Immune-manipulating parasites, bacterial genomes married to disease processes and viruses that bounce off already-infected cells make for an infectious episode of the Naked Scientists this week. Also up for analysis, why the eyes vote no to long space journeys; the problem with prostate cancer prediction; why nanoparticles trigger bacteria to breed superbugs and the contagious question of which cancers you can catch...
In this episode
01:39 - Sequencing an Infection
Sequencing an Infection
with Dr Estee Torok, Addenbrooke's Hospital
Kat - DNA sequencing technology is developing at a phenomenal rate. Decoding the genetic sequence of the bacterium now takes just hours and costs a few hundred pounds. Ten years ago, this price tag was in the millions. This means that researchers can now begin to marry up the genetic code of an infecting bacterium with how it causes diseases and what treatments might work best. At Addenbrooke's Hospital here in Cambridge, microbiology consultant Dr. Estee Torok is using this technique to try to understand more about MRSA or as it's more formally known methicillin-resistant Staphylococcus aureus. Hello, Estee. Thanks for coming on the show...
Estee - Hello, Kat.
Kat - So, let's track back a little bit and find out what tests do we currently do when someone has a bacterial infection?
Estee - Okay, so the current tests that we do are, we normally take either a swab, or a sample of pus, or a sample of tissue from the patient. That's put in a little pot or a container, taken to laboratory where it's streaked out onto an agar plate and then that plate is incubated in an incubator at 37 degrees for 24 hours, and after that, we look at the plate to see if there are bugs growing on it. Once we see that there are bugs growing on it, we then have to do further tests that are usually biochemical tests, both to identify the bacterium and also to test it for susceptibility to antibiotic drugs. So the whole process takes at least 24 hours to culture the bug and then 24 to 48 hours to identify and get the full susceptibility results.
Kat - So this could be quite crucial if someone's is very, very sick...
 Estee - Absolutely, so when someone comes in the hospital and you suspect they have a bacterial infection, you obviously can't wait for the results of the microbiology test because your patient may not survive 48 to 72 hours, so what we tend to do is give patients empiric antibiotics, so we guess what the bug might be and what it should be susceptible to and then give them what we think is the right antibiotic. But we don't always get that right.
Estee - Absolutely, so when someone comes in the hospital and you suspect they have a bacterial infection, you obviously can't wait for the results of the microbiology test because your patient may not survive 48 to 72 hours, so what we tend to do is give patients empiric antibiotics, so we guess what the bug might be and what it should be susceptible to and then give them what we think is the right antibiotic. But we don't always get that right.
Kat - Why would it be useful to know what's actually in a bacterium's genes? What sort of genes do affect how bacteria infect us and what they do to us?
Estee - In terms of the bacterium, the genetic code will code for things like the structure of the bacterium, for factors that contribute to their pathogenesis, so how they attach to human cells, how they invade human cells. Also, they code for toxins that can cause detrimental effects to the host cells and obviously, they can code for antibiotic resistant genes.
Kat - Which is obviously a hot topic that we'll come to. So, you and your team are working out how to do genome sequencing on bacteria and tell us exactly what sort of technology this is? How quick is it to do a whole bacterial genome?
Estee - I'm working in Professor Sharon Peacock's group at the Department of Medicine, and what we're interested in is to see whether we can bring whole bacterium genome sequencing into the heart of a diagnostic microbiology laboratory. Genome sequencing has been going on, for example at the Sanger Centre, or at reference laboratories, but this has taked quite a long time and cost quite a lot of money in the past. What we're trying to do is to see if we can take some of the newer machines and if we can use them close to the patient in order to inform both clinical care and public health.
Kat - I've seen some of these little sequencers and they're sort of the size of a printer, a desktop printer! How much would it cost to sequence an infection, one person's infection roughly?
Estee - Well it sort of depends. The big sequencers that are used at the Sanger Institute are Illumina HiSeq machines and they are very large and often when we send samples to them, it can take 6 to 8 weeks for us to get a result, and that's relatively inexpensive so less than 50 pounds or so for a genome, but obviously, you can't wait that long. With the smaller desktop sequencers or bench top sequencers I guess you should call them, they're the size of about a printer or one of those expensive cappuccino machines, and we can get a result in 18 to 24 hours, and the costs of coming down. I think you'll have to ask the companies how much they're charging but I would say it's in the hundreds of pounds as opposed to in the thousands.
Kat - This certainly starts to make it seem like something we could do. So if you could start to bring this into the clinic, what would be the benefits of knowing the sequence, the genetic sequence of the bacterium that's infecting someone?
Estee - I don't think we'll ever be in a situation where we can sequence every single bug that comes in to the laboratory, but I think we'll focus on bugs where for example, you think that the patient may be part of an outbreak investigation or where it's important to know things about virulence or drug-resistance more quickly. So, you'll need to pick which bugs you do and then focus on getting those results more quickly in order to either determine whether bugs are related or not in the case of outbreak investigation, or what antibiotics to use in the case of drug-resistance and in the case of surveying the emergence of drug-resistance.
 Kat - And in the news this week, the World Health Organisation was really expressing concern about the rise in antibiotic resistance that we're seeing. They're warning of this perfect storm that might lie ahead of us as we're using more and more antibiotics to treat people and in food production. These bugs are becoming resistant to it and we're sort of losing out in this genetic arms race. So, how can the kind of research that you're doing help to point us towards ways to help to combat this resistance, and could you actually use the information you're getting to develop better treatments?
Kat - And in the news this week, the World Health Organisation was really expressing concern about the rise in antibiotic resistance that we're seeing. They're warning of this perfect storm that might lie ahead of us as we're using more and more antibiotics to treat people and in food production. These bugs are becoming resistant to it and we're sort of losing out in this genetic arms race. So, how can the kind of research that you're doing help to point us towards ways to help to combat this resistance, and could you actually use the information you're getting to develop better treatments?
Estee - Yes, because obviously, if you have the genetic code of the organism, you have all the information that's available in terms of the antibiotic resistance genes. You can then design drugs that can be target against those in a way that you can't when you don't have the genetic codes. The other thing I think it's important is that it gives you a chance to survey the emergence of, for example, new pathogens or more drug- resistant pathogens in real time without having to send off bugs to the reference lab which can take weeks to get a result.
Kat - In terms of what you're doing now, this is a sort of "how long is a piece of string question", but how soon do you think this kind of sequencing might start to become more widespread in hospitals across the UK?
Estee - That's obviously the major challenge and certainly, that's what our group is working on; evaluating some of these new technologies to see if we can use them within a diagnostic microbiology laboratory and developing ways in which to make them a bit more user friendly. At the moment in order to analyse bacterial genome sequences, you often need a bioinformatician who's very experienced at doing that to give you the results in a way that you can understand. And over time, it is hoped that the machines that come out will have onboard computers that will actually be able to be useful to the practicing clinical microbiologist or infectious diseases doctor who will then be able to understand the data without the need for having a scientist to interpret it for them. So, optimistically, I would say 5 years or so, but I don't really know how quickly things will change. Certainly, the technology is there but the translation aspect may take a bit of time because you obviously have to train - a.) Improve the instruments, b.) Train people to use them.
Kat - Patients coming into Addenbrooke's, are they're having their bacterial genome sequenced?
Estee - Well they will be. So we're about to start a study based at Addenbrooke's which will involve sequencing all the MRSA bugs that we collect over the course of a one-year period from patients admitted to Addenbrooke's. We're also collecting bugs from the East of England and also, nationally in order to look at the geography and the diversity of MRSA in the UK and then to look more closely in Addenbrooke's at transmission of MRSA, both within the hospital and between hospitals in the UK.

09:41 - The Evasive Tactics of the Schistosome parasite
The Evasive Tactics of the Schistosome parasite
with Ed Farnell, Cambridge University
Chris - Parasites are organisms that live in or on another organism. They steal nutrients to survive. Nice! But living inside a host species, as many of them do, comes with its own set of problems. Parasites have to find ways to avoid the immune system and we now know that parts of the immune system whose job it is to get rid of parasites are the same parts that also cause allergies. Dr. Edward Farnell from Cambridge University's pathology department works on this and he is with us. So Ed, what does the immune system actually do to try to get rid of a parasite? Let's start there.
Edward - Yes, so parasites are very difficult for the immune system to deal with as other things, such as viruses and bacteria, are very small and the normal immune response to those is to send cells along which are actually capable of gobbling them up. They gobble them up, envelop them and then drop a load of chemicals on them and get rid of them.
Chris - So that would be bacteria and viruses.
Edward - Yeah, bacteria and viruses. They're very small and the immune system can do that. With parasites, certainly with the parasites we study, the worms, they're large and multicellular and there's no cell in your body that can come along and actually gobble it up.
Chris - It would be like you trying to engulf a shark or a blue whale!
Edward - Basically, yeah. So, the body has developed a more recently evolved part of the immune system to fight parasites and instead of trying to envelop them, it sends along a specialised type of cell which, when it encounters the parasite and the parasite has been marked in a way that says "this is something to be destroyed by an antibody" these cells then release a lot of toxic chemicals onto the parasite which is hopefully what destroys the parasite.
envelop them, it sends along a specialised type of cell which, when it encounters the parasite and the parasite has been marked in a way that says "this is something to be destroyed by an antibody" these cells then release a lot of toxic chemicals onto the parasite which is hopefully what destroys the parasite.
Chris - But it clearly doesn't work very well because if you look at the world population, a very high fraction, we're talking 10s of percent, are thought to be carrying parasites, especially worms, at any moment in time. So these parasites are obviously pretty resourceful at getting around what the immune system has to throw at them.
Edward - Yeah, they're very, very good at getting around what the immune system has to throw at them and it's done using a special antibody called IgE which recognizes special surface structures on the parasite. But obviously, if it can't see these structures, if the IgE can't bind to those structures, then the cells can't release the chemicals and they can't destroy the parasites. And so, the parasites have developed lots of very clever ways of avoiding that process. So, the parasite I work on is Schistosoma mansoni which causes the Schistosomiasis. It's a huge problem.
Chris - Bilharzia is the other name
Edward - Yes and that actually has the adult worms which live in the bloodstream so they're exposed to the immune system all the time. They see the immune system all the time.
They actually have a special outside layer called the tegument which wraps around them and actually hides the majority of the things that IgE can react against in the worms. Then it takes in bits of your own blood and coats itself in your blood. Your body's immune system is also very good at not destroying yourself, and you tolerise to your own antigens when you're very, very small and so, the worms coat themselves in this to protect them.
Chris - It's a massive game of subterfuge isn't it? They're disguising themselves as you. So, where are you in terms of understanding what this IgE is actually doing when it's going wrong? In other words, is there a way of stopping it going wrong so that it then can begin to re-attack the worm again because that's the answer to getting rid of the parasite, isn't it?
Edward - Yes, so a lot of the work we look at is understanding which antigens the IgE is binding to and at what point the IgE, this antibody, actually sees them. So when another worm dies, a lot of the antigens which were previously hidden then become visible. And then these are present in other stages of the lifecycle. So for example, when the worm is invading through the skin, those may actually be present on the surface and then you raise a response then. So the more we understand about the infection of humans, the more we understand about how you defend against it.
Chris - So does that mean, as someone lives with their parasite load, and these worms slowly die off of old age and then expose the various factors they had previously hidden, and you get this immune response, does that mean that the immune system does then get better over time at attacking them and eventually, does clear the parasite and is then protected?
Edward - So we do see an age protection effect. So the older people get, the more protected they become against the parasite and there's also various other things to do with the occupation and how much they're exposed to the parasite throughout their life.
Chris - Okay, so if we can understand actually how it's doing this because one of the things it's doing is fiddling with this IgE system and IgE is very important with allergy because when it goes wrong, we get all kinds of allergies. Does this mean then if you can work out how the parasite subverts that system, you could basically do the same trick in people who have too much IgE because they're allergic to things and deal with the allergy problem?
Edward - Yes, certainly, what we're trying to do at the moment on the current projects I'm working on is we're really interested in what the IgE is actually binding to on the parasite, and what we think is actually the targets that you see, the allergens that you see, look like parasites to your immune system. So that's why we're reacting against them. So we're looking at the moment on all of the known allergen structures and we're seeing if we can actually find the examples of IgE binding to those in a parasitic infection.
Chris - But what's intriguing is that if I have hay fever for example, which I do, I definitely get symptoms, but if I have a parasite, I don't necessarily get symptoms because of the parasite being there apart from the depleting effect it has and the debilitating effect it has on my body. So there's obviously something else to it.
Edward - Yeah, so there are factors that the parasites themselves also release which turn down the immune system and actually turn down the immune response, so some of our collaborators actually look at chemicals the parasite releases as it invades into the skin. And what you actually find is that some of these molecules that are secreted as the parasite comes suppress the local immune response. So you don't actually start to raise an immune response against that. But then there's also another effect which you see within the whole population where people actually raise different immune responses to the parasites. So genetic diversity means, some people respond to a parasite with a damaging reaction that looks very, very similar to severe allergy whereas other people actually, when they start to have very, very heavy parasitic burden start to turn down their immune system to stop themselves from hurting themselves.
Chris - Do we know why?
Edward - No, we don't. There's almost certainly a genetic component to it and there's almost certainly an exposure component to it. So it probably has a lot to do with when you were first parasitized, the state of your immune system as you're first parasitized, and then a whole host of other genetic factors which means that you may well produce what we call a modified response, whereby, you turn down your immune reactions which is beneficial to both you and the parasite. I mean, that's what the parasite wants as well. The parasites, they're very, very greedy. They want you all to themselves. They want to get in and stop other parasites from coming in and they also want to keep you as well as possible. So we could consider that to be what must be the normal thought of response.
Chris - Because this whole idea of the hygiene hypothesis where people who are infested with parasites have a lower risk of allergy. Is that real and is there something really going on? Is it that the parasite actively being in a person suppresses the immune response in this way or is it that the environment in which people acquire parasites makes people less likely to develop allergies overall?
Edward - I think it's much more complicated than we used to think it was. There have been several studies released that show that people are protected by having a worm parasite and the worm is doing something. There is some cross reaction between their immune system. There are other studies that actually show or suggest that having a worm might not be so great for you or it may not actually suppress any allergy at all, and the key to understanding that is really understanding the similarity between the structures of allergens and the structures of the antigens that are being reacted against on the parasite by the immune system.
Chris - So, how far away are you, do you think, from nailing this? So that we're in a position where we can say, "We understand what the parasite does to get around the immune response" and then we can turn the equation around and use this usefully to a.) Treat parasite problems and b.) Treat allergy problems?
Edward - With the best will in the world, I'd like to say we're really, really close and we'd love to go up to say, yeah we can do this, but I think we need to understand a lot more about the interactions because they're really complicated. The complex, but fascinating, interactions of how this happens.
Chris - Thank you very much. That's Ed Farnell. He is from the Department of Pathology at Cambridge University.

17:38 - Eyes vote no to space travel
Eyes vote no to space travel
Head scans of astronauts have shown signs of raised intracranial pressure affecting their eyes and pituitary glands, new research has revealed...
Writing in the journal Radiology, University of Texas Medical School, Houston radiologist Larry Kramer MRI scanned 27 spacefarers who had each notched up an average of 108 days in orbit.
The imaging showed signs of small cavities in the pituitary glands of three astronauts, flattening of the backs of the eyeballs in six cases, increased fluid around the optic nerves in nine individuals and bulges in the optic discs inside the eye in four cases.
These are features that would normally characterise increased intracranial pressure and they go some way towards explaining the basis for something that NASA has known for a while: that in the aftermath of exposure to microgravity, astronauts have an above-average chance of experiencing vision problems.
In fact, 30% of short-duration space trippers and 60% of longer-duration orbiters report sight loss symptoms.
The researchers speculate that the changes are caused by fluid shifts that occur inside the head in response to chronic microgravity exposure. Fluid that would normally collect in the tissues of the extremeties under the influence of gravity instead builds up inside the head. This is the same reason that astronauts often characteristically become temporarily puffy-faced in space.
But what is intriguing to NASA is not the 60% who do suffer problems but the 40% who don't. Studying them might reveal why they are relatively protected from the problem and lead to ways to protect those at risk, or identify individuals best suited for longer space missions.
Sending someone to Mars is likely to involve a year in space, so solving problems like this will be critical to the success such missions. In the meantime, NASA are proposing to carry out pre- and post-flight MRI scans of their astronauts on Earth and then monitor them in orbit using ultrasound scans of their eyes.
21:17 - Still no clear answers on PSA testing
Still no clear answers on PSA testing
March is widely recognised as prostate cancer awareness month - a disease that affects nearly 40,000 men in the UK every year, and many hundreds of thousands more worldwide. But while we have screening programmes over here for breast, cervical and bowel cancer, we don't have a national screening programme for prostate cancer, even though there is a blood test - the PSA test - which measures the levels of PSA, a protein produced by the prostate gland that can be raised in men with prostate cancer.
The reason for this is because doctors simply don't know how reliable the PSA test is. The problem is that PSA levels can be raised by a number of non-cancerous conditions, so it's not very specific. And there's also a small but significant proportion of men with prostate cancer who don't have raised PSA levels.
 And finally, even if a man does have a raised PSA level, and does have prostate cancer, it's hard to know whether it's an aggressive cancer that needs urgent treatment - and along with that comes the risk of serious side effects - or a slow-growing cancer that can be safely monitored over time. But if the test does pick up aggressive cancer at an early stage, it can save a man's life.
And finally, even if a man does have a raised PSA level, and does have prostate cancer, it's hard to know whether it's an aggressive cancer that needs urgent treatment - and along with that comes the risk of serious side effects - or a slow-growing cancer that can be safely monitored over time. But if the test does pick up aggressive cancer at an early stage, it can save a man's life.
Because of this confusion, many research teams around the world have been carrying out large studies to find out whether the benefits of PSA testing across large populations outweigh the risks. But, frustratingly, new results following up a big study of prostate screening don't provide any firm answers.
These are results from the European Randomised Study of Screening for Prostate Cancer - known as the ERSPC - which was set up in 1991 to try and figure out the effectiveness of PSA testing, looking at men in the Netherlands and Belgium, as well as Sweden, Finland, Italy, Spain and Switzerland.
These new results - following up more than 180,000 men after 11 years - have just been published in the New England Journal of Medicine. In the study, the men were divided into two groups - one were given PSA testing roughly once every four years, while the other group were left unscreened - but, of course, were free to go to the doctor if they noticed any unusual symptoms.
The researchers discovered that the death rate from prostate cancer was just over 20 per cent lower in the men who were PSA tested than untested men. But after further calculations, the scientists showed that in order to prevent one death from prostate cancer during the 11-year period, more than 1,055 men would need to be invited for screening, with nearly 40 cancers detected.
This chimes with results from the same study published two years ago. Meanwhile, a similar study in the US has also found that PSA testing has little impact on saving lives from prostate cancer, but may be leading to men being more likely to have treatment causing side effects such as impotence and incontinence for a cancer that may not be aggressive.
At the moment, men in the UK are advised to discuss PSA testing with their GP, who can outline the risks and benefits. But this study - and the others like it around the world - only serve to highlight the problems with the PSA test, and the urgent need to develop better tools for detecting aggressive prostate cancers at an early stage that will save lives, while avoiding men having to go through unnecessary treatment for tumours that are unlikely to be dangerous.
25:04 - How the Intestine Informs the Immune System
How the Intestine Informs the Immune System
with Dr Mark Miller, Washington University
Chris - This week, how the intestine tells harmless food items from potentially harmful bacteria and parasites has been revealed by researchers in the US. Professor Mark Miller at Washington University in St. Louis was using a technique called two-photon imaging to study the intestines of living mice. These animals were special because one class of their immune cells had been made to glow, so they could be seen easily. These are cells called dendritic cells or DCs and they're known as antigen presenting cells because their job is to educate the immune system about what it should attack or ignore. But when Mark then fed these animals samples of sugars that had glowing labels so that he could follow how those food components got moved across the intestinal wall and introduced to the immune cells, he wasn't quite expecting to see quite what he did...
Mark - The surprising finding was that the cell that does this is a highly secretory cell. It's called the goblet cell and its primary function is believed to be secreting mucus that provides a barrier or protection to the epithelial layer. What we found is that these cells, as they secrete, they also allow some of the luminal contents to be transported across the epithelium. So this is different from the process by which you would absorb nutrients or food. It's a way to deliver very concentrated amounts of an antigen to antigen presenting cells, and it's a new function that we've discovered for the goblet cell which has been studied for a very long time.
 Chris - Now you looked at one particular tagged antigen. Obviously, it's slightly more complicated when we're eating a balanced diet and there are lots of things coming in. So, have you got any idea as to whether or not those goblet cells discriminate between the good guys and the things we want to educate our immune system to ignore, and the bad stuff that we actually want the immune system to attack or do those goblet cells transfer everything and the immune system makes that decision?
Chris - Now you looked at one particular tagged antigen. Obviously, it's slightly more complicated when we're eating a balanced diet and there are lots of things coming in. So, have you got any idea as to whether or not those goblet cells discriminate between the good guys and the things we want to educate our immune system to ignore, and the bad stuff that we actually want the immune system to attack or do those goblet cells transfer everything and the immune system makes that decision?
Mark - That's a fascinating question. What we think is that their transporting mostly small soluble peptides, so these can be parts of a protein or intact proteins. It can also be things like sugars, so we're using dextran as one of our model antigens. So I think if there's any discrimination, it's that it would prevent something large like maybe an intact bacterium from getting across but allow these small soluble antigens to actually get across. But on the other hand, once the antigen comes across the epithelium, the antigen presenting cells themselves have specific receptors to take up sugars or certain proteins. So, even if there's a lack of specificity at the goblet cell step, the dendritic cell itself will be better at taking up certain substances and that could also lead to a different outcome in terms of an immune response.
Chris - If I have a healthy gut and I'm just presenting normal food stuffs then I can understand that being an absolutely perfect mechanism. But what about when I get a dose of Delhi belly or Montezuma's revenge, I get bacteria there that I shouldn't have or an overgrowth of even a parasite. Surely, there will then be antigens going across the wall of the gut. How do you stop the immune system saying these are friendly? How do you make the immune system on that occasion decide to attack that foreign antigen?
Mark - It's interesting that we've seen that this function of goblet cells appears to be downregulated when you have a pathogenic infection, so it's as if the barrier, the mucosal barrier, tightens up. So that's one response, but the fact that the antigen is coming in with a pathogenic organism will cause inflammation, pathogen associated molecular patterns will stimulate dendritic cells in such a way that they promote an inflammatory immune response. So again, that would happen at the level of the dendritic cell.
Chris - And what about in allergy states and other disease states? We know that there's an association if you give very young kids big doses of broad spectrum antibiotics that clear out lots of the bacteria that should be there then they're much more likely to develop allergy and diarrheal states later in life. Have you got any clues from the mechanism you've spotted how that sort of thing might actually be manifest and why it happens?
Mark - Yes, we do. So for example in the paper, we used germ-free mice that lack the normal flora that would colonise the small intestine. And in that case, we saw quite a lot of antigen being transported across. So, that experiment is showing that the normal flora or the bacteria in the gut may very well regulate this process. So depending on what species of bacteria are present or whether or not it's pathogenic or non-pathogenic, that could tie in very closely with how much of that antigen is delivered across the epithelium and change the character of the subsequent immune response.
Chris - And are you in a position now to manipulate this system? Do you understand what the trafficking system is, so that you can go in and intervene, and stop things that might provoke an allergy or indeed in someone who has an established allergy, stop it presenting itself to the immune system, so that person's allergy goes away?
Mark - We are definitely working in that direction. It turns out that we think this transport function is directly related to goblet cell secretion. And of course, we have both agonists and antagonists to either induce secretion or inhibit secretion. So, we are looking in various models right now to see how either shutting down this goblet cell mediated antigen transport or upregulating it may affect outcomes in models of inflammatory bowel disease for example.
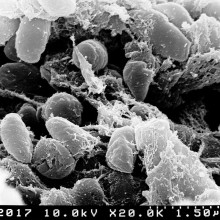
30:44 - Nanoparticles turn bacteria into superbugs
Nanoparticles turn bacteria into superbugs
Nanoparticles used routinely in wastewater cleanups can encourage bacteria to breed superbugs, new research has shown. The findings could have major implications for the way in which sewage should be processed in order to prevent the appearance of bacteria equipped to resist every known antibiotic.
 Writing in PNAS, Institute of Health and Environmental Medicine scientist Zhigang Qiu and his colleagues measured the amount of gene-swapping taking place between bacteria incubated with a range of common industrially-employed nano-particles. Alumina (Al2O3), titanium dioxide (TiO2), silicon dioxide (SiO2) and iron oxide (Fe2O3) were tested. All of them promoted the exchange amongst the bacteria of a test DNA plasmid called RP4, with alumina particles producing the largest effect - up to a 200-fold increase in the rate of successful DNA exchange compared with bugs grown without nanoparticles.
Writing in PNAS, Institute of Health and Environmental Medicine scientist Zhigang Qiu and his colleagues measured the amount of gene-swapping taking place between bacteria incubated with a range of common industrially-employed nano-particles. Alumina (Al2O3), titanium dioxide (TiO2), silicon dioxide (SiO2) and iron oxide (Fe2O3) were tested. All of them promoted the exchange amongst the bacteria of a test DNA plasmid called RP4, with alumina particles producing the largest effect - up to a 200-fold increase in the rate of successful DNA exchange compared with bugs grown without nanoparticles.
The gene transfers were also not limited to bugs of the same species. Mixtures of E. coli and Salmonella bacteria also increased their rates of gene swapping in the presence of the nanoparticles. The effect appears to be the result of biochemical stress. Electron microscope images show that the nanoparticles damage the bacterial cells, activating a range of protective responses, including a preponderance to swap sequences of genetic material.
Worryingly, the concentrations of nanoparticles needed to achieve this (about 2 million per millilitre) are much lower than those presently used industrially to scrub heavy metals and other contaminants from waste water. So present processing practices could be encouraging bacteria that harbour antibiotic-resistance genes to disseminate their dangerous DNA cargo.

33:10 - Predicting how drugs interact and driving flies to drink
Predicting how drugs interact and driving flies to drink
Predicting how drugs interact to produce side effects
Scientists have come up with a new way to anticipate previously hard to predict drug side effects. During their development, most drugs are tested in isolation, meaning a patient only takes one drug at one time. But, in the clinic, most patients end up being prescribed mixtures of different drugs at once to treat a range of diseases. And predicting how these mixtures might lead to side effects has always been extremely difficult. But now, writing in Science Translational Medicine, researchers at Stanford University have used data from four million patients to design a statistical system that can spot when potential problems might arise, including between combinations of drugs in routine current use.
Nicholas Tattonetti - We identified a potential interaction between thiazides that treat hypertension and selective serotonin reuptake inhibitors which are antidepressants, and we associated them with an increased prolonged QT interval and this is a clinical risk factor for heart arrhythmia. So it's a potentially important clinical variable.
Tatonetti NP et al (2012), Sci Transl Med, 4, 125, Data-Driven Prediction of Drug Effects and Interactions
A new uranium compound has been produced which could help mop up nuclear waste
A new form of uranium could make radioactive waste easier to reprocess in the future. Generating electricity from nuclear power inevitably produces radioactive materials that need recycling or long term storage. At the moment the estimated cost of clearing up just the UK's accumulating stock of nuclear waste is 70 billion Pounds. But now, Edinburgh University chemist Polly Arnold and her colleagues, writing in the journal Nature Chemistry, have discovered a new way to make clusters of uranium which may make these valuable radioactive materials easier to recycle.
Polly Arnold - One of the things that happens when you process nuclear waste is you need to separate out all the different metal components, the metal oxides. Things that can cause problems is when they cluster and form aggregates. But people don't know very much about how these form and then how to get rid of them. So the fact that we've seen this tiny beginning of a cluster suggests that it might lead us to think about new ways the clusters might form and then new ways to deal with the nuclear waste processing that goes on at the moment.
Arnold PL et al (2012), Nature Chemistry, 4, 221-227, Strongly coupled binuclear uranium-oxo complexes from uranyl oxo rearrangement and reductive silylation
Treating manic depression by increasing the strength and length of the body clock
Scientists have uncovered how the drug lithium, which can help sufferers of bipolar disorder, or manic depression, as it is also known, actually works. Lithium has been one of the main treatments for bipolar disorder for the last 60 years. But exactly how it works has remained a mystery. Now, writing in PloS One and using cells cultured from mice, scientists have shown that lithium achieves its therapeutic effects by strengthening the body's circadian clock, which it does by switching off a signalling enzyme called glycogen Synthase Kinase 3 beta. In people with mood disorders this enzyme has been shown to be overactive. The discovery could have implications for other neurological disorders.
Jian Li et al., (2012) PloS One Lithium Impacts on the Amplitude and Period of the Molecular Circadian Clockwork. e33292. doi:10.1371/journal.pone.0033292
Fruit flies deprived of sex turn to alcohol
Flies deprived of sex turn to alcohol, scientists have shown this week. One group of male flies were offered multiple mating opportunities. A second group were repeatedly rejected by a group of already sexually satisfied females. Provided with a choice of foods that either did or didn't contain alcohol, the flies that had been repeatedly rejected were much more likely to opt for the booze soaked dish.
Galit Ophir - This is a basic science study so it tells us better about how the brain represents social experience in terms of reward and it has implications into understanding better the mechanisms that are involved in social reward which is important to social related disorders and also to addiction. That study was published in the journal Science.
G. Shohat-Ophir, et al., (2012) Science, 335, 6074: 1351-1355. Sexual Deprivation Increases Ethanol Intake in Drosophila.

37:17 - The American Signal Crayfish Invasion - Planet Earth Online
The American Signal Crayfish Invasion - Planet Earth Online
with Martin Christmas, Environment Agency, Alison Dunn and Neal Haddaway, University of Leeds
Chris - Native white-clawed British crayfish are in trouble. Weakened by a parasite, this endangered species is being driven out of waterways by the North American signal crayfish. Planet Earth Podcast Presenter, Richard Hollingham, headed to Yorkshire to meet Alison Dunn and Neal Haddaway from the University of Leeds and, first, Martin Christmas from the Environment Agency. As they stood beside a stream (or as it's Yorkshire - a beck), Richard asked Martin what damage the American imposter was doing...
Martin Christmas - Signal crayfish because they're bigger, they're more aggressive. They tend to out compete for the same habitats, so white clawed crayfish get pushed out. Signal crayfish also bring with them the crayfish plague which they can carry but aren't so susceptible to, but native crayfish really do suffer from plague outbreaks, it wipe them out. So, those are two of the conservation issues we've got. Also, from the Environment Agency's perspective, signal crayfish are really good diggers, particularly in soft banks, and they can cause expensive problems in terms of undermining banks, bank collapses and maintaining flood banks is something that we invest a lot of money in every year.
Richard Hollingham - There are two things going on here, Alison, the fact that these signal crayfish are causing damage but also they are carrying this disease which affects the native crayfish.
Alison Dunn - There are two different diseases that are important for who wins that competitive interaction. There's the plague that Martin referred to, which the signal crayfish have brought to this country and it kills the white clawed. The other thing that's fascinating that we're looking at is a parasite called porcelain disease that changes how the native crayfish is able to feed. It affects its behaviour and its ability to catch its prey and as a knock on effect its ability to compete with the invader.
Richard Hollingham - It sounds a bit like the red squirrel, the native red squirrel and the grey squirrel, the American invader.
Alison Dunn - It is - it's a very similar situation. The plague is analogous to the squirrel pox virus, but the effect that we're looking at, the parasite that we're looking at - porcelain disease - is actually a native parasite and it only seems to affect the behaviour of the native crayfish. It makes it more sluggish, less able to catch its prey which Neil will tell us about.
Richard Hollingham - So, what does your research involve? What were you looking at?
Neil Haddaway - This particular research that we did was some lab based studies looking at the amount of food that the crayfish were eating. In particular we were interested in comparing the amount of food that the invasive crayfish ate, the amount of food the native crayfish ate and looking at the effect of the disease on the native. And what we found was that the invasive crayfish ate about 83% more food, but not only that it showed very little choice in what it was eating. The native crayfish with the disease ate, on average, 30% less and it also ate slower, so it was changing the way that it was eating. But not only this it was also changing it's choice of prey, so instead of going for fast moving prey the diseased crayfish were preferring animals that walked along the bottom as they were easier to catch.
Richard Hollingham - So really, the native crayfish was losing out food wise on all counts?
Neil Haddaway - Definitely, the native crayfish can't really compete when it comes to eating food with the invasive and the disease really did knock it for six.
Richard Hollingham - So how do you use research like this, Alison?
Alison Dunn - Understanding the processes going on in an ecosystem and understanding how important disease can be in modifying interactions, whether it's between an animal and its prey or between an animal and another one with which it competes are important to understanding how ecosystems are made up. And biological invasions are enormously important economically and in terms of biodiversity across the whole globe. In terms of losing our diversity of animals and plants they are second only after habit destruction as being a cause of loss of species across the globe. So by understanding in this small beck how disease modifies the interaction between the native and invasive we could develop our understanding at a much broader level of invasive species and the importance of disease.
Richard Hollingham - So coming back to the white clawed British crayfish, does that mean you can save it?
Alison Dunn - The white clawed crayfish will continue to decline both as a result of interactions with the signal and with the disease but I think the Environment Agency have strategies to try and slow the spread of the signal crayfish and to develop isolated populations where we can conserve our native species.
Richard Hollingham - So all is not lost?
Alison Dunn - No, all is not lost.
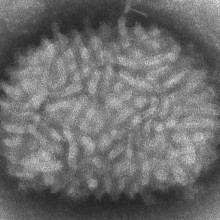
42:14 - The Spread of a Virus
The Spread of a Virus
with Geoff Smith, University of Cambridge
Chris - Now, we're turning to some of the tiniest organisms that are known to exist and those are viruses that have been very well studied over the last 50 years, so it's something of a surprise that no one had noticed that some viruses seem to be able to spread far faster than they ought to be able to. And now, Cambridge virologist Geoff Smith and his team have discovered why.
Geoff - We study pox viruses and the virus that we've used most in all that is called vaccinia virus and that was the virus that was used to eradicate smallpox from the whole world. One of the things that we started to do a few years ago was to look at how the virus spreads and how it seems to do so, so very quickly. And so, we just made some very simple measurements of the rate at which the virus would spread from one cell to another, across a lawn of cells that were just growing in the lab. So what we found was, it was spreading across each cell in just over one hour and when we got that measurement, we really stopped and scratched our heads because it didn't make any sense because we knew that the virus takes at least 5 hours to replicate in each cell. So before you can make any new virus particles after you've infected a cell, you have to wait 5 to 6 hours before the very first particles are made.
Chris - But in your experiments, it appeared that the virus was spreading 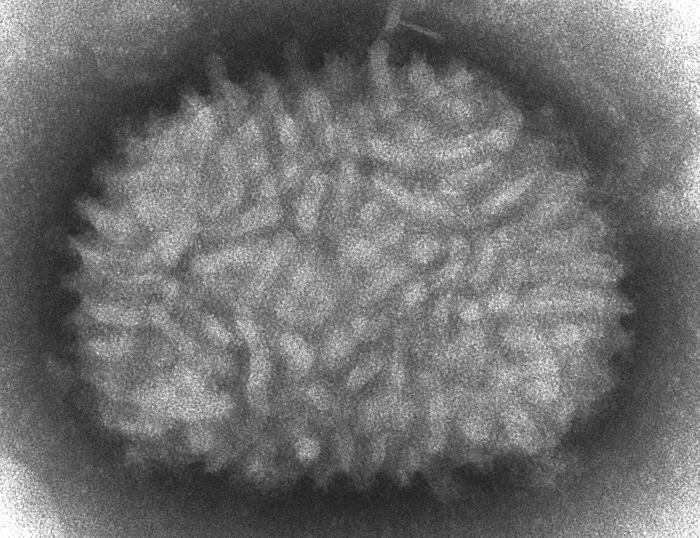 faster than it was being made.
faster than it was being made.
Geoff - That's right. The numbers didn't add up. The virus was spreading about 4 or 5-fold faster than could be predicted from what was known about its replication rate.
Chris - So how did you take that forward? Because that's obviously a really fundamental and important finding? It could either be totally wrong or there's something very ingenious going on.
Geoff - Well up to the time we did our study, it was thought that the viruses were only using the host cell transport machinery to accelerate the rate at which newly formed virus particles were being released from the cell in which they had been made.
Chris - So you make the virus in the cell then you use the cell's machinery to expel that virus onto an adjacent uninfected cell and that's why you get the spread?
Geoff - Yeah, there are two parts to the virus hijacking the cell biology to accelerate spread. The first is, that it uses a network of tubules called microtubules inside the cell which are the cell's normal transport machinery for moving cargo around inside the cell, and the virus hooks up to this transport system and exploits it to get the newly formed virus particles out to the cell surface rapidly. So that's the first part, and then once the virus gets to the cell surface, it then will induce the formation of a protein in the cell called actin which is important for giving the cell its structure and it induces the polymerization of actin so that these growing actin filaments will physically propel the virus away from the cell's surface towards uninfected cells that it can subsequently infect.
Chris - The virus comes out from the cell on almost like a stalk towards adjacent cells?
Geoff - That's right and then you can see these by videos. If you label up the virus in a way for instance by fusing it to a fluorescent protein, you can follow the virus moving around inside cells and between cells by live video microscopy. They make very good pictures.
Chris - I'm sure they do. So that much was known and that doesn't actually explain how you could marry up the observation you made, the viruses are spreading much more quickly than they should with actually what was going on then.
Geoff - That's correct because the ability of the virus to exploit actin to push the virus particle away from the cell in which it had been made only happens very late in infection, about 5 or 6 hours or later. So that is too late to explain the very rapid spread from cell to cell, so there had to be another mechanism.
By looking more deeply into what the virus was doing, we found that once the virus had left the original cell in which it had replicated, and it had then entered the adjacent cell, shortly after that cell was infected, the virus expressed two proteins which we knew formed a complex and that complex is transported to the cell's surface which essentially marked that cell as being infected already. And the consequence of that was that when additional viral particles trying to re-infect or superinfect that same cell came into contact with that complex on the cell's surface, that induced the formation of additional actin tails which repulsed the superinfecting virions and pushed them away towards uninfected cells.
Chris - Gosh! So it's almost like a virus comes in, lands on a cell that has already been infected but isn't yet making virus particles, can detect that the cell is infected and then bounces off in the direction of other potentially infectable, but so far uninfected, cells.
Geoff - That's exactly right and it's almost as if the virus infection is marking the newly infected cell as infeicted and saying to the additional viral particles, "Go away! There's no point comng here. I'm infected already. You need to go elsewhere." And in fact, this is a common theme in virology. Many viruses have mechanisms by which they restrict the re-infection of an already infected cell. But what was different in this study was that the virus didn't just stop the virus particles re-entering the same cell. They physically repelled them. So as you say, they bounced, or surfed, across the surface of the infected cell until they would come to a cell that was uninfected which there was no restriction upon them infecting.
Chris - Given that this gives the virus an advantage and you now know how it does it, disabling it could therefore be quite a good target to treat these viruses with drugs.
Geoff - Indeed so. We know for instance that if we remove the genes which encode the proteins that enable this rapid spreading mechanism to take place, number one, the virus spreads much more slowly in cultured cells and it is also a virulent. It can no longer induce disease. So that one could build attenuated vaccines perhaps in this way - that's one angle. A second one would be to design molecules which would interfere with this spreading mechanism. And in fact, you could take the parts of the proteins which mediate this mechanism themselves. And if we were to produce a large amount of that protein in soluble form, that would compete with the receptor bound on the cell's surface and so, prevent the rapid spread that the cell bound protein would otherwise induce.

How many strains of MRSA are there?
Estee - We don't actually know how many strains of MRSA there are. They're incredibly diverse organisms and there are likely to be hundreds of thousands really, but as we haven't sequenced all that many, we don't know exactly how many there are in the world.
Chris - It's not a new problem though is it, something that's been around for quite a few years, 30, 40 years.
Estee - Absolutely, but the previous typing methods that we have are relatively blunt instruments and they can group them into sort of groups of strains but we don't know how many individual strains there are.
Can we have multiple-bacteria infections?
Estee - At the moment, the whole genome sequencing that we're doing can only be done from a pure bacterial culture. We usually know in that situation that when we pick a colony, we pick one or two colonies from a plate, we can be pretty certain that it's a single strain, so a single bacterial isolate. However, patients can obviously present with polymicrobial infections and again, we'd be able to tell that from looking at the agar plate. There'd be bugs that look different on the plate. But at the moment when we sequence them, we pick one or two colonies of identical looking organisms and we know then that we're sequencing one strain.
How does the herpes virus hide when it's inactive?
It hides inside nerve cells. It exists as a small piece of DNA, which is just the genetic material for the virus, hiding inside the nerve cell's nucleus alongside the DNA in that cell. As a result, it's out of reach of the immune system and because it's not making any proteins that the cell can display on its surface, the immune system doesn't tend to touch it. It can then use the genetic recipe book in that DNA to come back to life later, producing new virus particles that go back down the nerve cell, come out on the skin, and produce an infectious lesion that is unsightly, but is also highly infectious and you can pass it on.
What are the most common illnesses caused by pathogens?
Edward - Parasites are very, very high on the list and certainly, worms at least. There's a great quote from a textbook about nematodes and roundworms:
["In short, if all the matter in the universe except the nematodes were swept away, our world would still be dimly recognizable, and if, as disembodied spirits, we could then investigate it, we should find its mountains, hills, vales, rivers, lakes, and oceans represented by a film of nematodes. The location of towns would be decipherable, since for every massing of human beings there would be a corresponding massing of certain nematodes. Trees would still stand in ghostly rows representing our streets and highways. The location of the various plants and animals would still be decipherable, and, had we sufficient knowledge, in many cases even their species could be determined by an examination of their erstwhile nematode parasites."
"Nematodes and their relationships" Cobb, N.A; Yearbook of the United States Department of Agriculture, 1914, pages 457-490.]
They're everywhere, they're in the soil, in plants, trees, animals, humans, parasites living free. They're incredibly ubiquitous."
Chris - Most toddlers I think pick up things, don't they? And what about TB Estee? Because this is a serious problem?
Estee - I would have to argue from the bacteriology side that 1 in 3 people in the world is infected with Mycobacterium tuberculosis. That's the bacteria that causes tuberculosis and I would have to be high up there on the list of common pathogens.
Can viruses combine to create a Super Virus?
Infectious diseases consultant Estee Torok answered this infectious question...
Estee - Absolutely. I mean, so there was a case report I think in the New England Journal of Medicine a few years ago of exactly that - a man who presented with HIV infection and then was subsequently re-infected with another strain and had a faster disease progression and died in fact.
Chris - Because once you've got one strain, if you add another one on top then they can share genes between the viruses and you end up with a virus that's got all of the worst bits of both.
Estee - Absolutely.
54:03 - Can you catch cancer?
Can you catch cancer?
Hannah - Let's kick off with sex and yes, cancer can be caused directly by infection with sexually transmitted viruses including the human papilloma virus that causes cervical, anal, and throat cancers. With more...
Margaret - Margaret Stanley in the Department of Pathology at the University of Cambridge. Human Papilloma Viruses are very, very common infections and there's a set of viruses that infect the genital tract and the oral cavity in men and women. 80% of us will have had, or will get, or currently have the infection. It's a very, very common infection, but only about .001% of people who are infected will actually develop the cancer. In women it develops into cancer of the cervix, the second commonest cancer in women worldwide. In men, a rare cancer, cancer of the anus and that's common in gay men. But also, cancer of the tonsil both in men and women but much more common in men than women - 5 times more common, and I have to say, increasing in incidence. What's important is how you get this virus - it's a sexually transmitted infection. And so, with changing sexual practices and behaviour, these cancers are actually becoming more common.
Hannah - And what about catching cancer through donated organs?
James - I'm James Neuberger. I'm Associate Medical Director of Organ Donation and Transplantation in NHS Blood and Transplant. In very, very rare cases, it is possible that the organ that is transplanted will contain cancer cells from the donor. We do our best to screen for this and prevent it, but we cannot prevent it entirely, however good our screening tests are. The second point is that immunosuppresion, which nearly all transplant recipients acquire lifelong, does carry an increased risk of some cancers and it's important that both the patients and their doctors are aware of this increased risk.
Hannah - There's also a risk of picking up cancer through viruses in blood like hepatitis B and C which can trigger cancers in some individuals and can be passed on in donated blood. Although thanks to screening programmes, the risk of this in developed countries is very low. And what about biting? Well, Tasmanian Devils catch cancer on the face through biting, physically transferring cancerous tissue from one Devil to the next. With more.. Elizabeth - Hello, my name is Elizabeth Murchison. I'm at the Wellcome Trust Sanger Institute near Cambridge. So it's actually one single cancer which is transmitted from one animal to another. The Devil's cancer is not recognised or rejected by the new host's immune system even though it's a foreign graft. There are some very rare examples of cancers which are transmitted in this way in humans and these are often associated with mothers getting cancer that are then transmitted through the placenta to the foetus, and even more rarely, vice versa - the foetus develops the cancer which is transmitted to the mother.
Hannah - So, Tasmanian Devils catch cancer through biting whilst humans can catch cancer through sex, transfer between mother and foetus, and there's also a small risk of catching cancer from a donated organ. Thanks to Dr. Elizabeth Murchison, Professor James Neuberger and Margaret Stanley for clearing up that contagious cancer question.










Comments
Add a comment