1
Chemistry / Re: Why does HNO3 exist but HPO3 does not?
« on: 29/11/2021 21:17:22 »
One can think of it as a hydration/dehydration equilibrium:
H2O + HXO3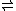 H3XO4
H3XO4
There are two reasons that the equilibrium favors the left-hand side when X = N, but the right-hand side when X = P, As, (or Sb, I believe):
1) (the simple answer, pointed out by Bored chemist) nitrogen is the smallest of the bunch (covalent radius of N = 75 pm, P = 106 pm, and As = 119 pm), so it is simply harder to fit the extra oxygen on there.
2) (the complex answer) the 2p orbitals on nitrogen and oxygen have fairly similar energies** (–13.1 and –15.9 eV vs vacuum, respectively) as well as very similar sizes (as noted in point 1), as well as shorter σ bonds (also due to point 1). This means that the p orbitals on neighboring O and N atoms can form very strong π interactions, and that the electrons in the π bonding and π anti-bonding orbitals would both be shared fairly equally.
In contrast, the valence of the phosphorus atom is a 3p orbital, with a much higher energy** (–10.2 eV; again comparing to –15.9 eV for O), and extends much farther away from the nucleus (but not towards a neighboring atom).

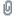 Screen Shot 2021-11-29 at 4.15.13 PM.png (42.56 kB . 794x624 - viewed 7327 times)
Screen Shot 2021-11-29 at 4.15.13 PM.png (42.56 kB . 794x624 - viewed 7327 times)
This means that in HNO3 the nitrogen atom has much more electron density compared to the P atom in HPO3 (the N is less electrophilic than the P, making the N less likely to accept electron density from another O), while the oxygen atoms bound to N have less electron density than those bound to P (the P-bound O atoms are more basic than N-bound, making them more likely to gain H+) and much stronger N=O bonds than P=O bonds (so it is easier to break the double bonds).

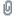 Screen Shot 2021-11-29 at 8.08.24 PM.png (45.63 kB . 546x680 - viewed 7302 times)
Screen Shot 2021-11-29 at 8.08.24 PM.png (45.63 kB . 546x680 - viewed 7302 times)
* https://www.schoolmykids.com/learn/interactive-periodic-table/covalent-radius-of-all-the-elements
** https://www.colby.edu/chemistry/PChem/notes/AOIE.pdf
H2O + HXO3
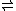 H3XO4
H3XO4There are two reasons that the equilibrium favors the left-hand side when X = N, but the right-hand side when X = P, As, (or Sb, I believe):
1) (the simple answer, pointed out by Bored chemist) nitrogen is the smallest of the bunch (covalent radius of N = 75 pm, P = 106 pm, and As = 119 pm), so it is simply harder to fit the extra oxygen on there.
2) (the complex answer) the 2p orbitals on nitrogen and oxygen have fairly similar energies** (–13.1 and –15.9 eV vs vacuum, respectively) as well as very similar sizes (as noted in point 1), as well as shorter σ bonds (also due to point 1). This means that the p orbitals on neighboring O and N atoms can form very strong π interactions, and that the electrons in the π bonding and π anti-bonding orbitals would both be shared fairly equally.
In contrast, the valence of the phosphorus atom is a 3p orbital, with a much higher energy** (–10.2 eV; again comparing to –15.9 eV for O), and extends much farther away from the nucleus (but not towards a neighboring atom).
This means that in HNO3 the nitrogen atom has much more electron density compared to the P atom in HPO3 (the N is less electrophilic than the P, making the N less likely to accept electron density from another O), while the oxygen atoms bound to N have less electron density than those bound to P (the P-bound O atoms are more basic than N-bound, making them more likely to gain H+) and much stronger N=O bonds than P=O bonds (so it is easier to break the double bonds).
* https://www.schoolmykids.com/learn/interactive-periodic-table/covalent-radius-of-all-the-elements
** https://www.colby.edu/chemistry/PChem/notes/AOIE.pdf
The following users thanked this post: Tomassci