On this weeks Pan-Continental Naked Scientists we bring you the latest science news from the AAAS conference in Boston. We hear about why cholesterol-lowering drugs are good for us but bad for bacteria, see the map that shows mankind's effect on the oceans and discover the surprisingly complex science of sand castles. Also in this week's show, we look into the future for organ transplants - how bone marrow grafts could make rejection a thing of the past, keeping organs alive outside the body and how we can grow a beating heart in the lab! Plus, in Kitchen Science, we find out how to prove that blood circulates around the body!
In this episode
Ocean Impact Map
A new study out this week has revealed that there is literally no-where to hide when it comes to the damaging affects we are having on the seas and oceans, and that over forty percent of the world's marine ecosystems are suffering from very high levels of threat.
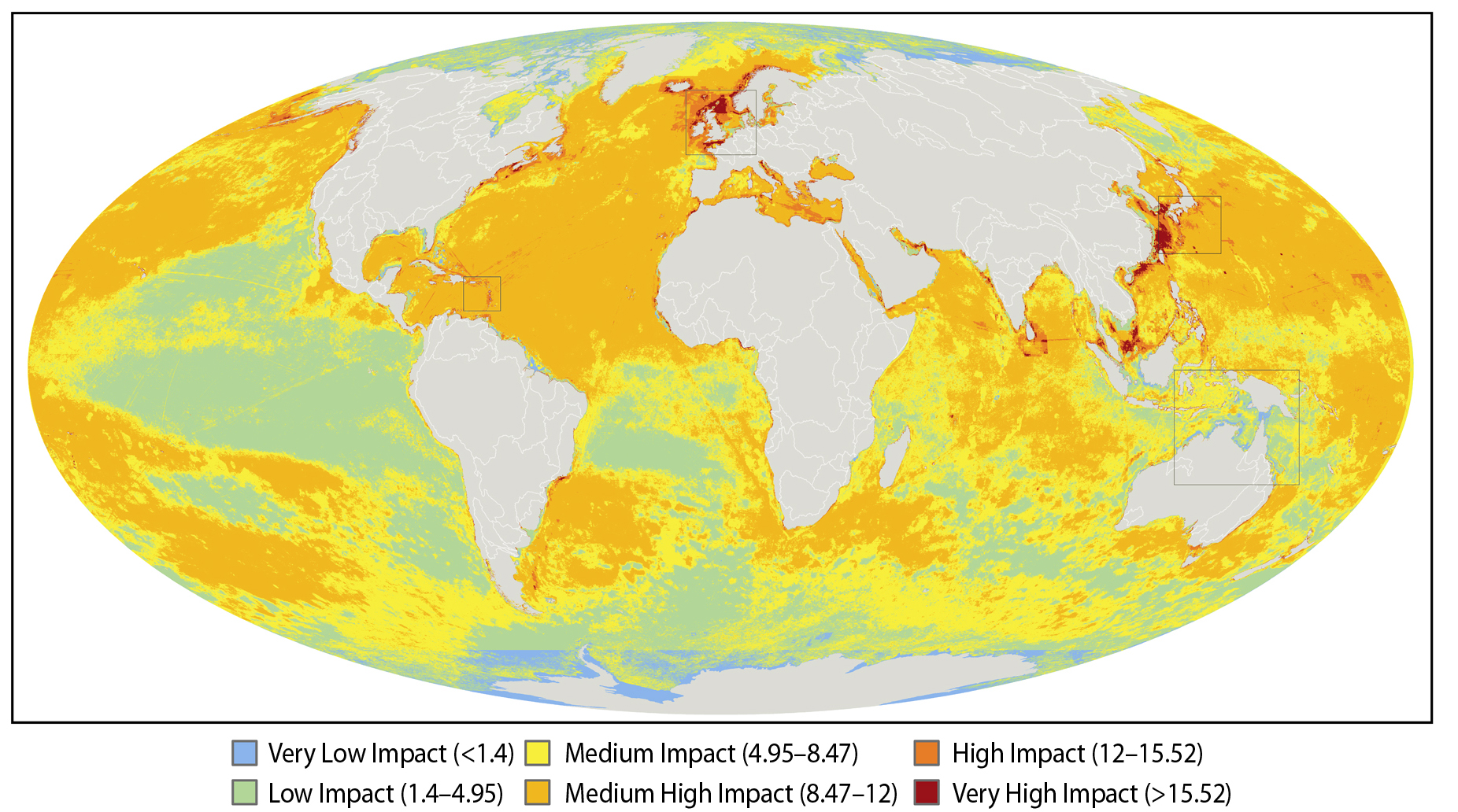
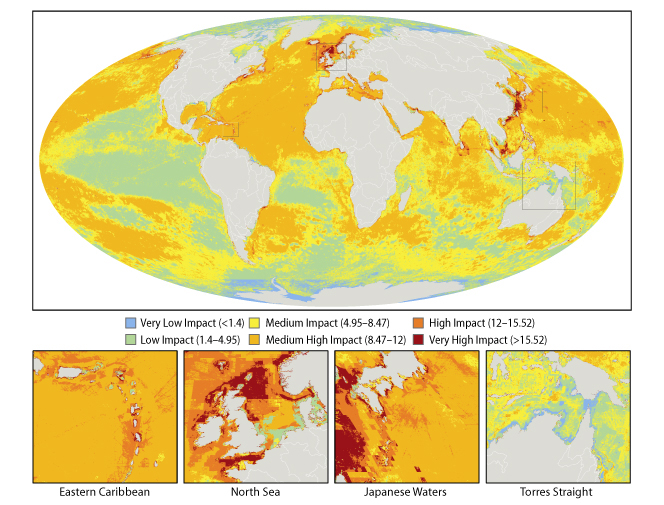 A team of 19 international scientists have drawn up the first ever map of the oceans showing where we are affecting them and by how much.
A team of 19 international scientists have drawn up the first ever map of the oceans showing where we are affecting them and by how much.
The study was presented this week at the AAAS in the United States - we'll be hearing more news from that meeting in a few minutes from Chris.
To gauge the global impact of human activities on the ocean the team looked at lots of different threats, including over-fishing, pollution, invasive species and global warming. They put together detailed information about the distribution of these threats across the water bodies of the world, and then added the affects of all these threats together, so that every square kilometre of the oceans was assigned an impact rating, ranging from low to high impact.
If you take a look at the map you'll see that a lot of the oceans are coloured in yellow and orange - these are areas with a medium to high level of impact. There is quite a bit of green which are areas with a relatively low impact going on, and just a few tiny patches that you can hardly see of blue, which are areas that suffer from only a very low impact, and these are clustered around the north and south poles. What are obvious are the patches of bright red, which are the really bad zones where multiple threats are having a great impact - these include the shores of the British Isles in the north sea, the Mediterranean, the Caribbean on the eastern seaboard of the United States, as well as in Asia and the Persian Gulf.
What this map does, apart from get us rather depressed about the state of the oceans, is hopefully something more positive - which is to give us see a bigger picture of what's going on and hopefully to allow us to better plan how best to protect the oceans from the ongoing impacts of the world's growing human population.

Science of Sandcastles
Here is a nice story showing the cutting edge science you can find in the the most everyday items. You have probably made lots of sand castles in your life, but have you ever wondered why it is so easy? It's not necessarily easy to make the fortification of your dreams, but it is very easy to make damp sand stick together, you don't have to mix sand with water in a specific recipe, it just seems to work. In fact if you mix between about 1% and 20% water by volume with your sand the strength of the mixture is almost constant. This is surprising because that is a huge change in the amount of water.
 You may not have asked this question but Mario Scheel in the Max Plank Institute in Gottigen, Germany did. Finding out the answer is quite difficult, because it is a three dimensional problem and sand is opaque so it is very hard to view what is going on. He and his colleagues managed to look inside a sand pile using X-ray microtomography. This is basically a miniature version of a CAT scanner you find in hospitals. It takes a series of X-ray images from different directions, and then uses a computer to put them together into a 3D model of what is going on.
You may not have asked this question but Mario Scheel in the Max Plank Institute in Gottigen, Germany did. Finding out the answer is quite difficult, because it is a three dimensional problem and sand is opaque so it is very hard to view what is going on. He and his colleagues managed to look inside a sand pile using X-ray microtomography. This is basically a miniature version of a CAT scanner you find in hospitals. It takes a series of X-ray images from different directions, and then uses a computer to put them together into a 3D model of what is going on.
They took many 3D images of piles of sand with different amounts of water and measured the properties of the mixtures.
The water seems to form little bridges between the sand grains, a bit like two trumpet bells glued to one another. The water's surface tension will then act to pull the two grains together giving the wet sand its strength. You would have thought that the more water you add the more bridges that form and so the stronger the sand and Mario found this was true up to about 1% water. Beyond this point bridges get close enough together that they start to merge forming bigger bridges and blobs of water. These blobs are weaker than the small bridges, so this cancels out the increase in water and leads to no change in strength.
This may sound like just fascinating news for the under eights, but understanding how granular materials behave is very important in understanding soils, concrete and the stability of slopes, amongst many other things.

Lowering cholesterol good for us, bad for bacteria
Scientists in the US have discovered a novel way to tackle problem superbugs like MRSA - by disabling their defences with a cholesterol-lowering drug.
 UCSD researcher Victor Nizet and his team initially noticed that bugs like Staph aureus (MRSA) produce a yellow-coloured family of antioxidants called carotenoids (also found in carrots), which protect them against free-radicals and other oxidising agents produced by the immune system to destroy bacterial cells. When the researchers knocked out the gene used by the bacteria to produce these chemicals the bugs became much more vulnerable to immune attack.
UCSD researcher Victor Nizet and his team initially noticed that bugs like Staph aureus (MRSA) produce a yellow-coloured family of antioxidants called carotenoids (also found in carrots), which protect them against free-radicals and other oxidising agents produced by the immune system to destroy bacterial cells. When the researchers knocked out the gene used by the bacteria to produce these chemicals the bugs became much more vulnerable to immune attack.
Looking for ways to neutralise the gene in bacteria that cause infections in patients, co-author Eric Oldfield realised that the building blocks used by the bacteria to make their carotenoid antioxidants were almost identical to the chemicals used in humans to produce cholesterol. In particular one gene, called squalene synthase was strikingly similar. Acting on this insight the researchers began to screen compounds developed previously as cholesterol lowering drugs that target this pathway. One agent, called BPH-652, was very effective at penetrating Staphylococcal cells and blocking their ability to produce carotenoids.
"We have found that the same golden armour used by Staph to thwart our immune system can also be its achilles heel" says Nivet. And because BPH-652 has previously been tested in human clinical trials it should be the costs and time required for it to enter the clinical should be lower.

Oestrogen in the water
Scientists in Canada have confirmed that oestrogens from OCPs in sewage water can spill over into rivers and feminise fish. Moreover it can have previously unrecognised impacts on other parts of the food chain.
 Karen Kidd is a researcher at the Uni of New Brunswick. She added oestrogens to the water of a lake in Canada for 3 years and monitored the fish population. The short-lived fish, like the fat head minnow, feminised and then showed dramatic reductions in population as clearly the affected males were breeding less effectively.
Karen Kidd is a researcher at the Uni of New Brunswick. She added oestrogens to the water of a lake in Canada for 3 years and monitored the fish population. The short-lived fish, like the fat head minnow, feminised and then showed dramatic reductions in population as clearly the affected males were breeding less effectively.
The populations of longer-lived fish were less impacted because they were less dependent on rapid breeding to replace their numbers. BUT, big fish, that were not affected directly by the oestrogens, showed a very large drop in population in subsequent years - because they had lost their food source of smaller fish.
Consequently there's a twist in the tale of this fish story because oestrogens, it seems, can have indirect effects on fish populations that were previously unrecognised. The good news is that oestrogens only last about 12 days in the environment, so if we sort out the sewage situation then the fish should bounce back, which is what happened within 3 years in the canadian lake after the oestrogen administrations were stopped.

17:17 - Avoiding Organ Rejection
Avoiding Organ Rejection
with Professor Megan Sykes, Harvard University
One of the biggest breakthroughs in the transplantation field has been the discovery of immuno-suppressants. These are drugs that can partially switch off the immune system to prevent it from rejecting what the body sees as foreign tissue or non-self in a donor organ. But this comes at a cost because the drugs are quite toxic and immuno-suppressed patients are also quite vulnerable to infections and cancers.
 Instead scientists have been searching for ways to persuade the immune system to accept the new foreign tissue in a donor organ as its own. Harvard's professor Megan Sykes and her colleagues have now managed to do that by giving patients a partial bone marrow transplant collected from the same donor as the kidney they're receiving. Somehow the new bone marrow re-educates the immune system so that it ignores the new kidney and the patients no longer require any kind of immuno-suppression.
Instead scientists have been searching for ways to persuade the immune system to accept the new foreign tissue in a donor organ as its own. Harvard's professor Megan Sykes and her colleagues have now managed to do that by giving patients a partial bone marrow transplant collected from the same donor as the kidney they're receiving. Somehow the new bone marrow re-educates the immune system so that it ignores the new kidney and the patients no longer require any kind of immuno-suppression.
Megan - Since the very first time allogeneic transplants were performed, that means transplants that were done one individual to another, we've known that there is this rejection response that will destroy the graft unless something is done to prevent it. So the success of allogeneic transplantation in patients in the last quarter century or so has depended on the use of immuno-suppressant drugs that suppress the immune system in a way that prevents the rejection of the graft.
Chris - There are some consequences of doing that though, aren't there?
Megan - Yes. The trouble with that is these immuno-suppressive drugs suppress all immune responses so that the immune system is very generally compromised. What that means is that the recipient is predisposed to developing infections and also cancers, diseases because it turns out the immune system is needed to protect us from developing cancers. These are very serious side-effects and in addition there are a number of metabolic side-effects associated with these drugs and other unpleasant side-effects that people would like to avoid.
Chris - Why can't we re-programme the immune system to try to ignore what we're putting into the body and say 'this kidney I'm putting in is friend, not foe, don't attack it?'
Megan - Well, that's exactly what we have been attempting to do for quite a long time now and that we seem to have achieved in a small group of patients in a pilot study. The approach that we have used involves use of bone marrow which contains cells that can form all of the blood-forming cells in the body. It's been known for quite some years now that if bone marrow of two different individuals exists in one recipient that the donor bone marrow will educate the immune system in a way that allows the immune system to regard the donor as self and so the situation that you just described is created. Any graft from that same donor is ignored because it looks like self. The immune system has been educated to think that graft is self.
Chris - So what you're saying is that if you gave someone a kidney transplant, if you gave them a bone marrow transplant at the same time you can change what the immune system recognises as friend and foe.
Megan - That's right. That's the idea. Now what I've just described has been well-established in animal models for a while now and we have a pretty good understanding of how it works in the animal models. The problem is, how do you go to an animal model from patients? Patients who get organ transplants in general do quite well early on, especially in the first two years. Another problem that I haven't mentioned yet with organs transplants that are performed is that despite all the chronic non-specific immuno-suppressive therapy there's a late phase of graft rejection called chronic rejection that really hasn't been improved by all this immuno-suppressive therapy so many grafts are lost in the 5, 10, 15 year period. If we had this state where the immune system is re-educated as we've described, not only would we not need immuno-suppressive drugs but this more chronic type of rejection would also be prevented.
Chris - Could you talk us through, step-by-step, what you did in the pilot study with these patients?
Megan - Yes. What it involves is giving the recipient some chemotherapy but at a dose that that is well below the dose that is used in a conventional bone marrow transplant and giving the drug that is the antibody that causes depletion of the rejecting lymphocytes, the T cells in the recipient and also affects the T cells in the donor bone marrow graft.
Chris - So you end up with a patient who, for a while at least has got 2 types of bone marrow. They've got the donor who's going to give them, say the kidney, and they've got their own bone marrow as well.
Megan - Right, that's exactly right.
Chris - Then you put in the donor organ and at that time the immune system now is being told because the bone marrow is the same, don't reject this organ.
Megan - Right, so the kidney and the bone marrow are given at the same time and the bone marrow is present in the circulation for a period of just a few weeks. Together the bone marrow derived cells in the kidney itself are doing some complex things that we're still trying to understand to re-educate the immune system and allow it to regard the kidney as self.
Chris - In the patients that you've tested this on so far what's been the outcome and are things still working for them now?
Megan - We've done five patients in this pilot study and four of them are currently doing very well. They've been off immuno-suppression for a number of years. One is approaching five years. Their kidneys are being accepted despite the lack of any immuno-suppressant drugs.
Chris - So you've proved that this can work, at least on a small scale, with kidney transplants. What about other organs, livers, hearts, lungs things like that.
Megan - The timing of the protocol is such that the organs and the bone marrow need to be transplanted at exactly the same time. Yet the preparation of the recipient has to begin five or six days beforehand. You need to know ahead of time that you're going to do the transplant. At the moment this protocol limits us to live donors and the types of organs that are transplanted from living donors right now include kidneys, partial livers and sometimes lungs but hearts at the moment would not be relevant with this protocol. However, the overall idea does work in animal models for any type of graft from the donor. One of the things that we're working on in our animal models is modifying the regiment so that it will be possible to time it in a way that any organ can be transplanted.
25:31 - Keeping Organs Alive
Keeping Organs Alive
with Dr Constantin Coussios and Professor Peter Friend, Oxford University
Helen - As
Megan Sykes was explaining, their system is currently only suitable for live transplant donors because patients have to be prepared for 5-6 days in advance before transplant for technique to work. So most donor organs wouldn't survive long enough.
But that could be about to change because two Oxford Scientists, Dr Constantin Coussios and Professor Peter Friend, have developed a way to keep organs in pristine condition outside the body. I wonder if perhaps we could start by outlining what is it that goes wrong with organs in transit. How can they get damaged?
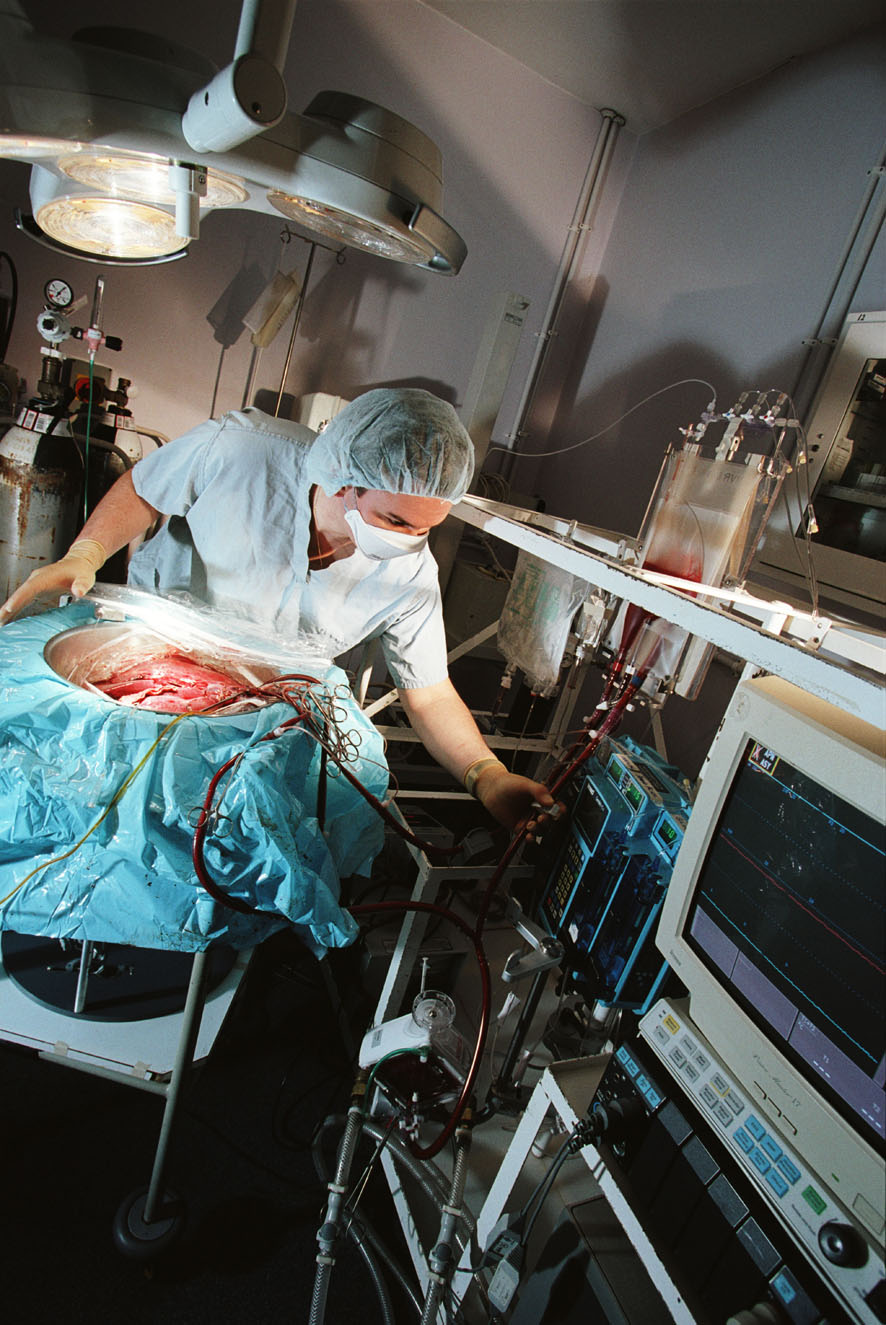 Peter - The problem is that we remove organs, we deprive them of oxygen, blood supply and to restrict the amount of damage that occurs we cool them down. We all know that when we put biological tissue, meat, into a refrigerator it lasts longer but it doesn't last forever but the cells continue to metabolise. They continue to function but at a much, much lower rate: about 10 times slower. There is still a limit to how long you can maintain a cell alive under those circumstances. At the moment for transplants, for kidneys, you can store a kidney for up to 24 hours but we do know the longer you store it the more problems there are likely to be thereafter.
Peter - The problem is that we remove organs, we deprive them of oxygen, blood supply and to restrict the amount of damage that occurs we cool them down. We all know that when we put biological tissue, meat, into a refrigerator it lasts longer but it doesn't last forever but the cells continue to metabolise. They continue to function but at a much, much lower rate: about 10 times slower. There is still a limit to how long you can maintain a cell alive under those circumstances. At the moment for transplants, for kidneys, you can store a kidney for up to 24 hours but we do know the longer you store it the more problems there are likely to be thereafter.
Helen - So, the longer you store it is it just more likely that it won't take when you transplant it?
Peter - Well it's got an increased risk of not working at all but if it does work it's likely to work less well and for a shorter time.
Helen - So at the moment the technology is just 'put the organs on ice.' Is that as simple as it is, really?
Peter - Well, the technology's essentially profusing the organ with a specialised solution which is designed to protect the cells from swelling and putting on ice to reduce the metabolic rate. That's, as you say is rather a simple concept and one that has worked quite well for a number of decades but it has severe limitations.
Helen - So, Constantin, perhaps I'll bring in you here as well. How are you addressing this problem, what are the technological changes that you've come up with to address this issue of organs surviving alive, if you like, outside the human body?
Constantin - We've long held this belief that cold is good but warm is better. In fact, organs are designed to be stored and operate at normal body temperature. We wanted to come up with a way to store an organ in such a way that it would never know it had left the human body. What we started working on was developing the technology that would allow us to maintain an organ at body temperature whilst profusing it with cold blood: feeding it such that it was undergoing the same function as it would be within the body.
Helen - So you've almost tried to trick it into thinking it hasn't left the body at all.
Constantin - Precisely. The major assumption in cold preservation as Peter just mentioned a moment ago you have damage in proportion to storage time whilst if you're able to maintain an organ in normal, active synthetic state what will happen is there'll be no such damage in proportion to storage time.
Helen - Ok so keeping the organs in a system that's tricking it into thinking it's still in a body. That's all sounds quite straight-forward. It isn't, is it?
Constantin - People have been trying to do it for over a hundred years and one of the major challenges which is particularly true for the liver, which is the main organ that we've been focussing on so far, is that it dramatically changes its vascular resistance to flow. Depending on how much we're eating or sleeping or drinking the amount of flow through the liver will allow through itself will change dramatically. The major innovation and the reason we believe what we're doing works much better than previous attempts is that it allows the organ to auto-regulate its blood supply. It's effectively a system designed around the organ which under no circumstances forces blood through the organ. What we end up with is very exciting. It is in fact a liver which manufactures bile outside the human body, just the way it would inside the human body itself.
Dave - So are you basically just pumping a constant pressure rather than just pushing a certain amount of blood through every second?
Peter - The object is to maintain completely normal physical parameters in terms of both flows and pressures. The liver, in particular is complex. It has a dual blood supply: one vein a high flow, low pressure and one artery with high pressure and lower flow - very complex. What actually happens inside the liver is relatively poorly understood in those terms. What we've done is to generate a system which places the liver in a completely physiological environment.
Dave - What is it which is limiting the life of the livers which you're using at the moment?
Peter - We haven't tested the hypothesis, as it were, to destruction so we don't know. We know that livers will survive and function apparently normal for periods of up to three days. We haven't done the ultimate experiment we'd like to see how long they will go but there are some complex biomedical engineering challenges in terms of, for example, damage to red cells in an artificial circuit which will become limiting at some point.
Constantin - The major limiting factor, if I may add, is the lack of sleep for the investigators concerned because at seventy-two hours the given team of a given size, eventually someone has to go to bed. We very much hope that with the advances of automation and integration that we're getting into we'll be able to test it to destruction.
Dave - If you can keep the liver for long periods of time does this mean you can match donor livers to recipients much better because you don't have to have them both ready at the same time?
Peter - Well, there are two huge potential advantages. One of which is that we can start to take organs that were previously discarded, organs that were damaged or organs that are causing concern as to whether they're going to work and we have good evidence now that we can actually resuscitate and improve the performance of an organ before it's transplanted. We can also measure how well it works before it's transplanted so there's no risk, as it were, to the patient receiving the organ. It's already been tested so that's a very large advantage. The other advantage which you were alluding to was if you could keep the organ for long enough you could start to do some very complex interventions into the matching between the donor and the recipient. You were hearing from professor Sykes a few moments ago about one very interesting intervention which is a bone marrow transplant. But, that requires time before the transplant takes place. This sort of technology starts to open up strategies of this sort, not just the one we've heard about but others which would improve the immunological outcome following transplant.
Helen - You're focussing at the moment on the liver and presumably would this kind of technology be applicable to other organs? Are there other organs that are even more of a challenge to keep alive outside the human body?
Constantin - Indeed. The principle as I said, the governing principle which has made this invention work is blood regulation. The principles behind it are certainly applicable to all organs that do not act as their own pump so all organs excluding the heart. In particular, the liver, the kidney, the pancreas, the small bowel and potentially the lungs in isolation are all potential candidates for use with this technology. The fundamental difference and the thing that makes this exciting is that in a cold preservation context you do not have a functioning organ during the period of preservation. In this paradigm you've actually got a fully functioning organ that can be quantitatively assessed prior to transplantation. That's really quite exciting.
Helen - So Peter, how close are we to seeing this actually happening in a hospital in emergency rooms: this technology might be applied?
Peter - There's been a lot of work on this working on an animal model. We're confident that the system works. We've gone all the way through with transplants. It's clearly an advance. We're currently in the process of developing prototypes which can be used in patients and clearly that's something which has to be developed but we are looking to carrying out limited clinical trials within the next year or two. It's not very far away.
34:46 - Building a New Heart
Building a New Heart
with Dr Steffen Kren, Univerity of Minnesota
A few weeks ago, we reported on the breakthrough where scientists in America had been able to
build a beating heart in the lab. We invited Dr Steffen Kren, from the University of Minnesota, to join us on the show.
Helen - I wonder if you could first of all tell us a little bit about your beating heart and what's been going on in your laboratory.
 Steffen - Sure. This is usually regarded as what's thought of as tissue engineering. The elements to create a tissue are typically cells which we can get from a variety or sources and a scaffold. In this case, we've devised a process whereby with diffusion profusion we can remove all the cells from a solid organ leaving the extra-cellular matrix, the protein that the cells excrete. This forms a remarkably detailed scaffold that represents the organ in three dimensions but it's completely a-cellular. Then using three-dimensional tissue culture techniques we can add cells back and create a new tissue that hangs on that scaffold.
Steffen - Sure. This is usually regarded as what's thought of as tissue engineering. The elements to create a tissue are typically cells which we can get from a variety or sources and a scaffold. In this case, we've devised a process whereby with diffusion profusion we can remove all the cells from a solid organ leaving the extra-cellular matrix, the protein that the cells excrete. This forms a remarkably detailed scaffold that represents the organ in three dimensions but it's completely a-cellular. Then using three-dimensional tissue culture techniques we can add cells back and create a new tissue that hangs on that scaffold.
Helen - So you basically make a mould, if you like, in the shape of the organ you want and then fill it in will cells. Is that right?
Steffen - Yes, that's right. It's even better, in a sense, than a mould because our process retains proteins that we believe guide cells to do what we need to do; that cue cells to - in the case of undifferentiated cells, perhaps - cue them to what they should turn into for that particular spot in the tissue.
Dave - Almost like an instruction manual inside the structure which you've got left which tells the stem cell, 'you should be part of a blood vessel?'
Steffen - That's right. We're hoping to (a) understand that cell signalling phenomenon and (b) use it to our advantage to create details in a tissue that we wouldn't be able to do just by providing cells alone.
Dave - So you've taken a heart. What kind of heart were you using?
Steffen - In this case, rat.
Dave - So you've taken a heart and in this case, cleaned it out of all the cells. Then you've just injected some stem cells and it suddenly grows back into a working heart?
Steffen - In this report, in Nature, we used neonatal cardiomyocytes. They're not stem cells, they're differentiated. They're young rat heart muscle cells. We sort of short-circuited the whole differential issue for this particular experiment. Are you following me?
Dave - Yes, that makes sense.
Steffen - Now the way we view this going forward in a transplant context, we would like the cells that we use to be autologous, to come from the patient.
Helen - How do you mean? Are you extracting the stem cells from the bone marrow, for example?
Steffen - Could be. Our laboratory has also found progenitor cells in adult cardiac tissue.
Helen - So actually from the heart that you're going to replace?
Steffen - This could end up a possible source. The way we see this as superior to cadaveric organ donation is that, in theory, grown from your own tissue the entire construct would be autologous save for the matrix itself. Worst case scenario the matrix is immunogenic that's rapidly turned over by smooth muscle cells. Perhaps over a relatively short course of immuno-suppression the autologous cells that we create turn over that protein matrix and it would become you in totality.
Dave - So the idea is you take someone else's heart, it doesn't have to be very good or it isn't working any more and then inject it with your own cells. Then it would grow into a heart and you could transplant that into you and it should start working. Is there any reason a human heart should be more difficult than a rat heart?
Steffen - Well, there's certainly a matter of scale and as a substitute we've used porcine tissue, cadaveric porcine hearts to be a point of principle in scaling this process to human-sized organs. Now at this point we've successfully de-cellulised a variety of porcine organs, not just the heart also kidney, liver, gall bladder - a variety of tissues. The scaling issue as far as the recent experiment is sort of beyond our mainly economic capabilities.
Dave - You need many more cells.
Steffen - Exactly. We would have to donate every square inch of tissue culture that we have to create the cells. At this point I don't really see that being a big issue.
Helen - How do you see the way forward in terms of, are we going to see the technology developed - actually used in clinical situations?
Steffen - Well, I think that even before that we're going to learn a tremendous amount of basic science about cell signalling. There may even be medical products along the way that are not entire replacement hearts. For instance, it may be that our tissue is a successful left ventricular patch in the case of an infarcted heart, for instance. So there may be steps along the way where this is useful technology, but stops short of a complete replacement heart.

40:45 - The Smell of Old Books?
The Smell of Old Books?
We put this question to Jana, Head of Laboratory for Cultural Heritage at the University Library of Slovenia.A smell or odour is caused by volatile compounds which we perceive by the sense of olfaction. An odour of a book is a complex mixture of odorous volatiles, emitted from different materials from which books are made. Due to the different materials used to make books throughout history, there is no one characteristic odour of old books. A professional perfumer has evaluated seventy odorous volatiles emitted from books and described their smells as dusty, musty, mouldy, paper-like or dry.
The pleasant aromatic smell is due to aromatic compounds emitted mainly from papers made from ground wood which are characterised by their yellowish-brown colour. They emit vanilla-like, sweetly fragrant vanillin, aromatic anisol and benzaldehyde, with fruity almond-like odor. On the other hand, terpene compounds, deriving from rosin, which is used to make paper more impermeable to inks, contribute to the camphorous, oily and woody smell of books. A mushroom odour is caused by some other, intensely fragrant aliphatic alcohols.
A typical odour of 'old book' is thus determined mixture of fragrant volatiles and is not dominated by any single compound. Not all books smell the same.
Can we make plastic without oil?
Most of the plastics that we use at the moment are manufactured from oil. You break up long chains, and you tend to get small gases, things like ethane. Out of an oil refinery you can cook up crude oil very hot and get some useful gases. You can then react them together and then stick them together and the little units stick together. They form long polymers which is what pretty all plastics are made of.
There has been a lot of research recently into other ways of making plastics in case we do run out of oil: using biological foodstuffs mostly and also so you can make plastics which are more biodegradable because we're making plastics from oil in a way very alien to organic processes. Organic things can't break them down very well. There have been various things including genetically engineering bacteria to produce precursors to making plastics or even whole plastics themselves. If you mash up a bacteria extracts with plastic you can get a beautiful plastic out. These plastics are doing the same job if not slightly better because they aren't going to persist in the environment and cause huge pollution like normal plastics do.
Why don't my saucepans dry in the dishwasher?
When things dry out you're making the water evaporate and this takes quite a lot of energy. So you've got a large amount of energy in a hot dishwasher, it's going to be 70, 80, 90 degrees in there. What you need to do is transfer that heat to the water. The best way of transferring that heat to the water, is if it's a thin sheet all over the surface is metal which conducts heat very well. So most of the heat should evaporate the water and it dries out really well. Things like china conduct heat reasonably well so if you've got hot china it's got quite a lot of heat in it and it can conduct out well and the water should dry out. It tends to be in fairly small globules. As he's saying TeflonÃ?,® is very, what's called, hydrophobic so it doesn't like water. The water tends to collect in great big globules so you've got to get lots of heat into a very small area which is difficult so heat's being lost through the rest of the pan. Also, the TeflonÃ?,® is quite insulating so it's hard for heat to get through it. It will tend to actually go out through the metal on the other side and so probably that's the reason why you're getting water left in your pans.

Why are yawns catching?
That's a very good question. Yawning is a really common reflex, actually that happens in lots of different animals. If you see someone yawning you want to yawn yourself which is very strange. We see it in all these animals including some that maybe aren't so social. Maybe it isn't something that's learned but maybe a bit more inherent. Perhaps it just starts to arrive at some point.Wild dogs in Africa get contagious yawning, where one will yawn and then the other will yawn and it tends to be about the time when they're going to bed. It's a nice way of getting the whole pack to decide to go to bed.
Often with very young babies, their eyesight isn't very good. They can't focus very well so it could be just she can't see you yawning. She doesn't learn how to yawn from that.
- Previous The Smell of Old Books
- Next DNA, Radio Waves and Watches










Comments
Add a comment