Stunning sapphires, dazzling diamonds and red rubies abound in this week's sparkling edition of the Naked Scientists in which we find out how gemstones are formed, what makes them so beautiful and why they're so rare. We also reveal the tricks used by experts to flush out fake stones, and discover how synthetic diamonds can make better lasers, more powerful electronics, sweeter tweeters and cutting-edge scalpels. Plus, why a mongoose could be your best friend in a minefield, how a good breakfast leads to more male births and, in Kitchen Science, how to grow some beautiful crystals at home!
In this episode

Best tool to find explosives? Mine's a mongoose!
Engineers in Sri Lanka have come up with a cheap but effective way to locate landmines - a mongoose tethered to a robot!
 Landmines are a serious problem. The United Nations estimates that there are over 100 million of them lurking in ground, worldwide. At the moment they are killing or maiming 2000 people every month. Part of the problem is that planting them costs comparatively little - often less than a pound each - but removing them again can cost up to £500, and the best mine clearing personnel can usually cover just 2 square metres of ground per day.
Landmines are a serious problem. The United Nations estimates that there are over 100 million of them lurking in ground, worldwide. At the moment they are killing or maiming 2000 people every month. Part of the problem is that planting them costs comparatively little - often less than a pound each - but removing them again can cost up to £500, and the best mine clearing personnel can usually cover just 2 square metres of ground per day.
But now help is at hand from an unusual source. University of Moratuwa engineer Thrishantha Nanayakkara and his colleagues have developed a robot which takes a trained mine-finding mongoose for a walk on a leash. The mongoose sniffs out the landmines by detecting traces of TNT which leaching out of mines, whilst the robot makes sure that the ungainly duo cover the ground systematically and thoroughly. The robot keeps a camera trained on its mine-detecting charge so that the operators can see when the animals locate buried ordnance, which they signal by standing up on their hind legs and sniffing.
At $3000 the system is low cost and covers ground quickly compared with other mine-locating strategies. But for those concerned about the welfare of the mongoose, a major plus is that, together, it and the robot weigh less than 10 kilos, which is too light to detonate a mine if it steps on one!

04:30 - Is it one of your favourite things?
Is it one of your favourite things?
If raindrops on roses are some of your favourite things, then here is some news for you: a study has come out this week that reveals why it is that drops of water cling to rose petals in that most beautiful of ways.
Lots of flowers and leaves are covered in spines and a layer of wax which repel water and cause it to slide off taking dust with it, a process known as self-cleaning. But this doesn't happen in roses, where water droplets cling on instead.
Now scientists from Tsinghua University in Beijing have worked out what is going on - rose petals are also covered in spines but they have no wax. Rows of spines along wide smooth furrows keep the water in spherical droplets and hold on to them
What's more the team made a cast of a rose petal out of polyvinyl alcohol and found that this manmade substance had the exact same ability to cling onto water as a real rose petal. So, a bit like geckos feet, the stickiness of petals relies on their physical structure and not so much the chemical composition of the material they are made of.
It is thought that rose petals might benefit from holding water droplets because it makes them glimmer and attract pollinating insects. Scientists also think that understanding the rose petals' stickiness could lead to some interesting developments in the lab, when tiny amounts of liquids need to be moved around without contaminating each other.

It's on the tip of my tongue - and that's where it'll stay if you're not careful
The claim that we learn from our mistakes would appear to be wrong, according to a new study from Canada.
 McMaster University researchers Karin Humphreys and Amy Warriner, writing in the Quarterly Journal of Experimental Psychology, have found that the worst thing to do when you experience a word on the tip-of-the-tongue experience, is to dwell on it - because otherwise you'll make it happen again.
McMaster University researchers Karin Humphreys and Amy Warriner, writing in the Quarterly Journal of Experimental Psychology, have found that the worst thing to do when you experience a word on the tip-of-the-tongue experience, is to dwell on it - because otherwise you'll make it happen again.
The team made the discovery by studying 30 students who were presented with statements provoking them to recall certain words. The statements were carefully chosen to elicit tip of the tongue experiences, mainly by focusing on rare words or expressions. After reading a statement the participants were told to press one of three buttons: Know, Don't Know and TOT (tip of the tongue).
When they experienced a TOT the team waited either 10 seconds or 30 seconds before telling them the word they were looking for.
Two days later the same students repeated the test using the same statements. In many cases the same statements provoked TOTs again, but more shocking was that on words in the first trial where the students were given the longest waiting times (30 seconds) before being told the word they were looking for, they were much more likely (50% more likely) to experience a TOT again.
The researchers think that this is because by trying to retrieve the TOT word the brain is actually learning to forget it. "Music teachers know this principle," says Humphreys. "They tell you to practice slowly. If you practice fast you'll just practice your mistakes". Instead, they urge those suffering a TOT experience to quickly ask a colleague for the missing word, or look it up on the Internet. Otherwise, says Humpreys, "you'll keep digging yourself the wrong pathway."

Cornflakes for boys
We know that many reptiles like crocodiles can choose the sex of their offspring by controlling the temperature their eggs are incubated at. And now it has been revealed that human mothers might also have some influence on whether they have a girl or a boy, not by controlling temperature but by whether or not they enjoy a bowl of cornflakes for breakfast.
 That's what researchers from the University of Exeter here in the UK found when they carried out a survey of over seven hundred expectant mums who didn't know whether they were carrying a boy or a girl. By asking them about what they ate around the time of conception, Fiona Mathews discovered that women who ate at least one bowl of cereal a day were more likely to have boys than women who frequently skipped breakfast.
That's what researchers from the University of Exeter here in the UK found when they carried out a survey of over seven hundred expectant mums who didn't know whether they were carrying a boy or a girl. By asking them about what they ate around the time of conception, Fiona Mathews discovered that women who ate at least one bowl of cereal a day were more likely to have boys than women who frequently skipped breakfast.
59% of women eating a good breakfast had boys compared to 43% of those who didn't eat cereal every day.
And this sort of thing has been noticed before. During wartime and times of stress, like the 9-11 bombings, there have been increases in the number of girls born compared to the number of boys.
In fact there is a global down turn in the number of boys being born, although it's only about a tenth of a one percent since the 1970s, it still adds up.
We don't know exactly what it causing these differences but it could be something to do with the body's way of dealing with tough times. The theory is that when there is plenty of food around, the best choice is to have boys since big strapping healthy males are best at producing their own offspring.
But when resources are limited, and mothers are going hungry, it is better to have females, since weedy males aren't much use. It is also possible that when a population has been affected by a disaster, like war, the quickest way to rebuild numbers is to have lots of females since ultimately they limit the number of children in the next generation.
Can robots smell out landmines?
Probably you could but you need to rely on some kind of electronic nose, these e-noses. I don't know how good our technology is at the moment with sniffing these things out with something that's portable and cheap. The benefit of combining a simple robot with a well trained Mongoose is that it's very low-cost and it is quite disposable. If you blow up a very expensive equipment obviously there's a greater cost to that.
Do chimpanzees have to trim their toenails?
Chris: I don't know, Helen, do you know this?Helen: I don't know actually, it's a good question. I can only imagine they do. I can imagine that they can chew them, they're quite bendy. They can probably chew their toenails. But we can find out. Anyone got any ideas? Chris: If you have any clues, do get in touch.

20:22 - Sparkling Science
Sparkling Science
with Ian Mercer, Gemological Association of Great Britain
Chris - Ian Mercer joins us now in the studio from the Gemological association of Great Britain. He's here to tell us more about it. Hello Ian.
Ian - Hello.
Helen - Thanks for joining us. Now first off what is it that makes a gem a gem and how do we know that one is a gem stone?
 Ian - Well, when you look at a gemstone and you think, "That's really beautiful. That really appeals to me and I'll get my husband to spend lots of money on it," well that's one of the attributes of gems. Also, they have to last quite a while, don't they? So they have to be a bit durable or hopefully, very durable. I guess pearls are less durable and diamonds are less durable but they are both gemstones. It's a bit variable.
Ian - Well, when you look at a gemstone and you think, "That's really beautiful. That really appeals to me and I'll get my husband to spend lots of money on it," well that's one of the attributes of gems. Also, they have to last quite a while, don't they? So they have to be a bit durable or hopefully, very durable. I guess pearls are less durable and diamonds are less durable but they are both gemstones. It's a bit variable.
Helen - It's not a strict definition of a certain chemical compound or anything like that?
Ian - It's not that strict and depends on those particular factors and also it helps if they're rare as well. Of course, it is quite rare to get beautiful big crystals. Therefore that's something else that people will pay for and then value. Also they've got to be acceptable, don't they? They've got to appeal to your fashion and appeal to your community or perhaps not come from living elephants and things like that. How acceptable are they? That really also is a factor. Really, I guess in the end it's something somebody pays a lot of money for.
Helen - Excellent. I have here in front of me in the studio a large lump of, what I think is very beautiful. Chris, I don't know if you agree?
Chris - Wow, is that yours?
Helen - No!
Chris - Is this your engagement ring?
Helen - No, Ian brought this in. It's a lump of - I'll describe it - raw crystals, I suppose. It's about the size of my hand, slightly pale blue in colour with some straight edges. What am I looking at, Ian?
Ian - You're looking at aquamarine crystals. They are beautiful gem quality crystals as grown in the Earth, very hot, underneath an area where there are volcanoes. That's just as it forms, hasn't been cut and polished.
 Chris - That's the size of Helen's fist, how much would that be worth? Not that I'm thinking of nicking it or anything...
Chris - That's the size of Helen's fist, how much would that be worth? Not that I'm thinking of nicking it or anything...
Ian - I would guess you could spend something like one hundred pounds on a group of crystals like that.
Chris - Why are they one hundred quid but a diamond that size would be unfeasibly expensive?
Ian - It's partly the rarity value that's really, how many diamonds do you get on the Earth's surface? Very few. How many big diamonds? Almost vanishingly few.
Helen - What's this made out of?
Ian - That is aluminium, beryllium silicate. It's got beryllium in it which is a strange, rather poisonous metal but these crystals are not poisonous. It's fairly rare and it's a little bit rarer when it's that beautiful blue. It's rarer still if it's in big crystals which are suitable for cutting. Of course, you can only cut a lovely gemstone out of a lovely crystal. You've gotta start with good to get good.
Chris - Chemically speaking, what actually are gemstones? What chemicals do you find in say, rubies and sapphires and emeralds and things?
Ian - Well, many of them are what we call silicates: a little silicon atom with four great big oxygen atoms around it. If you get those which are four cornered units, tetrahedral, they all link together often with metals and that makes up a nice silicate structure. We think of those as minerals or artificial crystals made as silicates. If those atoms come together really well, perfectly - nice orderly arrangement - then you get a nice crystal. You mentioned ruby and sapphire, those are oxides, they're relatively simple. That's corundum. Ruby and sapphire are both the same mineral, called corundum. If you have non-gem quality corundum as a sort of sand that's what many people think of as emery which is use for grinding.
Chris - Sand paper?
Ian - Yes, emery paper.
Chris - It's aluminium, isn't it?
Ian - That's aluminium oxide, yes.
Chris - Why is a ruby such a gorgeous red colour and it's aluminium oxide? A sapphire is that gorgeous blue colour and it's aluminium oxide. What's going on?
Ian - What's going on is impurities. You might think, "Well, how can you call it impure if it's so beautiful?" Well, they are metals which get trapped into that structure of aluminium oxide. In ruby it's chromium. In blue sapphire it's iron and titanium and there are many colours of sapphire, in fact. Many people don't realise sapphire could be any colour.
Helen - I've got a lovely blue one actually. I've got four on my finger which I rather like. You mention volcanoes. Is that where we find all of these gemstones? Is that where they're all formed?
Ian - Many are in volcanic districts. Strangely, those beautiful aquamarine crystals occurred in pockets around granite and granite when it's molten is a bit like porridge. It works its way up towards the Earth's surface. If it crystallises out nice and quickly around the edge of the granite then we've get those lovely big crystals. If it reaches the Earth's surface it forms terrible volcanoes, the most awful dangerous volcanoes ever. Luckily, most of it doesn't get to the surface. The stuff that stays down there might form gemstones.
Chris - How do we know where to look for different gemstones? In other words, because they require different conditions - quite specialist conditions to form - does that mean that there are hotspots for different types of gemstones around the planet's surface?
 Ian - There are hotspots. Yes. The type of deposit that forms that aquamarine is called a pegmatite and those are prevalent in certain places such as Madagascar, the Ukraine, Brazil, certain states of America and where there are past or present volcanoes.
Ian - There are hotspots. Yes. The type of deposit that forms that aquamarine is called a pegmatite and those are prevalent in certain places such as Madagascar, the Ukraine, Brazil, certain states of America and where there are past or present volcanoes.
Chris - If you haven't got a volcanoes there now but you did in the past, that means there presumably a hotspot there for finding things because those conditions exited there once if not today.
Ian - If there's the right type of rock, right type of volcanic province, yes. That's the place that geologists or gem prospectors are going to look. They're clues.
Helen - You touched already a little bit on rarity and what it is that we like. Am I right that in fact engagement rings: that rubies used to be the ones that people wanted because red colour was romantic, it was the colour of roses and hearts and love and things? It was only later that we were persuade that diamonds were a girl's best friend? I don't know if that's a story I picked up from somewhere.
Ian - Well, my wife's engagement ring is ruby. What can I say?
Helen - Wonderful.
Chris - What does a gemologist give to his wife on their engagement? Now there's a question!
How do geodes form?
We put this question to Ian Mercer:
Well, we were talking about volcanoes earlier. Now, if you think of a volcano after it erupted and all this black lava comes out and it starts cooling down then, as it cools, some of the gases start fizzing out. They form big bubbles and those bubbles gradually fill with more minerals as the water flows it cools the lava. These minerals line the side of what was the bubble and because the lava very often turns to a sort of soil and wears away you're left with this hard mass which was the lining of the bubble. Some of those when they're broken open you find that they're lined with these beautiful crystals, quite often amethyst which is purple quartz.

29:04 - Detecting a Dodgy Diamond
Detecting a Dodgy Diamond
with Philip Martineau, Diamond Trading Company
Meera - Diamonds are forever. A popular slogan and a James Bond advert but it's true. Some diamonds were made as long as four billion years ago and it's partly this resilience along with their incredible beauty that makes them so valuable in society. With so many fake diamonds or synthetics being made today how can you be sure that the large amount of money you're parting with isn't giving you a dodgy diamond? I set out to investigate and went to the De Beers office in London to meet with diamond research engineer, Philip Martineau, from the Diamond Trading Company. I asked Philip how companies like De Beers distinguished genuine gems from fake, synthetic ones. He showed me the two systems they use: DiamondSure(TM) and DiamondView(TM). These screen and analyse diamonds by their light absorption and fluorescence properties. The first stage of analysis uses DiamondSure(TM) - a rapid screening machine that identifies 98-99% of real diamonds.
 Philip - The idea underlying DiamondSure(TM) is that diamonds have resided deep within the Earth for hundreds of millions of years at high temperatures. In that time nitrogen impurities which are found in the vast majority of natural diamonds have aggregated together into groups of atoms which are more stable. In the process the way in which the stone absorbs light is changed in a way that we can detect and a way that's very hard to mimic in an artificial process on any practical timescale.
Philip - The idea underlying DiamondSure(TM) is that diamonds have resided deep within the Earth for hundreds of millions of years at high temperatures. In that time nitrogen impurities which are found in the vast majority of natural diamonds have aggregated together into groups of atoms which are more stable. In the process the way in which the stone absorbs light is changed in a way that we can detect and a way that's very hard to mimic in an artificial process on any practical timescale.
Meera - So DiamondSure(TM) rapidly screens diamonds using the patterns produced from light absorption, particularly the absorption resulting from nitrogen impurities present in the stone. Samples that don't pass this stage are referred to machine called DiamondView(TM).
Philip - In this instrument the stones are illuminated with ultraviolet radiation and the user can then collect the image with the resultant surface fluorescence. By studying that image you can then determine of a stone is a natural diamond or an artificial product of a synthesis process.
Meera - The surface fluorescence from the UV hitting the stone can be viewed on a linked computer monitor. The sample can also be rotated for a full 3-dimensional analysis. Philip had two samples with him when we met - one real and one fake. We put them to the test to find out if these machines are as accurate as they should be.
Philip - Yes, I've got two diamonds here. I'll put the first into the centre of the sample compartment. It says, "measuring, analysing." It gives a result: refer for further tests.
 Meera - So this one's not a real one?
Meera - So this one's not a real one?
Philip - we need to look at it in more detail. We'll put that to one side. Put the second on, press the test button: pass. That's a natural diamond. It doesn't need to be looked at in more detail. I'll take this across to the second instrument, DiamondView(TM). We switch on the UV radiation. You can see immediately we have different luminescence coming from different regions of the stone. We can see immediately that this is a synthetic. Because the synthetics are grown in a very different chemical environment from natural diamonds this is a high-pressure, high-temperature synthetic. It's been grown in a metallic environment. That gives rise to different shapes as the samples grow and therefore different tell-tale signals when you look at the sample after growth having polished the surface back. If we switch off the UV illumination you can see that it continues to glow after that. That phosphorescence is a very indicative thing as well.
Meera - Could we see a real one?
Philip - Yes ok. If I just switch on the UV radiation again you can see a sort of tree ring pattern here. The analogy we often use here is if you cut across a tree trunk you can see rings. Those rings correspond to a different stage in the growth history of a tree. In exactly the same way for this natural diamond here you have a central region where the diamond started to form spontaneously. As you go farther out you reach a ring where the luminescence intensity goes up significantly. We know that's because the environment in which the diamond was forming has changed. It may have moved about in the upper mantle or it may have been that it came into contact with a different sort of environment because of other material moving into that region. The important thing is that there's a series of those rings and they relate to the different stages in the diamond's growth. That's very indicative of a natural diamond formation.
 Meera - The complex growth pattern of a diamond as it formed and moved around the Earth's mantle can be seen using UV lasers. Synthetic diamond producers attempt to mimic these conditions associated with diamond formation for technological purposes as well as jewellery imitation. How do they do this?
Meera - The complex growth pattern of a diamond as it formed and moved around the Earth's mantle can be seen using UV lasers. Synthetic diamond producers attempt to mimic these conditions associated with diamond formation for technological purposes as well as jewellery imitation. How do they do this?
Philip - They're made in a very different chemical environment from natural diamonds. There are two basic techniques: high pressure-high temperature synthesis where they're formed from carbon dissolved in metal solvent catalysts. The metals generally used are some combination or other of iron, cobalt and nickel. The second method is chemical vapour deposition. There the synthetic material is produced from carbon containing gases such as methane, usually diluted in hydrogen. There the synthesis takes place at low pressures, usually at about a tenth of atmospheric pressure. Again, a very different environment and it's those differences in the environment which lead the differences which enable us to identify the material and distinguish it clearly from natural diamond.
34:49 - Diamonds in Industry
Diamonds in Industry
with Chris Wort, Element 6
Chris - Diamonds also have some technological applications as well. Chris Wort is from Element 6 which is a diamond manufacture and technology company. He's here to tell us a bit more about some of these applications. Hello Chris.
Chris W - Good evening, Chris.
Chris - Thank you for joining us. What sorts of things can you do with diamonds apart from putting them on your fingers?
Chris W - That's a very good question. Diamond is really quite an exceptional material. It has properties other than its beautiful brilliance that's used for the gem side of it. It's very very hard and people know that diamond will scratch glass but it will scratch absolutely everything else. They were the traditional abrasive type of applications of cutting and polishing. It's used for rock drilling, for oil prospecting where you're basically just using the hardness of diamond. In addition, diamond also has very high thermal conductivity that can be used for thermal management, for example, of electronic devices.
Chris - So in other words you could use it for heat sinks or something? You could draw heat away from things with it?
 Chris W - Well, it's really heat spreaders so it's exceptionally good at removing heat from hotspots and for example silicon devices. It's also optically transparent so you can use it for laser windows.
Chris W - Well, it's really heat spreaders so it's exceptionally good at removing heat from hotspots and for example silicon devices. It's also optically transparent so you can use it for laser windows.
Chris - How does that work then? Tell us about the lasers.
Chris W - You can synthesis the diamond as a fully crystalline plate and when you get the conditions correct this plate is perfectly transparent to certainly infra-red radiation. If you can polish it flat and parallel you can then use it as a laser exit window. Because it has a very high thermal conductivity and a very small thermal expansion coefficient you really get very little deviation of the beam.
Chris - In other words, when you make a laser beam out of a diamond it doesn't change its shape very much when it gets hot and also because it's very, very good at conducting wavelengths of all types of light then it doesn't hold anything back. Everything just goes straight through.
Chris W - Yes, that's pretty much it. You get a much better beam quality through a diamond window than any other laser windows.
Chris - The thing is that diamonds are obviously not trivial to find and not found in big enough quantities to make lasers so how are you getting round that problem?
 Chris W - In this particular case the diamond is synthesised by a technique called chemical vapour deposition. That's where you use carbon in its gaseous form. You excite it to a relatively high temperature of a few thousand centigrade and if you get the conditions right the carbon effectively condenses on a substrate. It forms as a diamond layer.
Chris W - In this particular case the diamond is synthesised by a technique called chemical vapour deposition. That's where you use carbon in its gaseous form. You excite it to a relatively high temperature of a few thousand centigrade and if you get the conditions right the carbon effectively condenses on a substrate. It forms as a diamond layer.
Chris - How big are diamonds you can make with this technique? Are you talking a pinhead or can you literally make sheets of diamond like that?
Chris W - No. You can certainly make six inch discs of polycrystalline diamond plates and you can make it millimetres thick. Equally you can coat surfaces with diamonds; use its very hard wear-resistant properties, if you like. It's basically many inches in diameter and many millimetres thick.
Chris - What is it about the chemistry of diamonds that gives them these amazing properties?
Chris W - Well, basically it's a very small atom and very tightly covalently bonded in a cubic lattice.
Chris - What does that mean to the average person in the street?
Chris W - It's very hard and very tightly bonded and very stiff.
Chris - If you were to sort of zoom in with a very powerful microscope and look inside a diamond at the arrangement of the atoms - just very simply - how are they organised?
Chris W - They're in a tetrahedral arrangement so each carbon is attached to four others. It's very stiff and because the carbon-to-carbon bond is short it's a very tight bond. Unlike other materials we've been discussing on this programme diamond is just one element. It is just carbon but it is carbon arranged in a particularly stiff, lattice structure.
Chris - It's interesting because I had a barbecue the other day and I burned another form of carbon, graphite, charcoal and that's certainly not very hard. Why is it so different between diamond and charcoal?
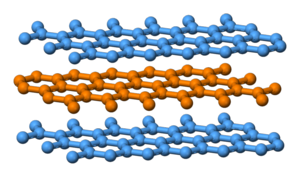 Chris W - It's a different form of carbon. In graphite and charcoals you don't have this tetrahedral bonding. It's actually in hexagonal plates. Although they're quite strong in one direction they're very weakly bonded in the other direction. In actual fact graphite can be used as a very good lubricant for that purpose.
Chris W - It's a different form of carbon. In graphite and charcoals you don't have this tetrahedral bonding. It's actually in hexagonal plates. Although they're quite strong in one direction they're very weakly bonded in the other direction. In actual fact graphite can be used as a very good lubricant for that purpose.
Chris - If we took a lump of diamond and heated it up on a barbecue could you burn it?
Chris W - Oh yes, it will burn. It is, after all, coal. It will burn like coal. If you get it hot enough it starts to oxidise and form carbon dioxide.
Chris - It'd be a pretty expensive experiment to do I suppose. So what other things are you doing at Element 6? What other applications? Some of the things on your websites suggest things like making speakers for hi-fis.
Chris W - It's actually tweeters. This is again using chemical vapour deposition to lay down a very thin layer of diamond in not quite a flat plate but it's a very shallow dome. Because diamond is very stiff and it is relatively low-density you can make a small piston that will displace air for the tweeter in a hi-fi system.
Chris - What sorts of sound frequencies will it produce when you do that?
Chris W - Well, it'll go up to substantially higher frequencies than, for example, a conventional aluminium tweeter just because of its rigidity and its low mass. It has what we call a break-up frequency in excess of 70kHz which is well-beyond the audible range of humans.
Chris - That's ok if you're a bat, isn't it? What about for a human?
Chris W - Well, for humans in actual fact the break-up frequency is important because you get harmonics that occur at lower frequencies. It's the harmonics that the human ear can detect at the low 20kHz that make a clattery tinny sound. If you have a higher break up frequency you actually have a more perfect sound in the whole of the audible range.
Chris - Lastly, one other cutting edge piece of technology you're developing is things to make scalpels even sharper.
Chris W - Yes, traditionally ophthalmic surgery knives have been made from natural diamonds. We're also able now to make a crystalline diamond which will give an almost anatomically sharp edge. It's extremely good for precision surgery.
Are there precious stones in Edinburgh?
We put this question to Ian Mercer:
Edinburgh actually is partly built on, or the castle is certainly built on, the remains of a volcano. Actually it's a basalt volcano. Yes, you can get gems in basalt but if you search all the basalt around the world looking for gems you'll only find gems in relatively few, relatively speaking. Before you try to seek permission to dig up Edinburgh I'd think about the geology first.
Chris: There's probably better things to find in Edinburgh like whiskey, for example!

Running Out of Oil?
Alistair Crosby, University of Cambridge:
Predictions of the end of oil have a long and undistinguished history. In 1874 the state geologist of Pennsylvania said that all the oil would be gone by 1878. Needless to say, it wasn't. In the 1970s pundits predicted we would run out by they year 2000 and they were wrong too. The reason why current predictions of peak oil production are almost certainly wide of the mark is that its price has increased hugely in the last ten years with no reduction in demand. This allows the production of reserves previously considered infeasible. The greater the price, the greater the fraction of a given oil field that can be extracted out for profit.
In other words, peak oil depends on price.
A good example is the tar sands of Venezuela. It is only economic to extract the heavy oil when prices are more than $30 per barrel. The sands in these two countries alone contain more oil than the conventional reserves of the rest of the world combined. More importantly high prices allow exploration of previously inaccessible areas such as the deep continental shelves of West Africa and Brazil. Exploration here is amazingly expensive. To drill a single well can cost upwards of a $100,000,000 but the rewards are immense. It may not be politic to say so, but global warming will also keep us in oil. As sea ice melts huge swathes of the arctic will become accessible and may contain reserves as large as anything in the Atlantic. It is no coincidence that Russia, America, Denmark and Canada re all aggressively staking their claims. In the end of course, we will reach the end of what can be viably extracted although probably not in our lifetimes. By then I think we will no longer care. As Sheikh Yamani famously said, "The stone age did not end because we had a lack of stones and the oil age will not end because we have a lack of oil." We have not invented oil's replacement yet, but I think it is only a matter of time.

What is tanzanite and where is tanzanite found?
We put this question to Ian Mercer:
The short answer is yes, that is true. Tanzanite is a really beautiful gemstone. It's a gem variety called zoisite which really occurs as a sort of powdery crust - greeny blue and brownish colours. Certainly not gem quality. That place in Tanzania is the only place in the world where quantities of gem quality zoisite form. Chris: Chemically what is it? Ian: It's another of these silicates. It's a pretty complex silicate in fact. We won't go into the formula because it would take longer than a minute. It's an orthorhombic silicate. One of its attributes is that it changes colour as you turn the stone around. It's what's called dichroic. It's treated in order to improve that blue colour as with most gem stones.
What is iolite and where is iolite found?
We put this question to Ian Mercer:
This is an interesting stone. This forms at very high pressure. This isn't a volcanic stone, this is a gemstone that forms as a mineral in rocks that are squeezed in between something. This is a stone which is intensely dichroic. It changes colour completely as you turn it round. Chris: Where do you find it?Ian: That comes form all sorts of places like Norway and Africa. Anywhere where deep-seated rocks are pushed up towards the surface.
- Previous Running out of Oil
- Next The science of cancer and Teleportation.











Comments
Add a comment