least in this part of the work, but it's part of what we are doing now. So, what are the signals that attract the macrophages to these senescent cells?
Chris - And if you kill off the macrophages or inhibit them, or prevent macrophages from being there, do you see what one would predict would happen? Which is that you would see a population of highly senescent cells and there would be an impact on regenerative effort if you then come along and repeat, say, the removal of a limb under those settings.
Maximina - We do see exactly what you described.
Chris - So, how did you find that the macrophages were doing this important role in the first place? What lead you to make that conclusion?
Maximina - So, we used a very nice set of tools which allow us to, first, label the macrophages. So, we know where they are within a tissue and we realized that they were being recruited to the sites of senescent cells. Whether these were the endogenous senescent cells that appear in regeneration or whether these were senescent cells that we implanted within those tissues.
Chris - And do we know why this happens in a salamander but it appears to have been abandoned as you go up the evolutionary tree. Why don't humans have this amazing ability?
Maximina - Well, you're quite right and it's been shown that these senescent cells are involved in many age-related disorders. Now, we don't know exactly why this is happening, what's the difference the salamander and humans. But what we think is that differences in the immune system between salamanders and humans could be playing a role. And, clearly, if we can find what these differences are then we can perhaps design therapies and strategies to eliminate senescent cells in humans eventually.
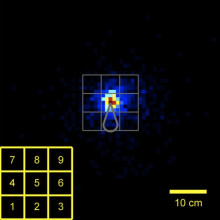
11:40 - Mouse ultrasound
Mouse ultrasound
with Dr Joshua Neunuebel, University of Delaware
A few years ago, scientists discovered that mice squeak at each other using ultrasound. But studying why they produce these vocalizations, which animals make them and when, and in response to what stimuli has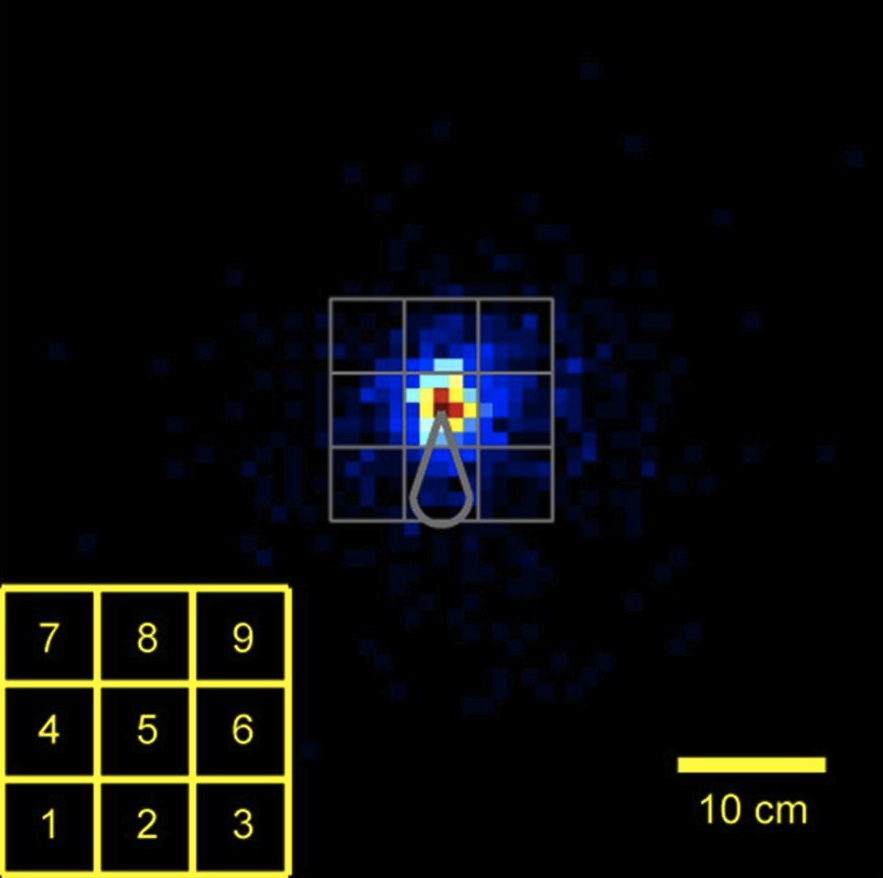 always been a major challenge. And this is owing in part to the constraints of how ultrasound behaves and how to detect it. Now, Joshua Neunuebel from the Janelia Farm Research Campus has developed a system to allow him to localize the sources of these sounds in 3D space and in real time. And he's already busted one myth. Both sexes, not just the males, make them. He explained to Chris Smith...
always been a major challenge. And this is owing in part to the constraints of how ultrasound behaves and how to detect it. Now, Joshua Neunuebel from the Janelia Farm Research Campus has developed a system to allow him to localize the sources of these sounds in 3D space and in real time. And he's already busted one myth. Both sexes, not just the males, make them. He explained to Chris Smith...
Joshua - They're emitting these sounds ultrasonically, and so these are really high pitched sounds. And we can't hear them, and so, it requires specialized microphones to actually detect these sounds. And so, we can't really put a microphone on the mouse to figure out which one's vocalizing. And so, we decided to actually just take advantage of physics. Sound travels at 340 meters per second. And the closer you are to this, the sooner you're gonna be able to detect these sounds. And so, we decided to use math and physics to sort of pinpoint where the sound was coming from.
Chris - This gives you an insight then into which animal is producing a sound in what sort of setting without actually having to interfere with the animals at all.
Joshua - Yes, exactly.
Chris - And what does it show then, when you do that?
Joshua - So, the field has believe when you put a male and a female together, that it's always the male that produces these vocalizations. And the reason for that is if you anesthetize the female and you put a male in, he'll run around and vocalize. But if you do the opposite and you knock out the male and put a female in, she doesn't vocalize. And so, the field has believed that males produced these vocalizations. And so, what we showed and with normally freely behaving animals, females do vocalize.
Chris - Tell us a bit about your sort of set up then. How do you physically do these recordings?
Joshua - What we do is, basically, we build a microphone array and that means we have multiple microphones positioned around the arena where the animals run around. And normally, ultrasound in incredibly reflective, so if it hits a plastic wall or hits something hard, it's gonna be bounced back. If that's occurring, it makes it really difficult to localize the sound. So, what we did was we built this acoustically transparent cage. So, the ultrasound can actually pass through this cage because the cage is made out of this nylon mesh, and then it passes through and gets detected by these microphones on the outside in the periphery. And there's also some foam on the outside that helps absorb this and prevents the bounce back.
Chris - And how many animals can you resolve at once? Is it just pairs that you can accurately resolve with this, or could you go to bigger groups?
Joshua - In the paper that we published, we went up to four animals. So, it's two males and two females.
Chris - So, this is quite literally a triangulation effect. You basically are recording from multiple microphones and you can time how long it takes the sound to arrive at each of the different ones, and you can match them up and you can say, "Right. Okay. That must be coming from that animal, that animal, or that animal."?
Joshua - Precisely. Yeah.
Chris - So, what problem can you now apply this to? So, you've done the initial detection right. We've shown that for the first time that the male and the females, they're both vocalizing. But what will you be able to now test next? What's the next step?
Joshua - So, there's a lot of different steps, actually. And so, I just actually started my first faculty position this year and so, what I want to do is I want to start applying this to different autism models. And so, if you look at a lot the autism mouse models out there, you see impairments in social behavior and you see impairments in, what they say, communication. And that means there's a reduction in the number of vocalizations. But we don't really know if there's a deficit in vocal interaction between these animals. So, I'd like to actually go in and use autism models and see if there are impairments in vocal communication.
Chris - What about if the mice don't feel like talking?
Joshua - Well. It's gonna be a bad day in the house.
Chris - Does that happen? How often do they produce these sounds so that you get meaningful data?
Joshua - It depends on the conditions. And usually, if you pair a male and a female mouse together, they will vocalize. And so, as long as they're together, you're gonna get some data. Maybe not as much as you want but you'll get data. One of the things we did, for male mice, they'll vocalize to female urine. So, they don't need a partner in there. Like, they habituate to those conditions very quickly. And so, that's the more variable part. How often are you going to get vocalizations to, like, external stimuli or a different stimuli. But if put a male and a female together, you'll get some data to look at.

16:50 - Drosophila duet: mating flies harmonise
Drosophila duet: mating flies harmonise
with Prof Mala Murthy, Princeton
Mala Murthy has been studying a species of fruit fly called, Drosophila Virilis, where the two sexes sing to each other during courtship. She's trying to understand neurologically what underpins their courtship ritual,  and ultimately, how we humans also harmonize. She explains to Chris Smith...
and ultimately, how we humans also harmonize. She explains to Chris Smith...
Mala - The male and female of this particular species, Drosophila Virilis, have an interesting courtship behavior where while the male is singing to the female, and she's singing back to him, he's constantly interacting with her in a kind of salacious way. He's licking her genetalia with his mouth parts and he's rubbing his front legs against her abdomen. And what we found is that the timing of these two contact behaviors, the licking of her genetalia and the touching of her abdomen, they're precisely timed with her song. And that she's actually using this information in combination with his song to determine how much song she produces and when she produces that song in relation to him.
Chris - Is this mediated or generated at the level of higher centers in the nervous system, or is there some kind of central pattern generator which is integrating the input from the male - the touching and the licking - with the sounds. So, that then that triggers the correct output at the correct time of her own song.
Mala - That's exactly right that there's this sort of integration going on. But interestingly, the tactile inputs - the licking and the rubbing - are coming from her abdomen, and the auditory inputs are coming from her antenna, which are on the head. And so, the auditory information comes in via the brain, whereas, we found that the tactile information comes in directly to her ventral nerve cord, which is the fly's equivalent of its spinal cord. And so, somewhere in that spinal cord equivalent, she's integrating this tactile information with the auditory information from the brain to pattern her song. And so, we think in that part of the nervous system, in this ventral nerve cord, there is this, as you say, a central pattern generation for her that guides the timing and the amount of her song and these pieces of sensory information interact with it.
Chris - Are both necessary? What I'm getting at is if I were to remove the antennae, or remove the input from the antennae and leave just the rubbing or the licking, for example, or I divorced the licking from the antennal detection sound, would I still get the duetting off of the female, or do you need all of those inputs to sync up in order to get the output?
Mala - The experiments we did are just as you described. We remove each of these sources of sensory information and ask what happens to the behavior. And we found that the tactile information, the rubbing and the licking, is the most important for song timing but the auditory information sculpts how much song she produces, and so, therefore, also end up being critical for the duet. And so, both of these sensory cues play a role, although they're sort of biased towards the tactile information, she relies more heavily on that and that's really novel. I think most studies of duetting have focused exclusively on acoustic interactions which makes sense, you know, that ultimately the behavior is about the two acoustic signals from these two animals and how they interact. But what we found is this role for a non-acoustic signal in timing the duet and I think that will be important to consider going forward.
Chris - Flipping it around, how does the male coordinate all of that? Is it the same system just running in reverse, so what would be the pattern generator receiving inputs in the female is the same circuitry but it's running backwards and just generating an appropriately synced up output in the male which is then, obviously, supplied to the female.
Mala - The songs the male and female produce are different. They have different timings and they're recognizably different. And the males are actually capable of producing female-like songs. So, it's as if they have two circuits - one to generate a male-like song with male timing and the other to generate a female-like song. And the only way we can get the males to produce these female-like songs is if we paired them with another male and these males then received the courtship licking and rubbing, and the acoustic input, the auditory input from the other male. And when he got those pieces of sensory information combined, just as the female normally does, it then drove this other circuit to produce the female-like song. So, it's as if the males have sort of both, sitting there within the nervous system and they choose which one to trigger based on the sensory information they receive.
21:47 - Chemical Harpoons: bacterial anchors
Chemical Harpoons: bacterial anchors
with Professor Ulrich Schwarz-Linek, University of St Andrews
To invade or even just to colonize our bodies, microorganisms need to be able to adhere to surfaces to stop themselves being, quite literally, washed away. Previously, microbiologists had identified the bacterial equivalent of Velcro, sticky materials which could interact reversibly with cell surfaces to help microbes to cling on.
But now, Uli Schwarz-Linek at Saint Andrew's has discovered an entirely new form of adhesion system. This one's the microbial equivalent of super glue. It's a chemical entity that forms a permanent covalent bond to a host's surface, locking the bug in place. He explains to Chris Smith...
Uli - What we're interested in are ways in which microorganisms set up home in our bodies and attach to our tissues, and we are interested in discovering the exact mechanisms by which they achieve that, because if they can't adhere to our tissues, they're simply flushed away by swallowing or a coughing.
Chris - And if we can understand how these things work, that may give us an inroad into designing novel therapeutics, wouldn't it? Because we would be able to potentially dislodge and detach these organisms from the surfaces they're trying to cling on to.
Uli - Absolutely! That is the point. Yes.
Chris - What's new here then? Because we've known for a long time that these microorganisms must have an ability to cling on to surfaces, and we know that they produce little extensions from their surfaces that are almost like miniature molecular grappling hooks that can cling on to things.
Uli - The main difference is that all that has been known previously about how microorganisms bind to human tissue. If you take a very close look at it at great magnification, if you'd like, it looks a little bit like Velcro as you say, their little hooks out to fit together with loops, if you like, on the host's surface. What we hypothesize most that the bacteria have developed a molecular tool, a molecular weapon that, if you compare it to Velcro, it's a little bit like super glue. You do not need large areas of contact to provide strong binding. But instead, you have an extremely strong interaction that doesn't require a large surface.
Chris - Is that not potentially deleterious for the bug though? Because as anyone who has used super glue knows - yes, it's great for gluing the heel back on your shoe when you need it in a hurry but get it on two fingers next door to each other and you've got a problem. It's a trip to casualty. Does there not come with this a danger that the microbes could end up sequestered permanently where they don't want to be?
Uli - To be honest, we do not know. However, what know is that many of bacteria that we are working on have also already come up with a solution to that problem because they have tools to cut off the link again.
Chris - What is the molecule, and how do they do this?
Uli - The specific class of molecules we were working on is a protein that is anchored on the surface of the bacteria, and these are rod-like molecules. And the very tip contains what we refer to now as a chemical harpoon. It will recognize the host's surface and then form a chemical bond. It will undergo a chemical reaction and the chemical reaction means there is a very strong connection formed that cannot be broken easily anymore.
Chris - This is what chemists refer to as a covalent linkage or covalent bond, isn't it? What's performing the covalent linkage? What chemicals are involved?
Uli - Yes, this is a covalent bond, and all you need is a particular bond within the protein and that is really the starting point, the discovery of this unusual bond which we call a thioester. This thioester will react specifically with certain groups on other molecules and these groups are, as we discovered, a side chain of one particular amino acid and that amino acid is called lysine.
Chris - Do all bacteria, do you think, have this or is it just a sub-set?
Uli - As far as we can tell for now, it is a sub-set of bacteria but it's a large sub-set. We found these proteins exclusively in the class that we would call the gram-positive bacteria.
Chris - How do the bacteria keep their powder dry, so to speak? When they're making this intensely sticky chemical, how do they stop it accidentally going off in their face? So, in other words, it's not armed and dangerous until it's deployed onto the surface of the microbe in the right context at the end of that rod?
Uli - We are not entirely sure how that occurs yet, but our hypothesis now is that for the reaction to occur what you need to have is a very specific binding partner. If you mix our bacterial proteins with any other protein, there's no reaction whatsoever. And it's only when the very specific binding partner is found that the reaction occurs. How exactly that happens, we do not understand yet.
Chris - Is it inducible? Can the microbes upregulate and downregulate it? So that they can effectively express it when they want it?
Uli - The answer to that I think is yes, at least for some of the proteins that are better understood. The protein we very much focused on belongs to bacteria called Streptococcus pyogenes. And we know that this chemical harpoon protein we were studying is made by the bacteria particularly when they are exposed to oxygen when the bacteria are about to enter the body. And these bacteria target our tonsils in the throat in particular.










Comments
Add a comment