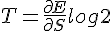Post by: hamdani yusuf on 20/09/2020 08:03:23
Quote
Temperature is a measure of the average kinetic energy of all the molecules in a gas. As the temperature and, therefore, kinetic energy, of a gas changes, the RMS speed of the gas molecules also changes. The RMS speed of the molecules is the square root of the average of each individual velocity squared.
In wikipedia,
https://en.m.wikipedia.org/wiki/Kinetic_theory_of_gases
(https://upload.wikimedia.org/wikipedia/commons/6/6d/Translational_motion.gif)
Quote
The kinetic theory of gases is a historically significant, but simple, model of the thermodynamic behavior of gases, with which many principal concepts of thermodynamics were established. The model describes a gas as a large number of identical submicroscopic particles (atoms or molecules), all of which are in constant, rapid, random motion. Their size is assumed to be much smaller than the average distance between the particles. The particles undergo random elastic collisions between themselves and with the enclosing walls of the container. The basic version of the model describes the ideal gas, and considers no other interactions between the particles and, thus, the nature of kinetic energy transfers during collisions is strictly thermal.average kinetic energy per molecule of ideal gas.
(https://wikimedia.org/api/rest_v1/media/math/render/svg/0271951ad96df02e2970eb811c2efb3139da2bc4)
For polyatomic gases, more energy is required to increase the temperature by the same amount. It means some amount of kinetic energy don't contribute to the increase of temperature.
The measurement of temperature should exclude contribution of the system's total translational and angular momentum. It should also exclude rotational and vibrational movement of individual particle.
The question thus becomes, what kind of movements are left to constitute the measure of temperature?
Post by: Bored chemist on 20/09/2020 11:14:19
So you can choose any of them as a measure of temperature.
https://en.wikipedia.org/wiki/Equipartition_theorem
For some systems- far from equilibrium- the temperatures can be different- or even negative.
Post by: hamdani yusuf on 20/09/2020 11:49:56
For some systems- far from equilibrium- the temperatures can be different- or even negative.How is the temperature measured before reaching equilibrium?
Imagine ten giant molecules each forming 1 kg of metal balls contained in 1 cubic meter box floating in the space. They don't move relatively to each other nor to the box but rotate at 1 rps. Do their angular speeds affect the measure of the system's temperature?
Post by: Bored chemist on 20/09/2020 12:24:09
How is the temperature measured before reaching equilibrium?Sometimes you can do this spectroscopically.
Sometimes it's impossible.
Do their angular speeds affect the masure of the system's temperature?
Hypothetically, yes.
There's a school experiment "measuring the temperature of a flame"- it's not very accurate but...
You take a lump of metal (If i remember rightly we used a large hexagonal nut) and heat it in boiling water so it reaches 100C
And then you put it in a beaker containing 100 ml of water at zero degrees and you measure the temperature rise of the water.
Then you heat the same lump of metal in the flame until it gets as hot as the flame (that's an approximation but...).
Then you put it into a beaker with 100 ml of ice cold of water and measure the temperature rise.
The temperature of the flame can be calculated from the ratio of the temperature changes etc.
It's not a very accurate measurement but it illustrates the point.
You can measure the temperature of your system of a box of metal balls floating in space by filling the box with water.
Viscous drag will slow the balls down and warm the water up.
And that lets you calculate the initial temperature of the box + contents.
Post by: hamdani yusuf on 20/09/2020 14:39:33
You can measure the temperature of your system of a box of metal balls floating in space by filling the box with water.That would convert mechanical kinetic energy into thermal energy. In Joule's experiment, it was accompanied by increase of temperature. It means that the end temperature is different than initial temperature, which is the one we want to determine.
Viscous drag will slow the balls down and warm the water up.
Post by: Bored chemist on 20/09/2020 15:11:14
That would convert mechanical kinetic energy into thermal energy.Is there a difference?
It means that the end temperature is different than initial temperature, which is the one we want to determine.So, it is exactly the same as the experiment to measure the temperature of a flame,
Post by: hamdani yusuf on 21/09/2020 04:03:58
an object with high mechanical energy but low thermal energy is cool.That would convert mechanical kinetic energy into thermal energy.Is there a difference?It means that the end temperature is different than initial temperature, which is the one we want to determine.So, it is exactly the same as the experiment to measure the temperature of a flame,
an object with low mechanical energy but high thermal energy is hot.
Post by: Bored chemist on 21/09/2020 08:33:59
But, as we have seen, if the "molecules" are a Kg, the distinction isn't clear.an object with high mechanical energy but low thermal energy is cool.That would convert mechanical kinetic energy into thermal energy.Is there a difference?It means that the end temperature is different than initial temperature, which is the one we want to determine.So, it is exactly the same as the experiment to measure the temperature of a flame,
an object with low mechanical energy but high thermal energy is hot.
In a more realistic scenario, the spheres wouldn't be stationary in their box- the uncertainty principle forbids it- so, from time to time they would bump into each other and they would convert rotational energy into translational and vibrational energy.
After a while, they would reach equilibrium where, on average, their energy is divided equally among all the available degrees of freedom.
And that's a temperature.
Post by: hamdani yusuf on 02/10/2020 07:50:44
In a more realistic scenario, the spheres wouldn't be stationary in their box- the uncertainty principle forbids it- so, from time to time they would bump into each other and they would convert rotational energy into translational and vibrational energy.I think it's possible to stabilize the system for a long time before the balls bump into each other, which makes the equilibrium has to wait even longer. Does it mean that the system's temperature can't be defined yet?
After a while, they would reach equilibrium where, on average, their energy is divided equally among all the available degrees of freedom.
And that's a temperature.
Post by: Bored chemist on 02/10/2020 08:25:34
Post by: hamdani yusuf on 05/10/2020 02:51:59
You can have a system with more than one temperature.What if all of those balls rotate at the same angular speed? How many temperatures are there?
Post by: Bored chemist on 05/10/2020 08:40:03
The best you could do would be , as I said, to average the energy somehow, for example by adding water.
Even a little gas would do, if you waited long enough.
Post by: hamdani yusuf on 09/10/2020 10:46:00
Box A contains 1 mole of Hydrogen gas (H2) at STP.
Box A contains 1 mole of Helium gas (He) at STP.
Both of them are sealed from environment.
A rotating ball with 1kJ of rotational energy is introduced into each of those containers.
When the balls stop spinning, their kinetic energies have been transfered to those gases. But since their Molar heat capacities are different, their final temperature would be different. (H2)=28.836 J/(mol·K) (He)=20.78 J/(mol·K)
Helium will have higher temperature than Hydrogen, despite having higher mass.
It means that Helium will have higher thermal energy than Hydrogen. I conclude that in Hydrogen, there are some kinetic energy which are not converted into thermal energy.
Post by: Bored chemist on 09/10/2020 12:30:19
Some of the heat in the hydrogen is in the form of vibrational or rotational energy of the molecules.
Post by: hamdani yusuf on 10/10/2020 02:26:06
In case of solids, the temperature is almost exclusively determined by vibrational motion.
This brings us back to the original question, what is temperature? Kinetic gas theory suggests that it's a form of kinetic energy. But heat transfer shows that it behaves like potential energy. The energy flow is similar to how electric batteries behave when connected to other batteries in parallel. Or how the liquid flow in connected vessels.
Post by: Bored chemist on 10/10/2020 12:03:52
It only means that in case of gases, they contribute less than translation.They all contribute the same energy per degree of freedom.
Post by: hamdani yusuf on 11/10/2020 03:05:33
Post by: Bored chemist on 11/10/2020 11:07:53
For a cold diatomic gas like nitrogen there's not enough to excite vibrations and so the only contributions to the heat capacity are translation and rotation.
That's 3 translational degrees and 2 rotational ones (rotation about the axis between the centres of the two atoms doesn't count) making 5 in total
When the gas is hot there is enough energy to get the molecules vibrating and that adds some more degrees of freedom into which energy can be placed.
That adds another degree of freedom , making 6 in total.
So the calculated heat capacities under those conditions are 2.5kT and 3 kT
But, at an intermediate temperature some, but not all, of the molecules will have enough energy to induce vibrations.
And under those conditions the average number of degrees of freedom will be somewhere between 5 and 6.
Post by: hamdani yusuf on 04/11/2020 02:36:09
But their atomic mass are very different, (4 for Helium and 222 for Radon)
Their densities also vary significantly (0.1786 g/L for Helium and 9.73 g/L for Radon).
Their temperatures are almost entirely determined by their average translational motion.
Increasing one mole of Helium by 1 Kelvin increases its internal energy by 20.78 Joule. Since the kinetic energy is ½mv², the average velocity of Helium atoms would increase by √(2 * 20.78 / 4 * 1000) = 101.93 m/s
For Radon, the internal energy will increase by 20.786 Joule.
The average atom velocity would increase by √(2 * 20.786 / 222 * 1000) = 13.68 m/s
Post by: Bored chemist on 04/11/2020 08:55:55
But their atomic mass are very different, (4 for Helium and 222 for Radon)
Their densities also vary significantly (0.1786 g/L for Helium and 9.73 g/L for Radon).
And the ratios are nearly constant
4/ 0.1786=22.40 litres per mole
222/ 9.73=22.82 litres per mole
Post by: puppypower on 04/11/2020 14:29:24
Water has an unusually heat capacity and boiling point, for a molecule so small in size. If you compare water; H2O, to methane; CH4 and ammonia; NH3, which all have the same molecular weights, water is in a different category. Water has more ways to tie up energy, so the temperature rise per unit of energy input is much lower. Temperature appears to be connected to saturation of available energy states, with some materials having more states needing saturation.
The greenhouse affect is based on CO2 and H2O having higher heat capacity than the rest of the gases in the atmosphere. Once these big dogs are saturated, any excess energy given off will heat the rest of the gases to a higher temperature.
Post by: Bored chemist on 04/11/2020 14:44:12
There is also a consideration called heat capacity.Did you think you were adding anything by saying that?
The grown ups have been discussing heat capacity in this thread for a while.
But since their Molar heat capacities are different
the only contributions to the heat capacity are translation and rotation.
the calculated heat capacities under those conditions are 2.5kT and 3 kT
Let's consider the molar heat capacities of noble gases
If you compare water; H2O, to methane; CH4 and ammonia; NH3, which all have the same molecular weights,In the very real sense that 18, 16 and 17 are the same number.
Did you consider not posting tosh?
The greenhouse affect is based on CO2 and H2O having higher heat capacity than the rest of the gases in the atmosphere.No, it is not.
that's utter hogwash.
Water has more ways to tie up energy, so the temperature rise per unit of energy input is much lower.Since the thread is talking about the vapour phase that's simply wrong (water vapour has a lower heat capacity) and complicatedly wrong ammonia has more ways to distribute the energy).
More interestingly, it's wrong for liquid phase material too.
Water at the BP 4.1813 j/g/k
liquid ammonia 4.700 j/g/k
(from wiki)
https://en.wikipedia.org/wiki/Table_of_specific_heat_capacities
So, as one might expect from a fool who believes in homoeopathy, you are wrong on essentially every "point" you have made.
Post by: hamdani yusuf on 05/11/2020 08:31:16
Their temperatures are almost entirely determined by their average translational motion.By contrasting those average speed values I was trying to figure out if they have anything to do with thermal radiation of gases, which are significantly different than black body radiation. Let's say those noble gases are contained in spherical containers with the same volume. With higher average speed, the gas particles would bounce more often with container wall, hence producing higher frequency of radiation, even though their temperatures are the same. I wonder if there are experimental evidence regarding this issue.
Increasing one mole of Helium by 1 Kelvin increases its internal energy by 20.78 Joule. Since the kinetic energy is ½mv², the average velocity of Helium atoms would increase by √(2 * 20.78 / 4 * 1000) = 101.93 m/s
For Radon, the internal energy will increase by 20.786 Joule.
The average atom velocity would increase by √(2 * 20.786 / 222 * 1000) = 13.68 m/s
Post by: Bored chemist on 05/11/2020 08:47:47
https://en.wikipedia.org/wiki/Spectral_line#Broadening_due_to_local_effects
At high enough temperatures and pressures the light emitted will be pretty close to black body radiation.
https://en.wikipedia.org/wiki/Xenon_arc_lamp
Post by: hamdani yusuf on 09/11/2020 04:47:05
Let's consider the molar heat capacities of noble gases which are almost uniform and very close to 21 J/(mol.K).From those results, we can infer that temperature is proportional to particle's mass and square of particle's speed. From previous information we also obtain that different type of motions contribute differently to the temperature of a system. Thus,
But their atomic mass are very different, (4 for Helium and 222 for Radon)
Their densities also vary significantly (0.1786 g/L for Helium and 9.73 g/L for Radon).
Their temperatures are almost entirely determined by their average translational motion.
Increasing one mole of Helium by 1 Kelvin increases its internal energy by 20.78 Joule. Since the kinetic energy is ½mv², the average velocity of Helium atoms would increase by √(2 * 20.78 / 4 * 1000) = 101.93 m/s
For Radon, the internal energy will increase by 20.786 Joule.
The average atom velocity would increase by √(2 * 20.786 / 222 * 1000) = 13.68 m/s
T=C.∑m.vn².εn
where
C is a proportionality constant.
m is particle's mass
vn is particle's speed in corresponding degree of freedom.
εn is effectiveness of each degree of freedom to affect system's temperature.
Post by: Bored chemist on 09/11/2020 09:02:30
Quantum mechanics modifies that and considers how likely it is that a given degree of freedom is actually excited.
we can infer that temperature is proportional to particle's mass and square of particle's speed.For a given number of gas molecules and a given energy- which is a rather odd set of data to have.
Post by: hamdani yusuf on 17/11/2020 07:34:04
https://en.wikipedia.org/wiki/Equipartition_theorem#History
Quote
The equipartition of kinetic energy was proposed initially in 1843, and more correctly in 1845, by John James Waterston.[15] In 1859, James Clerk Maxwell argued that the kinetic heat energy of a gas is equally divided between linear and rotational energy.[16] In 1876, Ludwig Boltzmann expanded on this principle by showing that the average energy was divided equally among all the independent components of motion in a system.[17][18] Boltzmann applied the equipartition theorem to provide a theoretical explanation of the Dulong–Petit law for the specific heat capacities of solids.
The history of the equipartition theorem is intertwined with that of specific heat capacity, both of which were studied in the 19th century. In 1819, the French physicists Pierre Louis Dulong and Alexis Thérèse Petit discovered that the specific heat capacities of solid elements at room temperature were inversely proportional to the atomic weight of the element.[20] Their law was used for many years as a technique for measuring atomic weights.[11] However, subsequent studies by James Dewar and Heinrich Friedrich Weber showed that this Dulong–Petit law holds only at high temperatures;[21] at lower temperatures, or for exceptionally hard solids such as diamond, the specific heat capacity was lower.[22]
Experimental observations of the specific heat capacities of gases also raised concerns about the validity of the equipartition theorem. The theorem predicts that the molar heat capacity of simple monatomic gases should be roughly 3 cal/(mol·K), whereas that of diatomic gases should be roughly 7 cal/(mol·K). Experiments confirmed the former prediction,[3] but found that molar heat capacities of diatomic gases were typically about 5 cal/(mol·K),[23] and fell to about 3 cal/(mol·K) at very low temperatures.[24] Maxwell noted in 1875 that the disagreement between experiment and the equipartition theorem was much worse than even these numbers suggest;[25] since atoms have internal parts, heat energy should go into the motion of these internal parts, making the predicted specific heats of monatomic and diatomic gases much higher than 3 cal/(mol·K) and 7 cal/(mol·K), respectively.
A third discrepancy concerned the specific heat of metals.[26] According to the classical Drude model, metallic electrons act as a nearly ideal gas, and so they should contribute (3/2) NekB to the heat capacity by the equipartition theorem, where Ne is the number of electrons. Experimentally, however, electrons contribute little to the heat capacity: the molar heat capacities of many conductors and insulators are nearly the same.[26]
Several explanations of equipartition's failure to account for molar heat capacities were proposed. Boltzmann defended the derivation of his equipartition theorem as correct, but suggested that gases might not be in thermal equilibrium because of their interactions with the aether.[27] Lord Kelvin suggested that the derivation of the equipartition theorem must be incorrect, since it disagreed with experiment, but was unable to show how.[28] In 1900 Lord Rayleigh instead put forward a more radical view that the equipartition theorem and the experimental assumption of thermal equilibrium were both correct; to reconcile them, he noted the need for a new principle that would provide an "escape from the destructive simplicity" of the equipartition theorem.[29] Albert Einstein provided that escape, by showing in 1906 that these anomalies in the specific heat were due to quantum effects, specifically the quantization of energy in the elastic modes of the solid.[30] Einstein used the failure of equipartition to argue for the need of a new quantum theory of matter.[11] Nernst's 1910 measurements of specific heats at low temperatures[31] supported Einstein's theory, and led to the widespread acceptance of quantum theory among physicists.[32]
I find these data surprising.
https://en.wikipedia.org/wiki/Deuterium#Data_for_elemental_deuterium
Quote
Specific heat capacity at constant pressure cp: Gas: 5200 J/(kg·K)Since molecular weight is 4 g/mol, molar heat capacity of deuterium is
5200 * 4 / 1000 = 20.8 J/(mol·K)
https://en.wikipedia.org/wiki/Hydrogen
Quote
Molar heat capacity (H2) 28.836 J/(mol·K)Since molecular weight is 2 g/mol, specific heat capacity of hydrogen is 28.836 * 1000 / 2 = 14418 J/(kg·K), which is much bigger than deuterium.
Molar heat capacity of deuterium is significantly different than hydrogen. It's surprising because in case of noble gases, different atomic masses don't seem to affect molar heat capacity.
Post by: Bored chemist on 17/11/2020 08:45:33
There's also this complication.
https://en.wikipedia.org/wiki/Spin_isomers_of_hydrogen
Post by: hamdani yusuf on 17/11/2020 10:26:35
Quote
Data for elemental deuteriumThe data is either at triple point or STP.
Formula: D2 or 2
1H
2
Density: 0.180 kg/m3 at STP (0 °C, 101.325 kPa).
Atomic weight: 2.0141017926 u.
Mean abundance in ocean water (from VSMOW) 155.76 ± 0.1 ppm (a ratio of 1 part per approximately 6420 parts), that is, about 0.015% of the atoms in a sample (by number, not weight)
Data at approximately 18 K for D2 (triple point):
Density:
Liquid: 162.4 kg/m3
Gas: 0.452 kg/m3
Viscosity: 12.6 μPa·s at 300 K (gas phase)
Specific heat capacity at constant pressure cp:
Solid: 2950 J/(kg·K)
Gas: 5200 J/(kg·K)
Post by: alancalverd on 17/11/2020 10:33:41
Post by: hamdani yusuf on 19/11/2020 01:12:40
https://webbook.nist.gov/cgi/cbook.cgi?ID=C7782390&Mask=1&Type=JANAFG&Table=on#JANAFG
At 300K, the molar heat capacity of deuterium is 29.19 J/(mol*K)
At 300K, the molar heat capacity of hydrogen is 28.85 J/(mol*K)
At 300K, the molar heat capacity of helium is 20.79 J/(mol*K)
https://webbook.nist.gov/cgi/cbook.cgi?ID=C1333740&Mask=1&Type=JANAFG&Table=on#JANAFG
https://webbook.nist.gov/cgi/cbook.cgi?ID=C7440597&Mask=1&Type=JANAFG&Table=on#JANAFG
Post by: alancalverd on 19/11/2020 01:33:16
Post by: Bored chemist on 19/11/2020 10:19:29
Noble gases are monatomic, so essentially billiard balls, whereas H2 and D2 are dumbell molecules withall sorts ofsix ways of storing and exchanging energy.
Three translations, a vibrational mode and two (degenerate) rotational ones.
and in the case of a "bent" molecule like H2O thereare umpteenis one morebending and asymmetric stretchingrotational modesavailable
Post by: Bored chemist on 19/11/2020 10:29:45
https://webbook.nist.gov/cgi/cbook.cgi?ID=C7782390&Mask=1#Thermo-GasFor helium, only the translational modes exist, so it has the lowest heat capacity of these gases. (And the same as neon, argon etc)
https://webbook.nist.gov/cgi/cbook.cgi?ID=C7782390&Mask=1&Type=JANAFG&Table=on#JANAFG
At 300K, the molar heat capacity of deuterium is 29.19 J/(mol*K)
At 300K, the molar heat capacity of hydrogen is 28.85 J/(mol*K)
At 300K, the molar heat capacity of helium is 20.79 J/(mol*K)
https://webbook.nist.gov/cgi/cbook.cgi?ID=C1333740&Mask=1&Type=JANAFG&Table=on#JANAFG
https://webbook.nist.gov/cgi/cbook.cgi?ID=C7440597&Mask=1&Type=JANAFG&Table=on#JANAFG
At 300K there's enough energy to excite the translational and rotational modes, but barely enough to excite the vibrational one.
However, the energy needed to excite the vibration in deuterium is a bit lower because the vibrational frequency is lower (the spring is pretty nearly the same, but the masses are larger).
So slightly more energy goes into getting the molecule vibrating, That's why D2 has a slightly greater heat capacity.
Post by: alancalverd on 19/11/2020 11:56:27
is a nice illustration of a phenomenon that occurs in Cambridge and Los Angeles, but not in Oxford, apparently ???
Post by: Bored chemist on 19/11/2020 12:26:58
http://www.chem.ucla.edu/~harding/IGOC/A/asymmetric_stretching.html#:~:text=Asymmetric%20stretching%3A%20Simultaneous%20vibration%20of%20two%20bonds%2C%20with,is%20contracting.%20Asymmetric%20bond%20stretching%20in%20water%20.Are you saying that you think that Cantabrian diatomics like H2 and D2 have an asymmetric stretch, or do you think that linear molecules can't?
is a nice illustration of a phenomenon that occurs in Cambridge and Los Angeles, but not in Oxford, apparently ???
The only change that being bent or linear makes is the removal of the rotational energy about the linear axis.
So
in the case of a "bent" molecule like H2O there is one more rotational modes availablecompared to a bent but otherwise similar molecule.
There are two approaches to this, in one case you count the atoms, multiply by 3 and then subtract 5 or 6 (depending on whether it's linear or not).
https://en.wikipedia.org/wiki/Molecular_vibration
In the other approach, you count the spectroscopists writing the posts.
Post by: alancalverd on 19/11/2020 14:33:04
Are you saying that you think that Cantabrian diatomics like H2 and D2 have an asymmetric stretch, or do you think that linear molecules can't?My original statement referred explicitly to H2O, which seems to have the same properties at UCLA as in Cantab. I can't imagine an asymmetric stretch in a diatomic molecule, but then I'm not a chemist! There being no suitable emoticon, semper lingua in buccam, socius.
The only change that being bent or linear makes is the removal of the rotational energy about the linear axis.
Post by: Bored chemist on 19/11/2020 15:55:09
My original statement referred explicitly to H2O,And it has 1 more mode than CO2 (where the difference is that the molecule is linear rather than bent), or 4 more modes than H2.
Yet you talked about
umpteen more bending and asymmetric stretching modes available,
What's your idea of the cut off for calling something "umpteen"?
Is it 1,2,3 or 4?
Post by: Bored chemist on 19/11/2020 16:00:36
semper lingua in buccam, socius.
My Latin is practically non existent but Google thinks you said "Always tongue into my head member".
I suggest you :-X
Post by: alancalverd on 19/11/2020 16:12:14
Post by: Bored chemist on 19/11/2020 17:02:31
What's your idea of the cut off for calling something "umpteen"?
Is it 1,2,3 or 4?
Post by: hamdani yusuf on 20/11/2020 07:14:38
Temp (K) Hydrogen Deuterium Helium Argon Radon
300 28.85 29.19 20.79 20.79 20.79
1000 30.20 31.64 20.79 20.79 20.79
3000 37.09 38.16 20.79 20.79 20.79
6000 41.97 42.25 20.79 20.79 20.79
From the table we can conclude that increase of temperature also increases the portion of rotational and vibrational movements in kinetic energy of diatomic gases. In noble gases, those types of motion are virtually non-existent.
Post by: hamdani yusuf on 24/11/2020 06:37:13
Here is a more complete table of molar heat capacity I compiled from NIST website.If we compare the results for hydrogen and deuterium, we get that particle's mass affect the portion of rotational and vibrational movements in kinetic energy of diatomic gases. But the mass doesn't affect molar heat capacity of noble gases whose motions are restricted to almost exclusively translational.
Temp (K) Hydrogen Deuterium Helium Argon Radon
300 28.85 29.19 20.79 20.79 20.79
1000 30.20 31.64 20.79 20.79 20.79
3000 37.09 38.16 20.79 20.79 20.79
6000 41.97 42.25 20.79 20.79 20.79
From the table we can conclude that increase of temperature also increases the portion of rotational and vibrational movements in kinetic energy of diatomic gases. In noble gases, those types of motion are virtually non-existent.
From those results, we can infer that temperature is proportional to particle's mass and square of particle's speed. From previous information we also obtain that different type of motions contribute differently to the temperature of a system. Thus,In case of noble gases, we can put vn close to 0 for rotational and vibrational motion.
T=C.∑m.vn².εn
where
C is a proportionality constant.
m is particle's mass
vn is particle's speed in corresponding degree of freedom.
εn is effectiveness of each degree of freedom to affect system's temperature.
In diatomic gases, εn for rotational and vibrational motion don't seem to be constant, but they're affected by temperature instead.
Post by: Bored chemist on 24/11/2020 09:00:43
In diatomic gases, εn for rotational and vibrational motion don't seem to be constant, but they're affected by temperature instead.It's one aspect of the quantisation of energy.
Post by: hamdani yusuf on 25/11/2020 02:37:05
How can energy quantisation help to explain the phenomenon I described above?In diatomic gases, εn for rotational and vibrational motion don't seem to be constant, but they're affected by temperature instead.It's one aspect of the quantisation of energy.
Post by: Bored chemist on 25/11/2020 08:37:25
In a classical world,you could have the molecules rotating at any speed, including very low ones.
And so, even at very low temperatures the heat capacity would include a contribution from rotation.
But in the quantum world, only certain rates of vibration are allowed.
So, unless kT is big enough, it will not be able to excite the rotation of the molecule and that will reduce the heat capacity of cold molecules.
The same is also true of vibrations and of electronic excitations.
Post by: hamdani yusuf on 08/12/2020 13:46:46
300 20.79 28.85 29.19 29.12 29.39 31.38
1000 20.79 30.20 31.64 32.69 34.86 37.09
3000 20.79 37.09 38.16 37.03 39.87 37.54
6000 20.79 41.97 42.25 38.27 44.39 28.99
Let's compare heat capacities of other light diatomic gases: N2,O2 & F2.
Nitrogen with triple bond has low heat capacity at low temperature, but increase more steadily.
Oxygen with double bond increase more heat capacity as temperature raised.
Fluorine with single bond initially increase heat capacity quicker, but then decline at high temperature.
Post by: Bored chemist on 08/12/2020 14:27:40
https://en.wikipedia.org/wiki/Singlet_oxygen
which complicates things.
I'm not sure how hot you have to get fluorine before it dissociates into two atoms.
Post by: hamdani yusuf on 10/12/2020 01:56:34
Post by: hamdani yusuf on 20/01/2021 08:28:19
Post by: hamdani yusuf on 21/01/2021 01:14:00
In vibrational motion, the kinetic energy is continuously exchanged with potential energy. So for a collection of particles with random phases of vibration, only some part of the system's total energy is manifested as kinetic energy at any given time. That's why gases capable of vibrational motion shows higher heat capacity than those with pure translational motion.Similar thing may apply to rotational motion too. But it's not obvious how potential energy is related to rotational motion. Apparently, potential energy must be derived from force and distance.
Let's try to analyze this scenario.
(https://upload.wikimedia.org/wikipedia/commons/thumb/f/f0/Rotating_spheres.svg/180px-Rotating_spheres.svg.png)
Two identical spheres, rotating about the center of the string joining them. Because of the rotation, the string is under tension.
Each sphere has mass (m) of 1 kg.
The radius of the trajectory (r) is 1 meter.
Moment of inertia (I) is ∑m*r² = 2 kg.m².
The angular velocity is (ω) 1 rad/s,
Hence the tangential velocity (v) is 1 m/s.
String tension = centripetal force = m*v²/r = 1*1²/1 = 1 Newton.
Angular momentum (L) is I*ω = 2*1 = 2 kg.m²/s.
Kinetic energy (Ek) = ½*I*ω² = ½*2*1² = 1 Joule.
Suppose the central point of the string is made of a retractable mechanism with negligible mass. The mass of the string is also negligible.
(https://images-na.ssl-images-amazon.com/images/I/61vgK7xv8BL._AC_SX679_.jpg)
Without significant external force, let's say using a timer inside, the lock mechanism of the retractable device is released.The string is then stretched so the radius of the new trajectory becomes 2 m.
The masses are conserved, 1 kg each.
Moment of inertia (I) is ∑m*r² = 1*2² + 1*2² = 8 kg.m².
Angular momentum (L) is conserved, still = 2 kg.m²/s.
The angular velocity is (ω) is L/I = 2/8 = 0.25 rad/s,
Hence the tangential velocity (v) is ω.r = 0.25*2 = 0.5 m/s.
Kinetic energy (Ek) = ½*I*ω² = ½*8*0.25² = 4/16 = 0.25 Joule.
String tension = centripetal force = m*v²/r = 1*0.5²/2 = 0.125 Newton.
We see there is a reduction of kinetic energy in the isolated system. If we assume that total energy is conserved, then there must be an increase in potential energy.
Post by: hamdani yusuf on 21/01/2021 03:39:58
We see there is a reduction of kinetic energy in the isolated system. If we assume that total energy is conserved, then there must be an increase in potential energy.But if the sequence is reversed, we don't see identical result. If we start from 2 meter radius and finish with 1 m, we would need energy to retract the string, because some force is required to oppose the string tension. Where did the energy go in the previous case?
Post by: hamdani yusuf on 23/01/2021 13:51:38
Without significant external force, let's say using a timer inside, the lock mechanism of the retractable device is released.The string is then stretched so the radius of the new trajectory becomes 2 m.Without energy dissipation, what we'll get is a reflection. The spheres will bounce back to their original radius.
When the lock is active, the spheres are moving in circular trajectory. But when it's released, they move linearly, tangent to the initial circle radius.
The distance of each spheres from the center vary between 1 and 2 meters. So the trajectory of each sphere, viewed from a stationary observer, is an isoscale triangle. The combined pattern will look like star of David.
Post by: hamdani yusuf on 24/01/2021 00:53:05
Post by: hamdani yusuf on 23/11/2021 06:35:09
Now I'd like to move on to other phases of matter. Particularly, I'm curious about what's the microscopic mechanism of emissivity factor ε. Black body radiation is just a special case where ε=1.
What's the minimum theoretical value of ε? What does it mean if a material has ε=0? What does it mean if a material has ε<0?
Quote
For surfaces which are not black bodies, one has to consider the (generally frequency dependent) emissivity factor ε(ν). This factor has to be multiplied with the radiation spectrum formula before integration. If it is taken as a constant, the resulting formula for the power output can be written in a way that contains ε as a factor:
(https://wikimedia.org/api/rest_v1/media/math/render/svg/dc5ba88162cd8c860f9982a73c3959b2f7ebf381)
This type of theoretical model, with frequency-independent emissivity lower than that of a perfect black body, is often known as a grey body. For frequency-dependent emissivity, the solution for the integrated power depends on the functional form of the dependence, though in general there is no simple expression for it. Practically speaking, if the emissivity of the body is roughly constant around the peak emission wavelength, the gray body model tends to work fairly well since the weight of the curve around the peak emission tends to dominate the integral.
Post by: Bored chemist on 23/11/2021 08:48:16
Particularly, I'm curious about what's the microscopic mechanism of emissivity factor ε.
https://en.wikipedia.org/wiki/Kirchhoff%27s_law_of_thermal_radiation
Post by: hamdani yusuf on 24/11/2021 03:32:37
I've read the link, which starts with this description:Particularly, I'm curious about what's the microscopic mechanism of emissivity factor ε.
https://en.wikipedia.org/wiki/Kirchhoff%27s_law_of_thermal_radiation
Quote
In heat transfer, Kirchhoff's law of thermal radiation refers to wavelength-specific radiative emission and absorption by a material body in thermodynamic equilibrium, including radiative exchange equilibrium.Unfortunately, I can't find the description of its microscopic mechanism, which will give us a way to increase or decrease the emissivity of a material at will.
A body at temperature T radiates electromagnetic energy. A perfect black body in thermodynamic equilibrium absorbs all light that strikes it, and radiates energy according to a unique law of radiative emissive power for temperature T, universal for all perfect black bodies. Kirchhoff's law states that:
For a body of any arbitrary material emitting and absorbing thermal electromagnetic radiation at every wavelength in thermodynamic equilibrium, the ratio of its emissive power to its dimensionless coefficient of absorption is equal to a universal function only of radiative wavelength and temperature. That universal function describes the perfect black-body emissive power.[1][2][3][4][5][6]
Here, the dimensionless coefficient of absorption (or the absorptivity) is the fraction of incident light (power) that is absorbed by the body when it is radiating and absorbing in thermodynamic equilibrium.
Post by: Bored chemist on 24/11/2021 20:51:55
The microscopic explanation of colour is quite well known.
Choosing a material which is a different colour is easy.
Changing the colour of a material is sometimes difficult, but not impossible.
https://en.wikipedia.org/wiki/Interference_filter
If the world you worked in was cold enough, you could easily build metamaterials with variable properties (in the microwave region) and vary their absorbance and therefore the emissivity
Post by: hamdani yusuf on 25/11/2021 03:07:35
Theoretically speaking, 0 emissivity means that there's no electromagnetic radiation from the surface. But 1000 °C temperature of the vessel means that if a small metal plate at room temperature touches the vessel, it will receive thermal energy from the vessel through conduction, and its temperature will increase toward 1000 °C.
Post by: Bored chemist on 25/11/2021 08:56:16
If I want to minimize heat loss from thermal radiation of a hot vessel, say 1000 °C, I must make the emissivity of its surface to near 0.
If the emissivity is near zero, that means (by Kirchhoff's law) that the absorptivity is also zero.
If the surface absorbs no light, then it must reflect it all.
That's why they silver the insides of thermos flasks.
Post by: puppypower on 25/11/2021 13:55:12
This paradox has to do with the second law, which states that the entropy of the universe has to increase. This net irreversible situation implies the universe is bleeding energy. Since entropy has to increase, the energy that is being lost into entropy is not net retrievable. We cannot undo the randomness of a state, and straighten things out, since the second law implies lost energy has to increase. Some energy within this entropic state is lost to the universe, via the added complexity and randomness, that also expresses a fixed temperature. There is a second way random affects add to a fix amount.
In the Gibb's Free Energy equation G=H-TS, where G is the free energy, H is internal energy or enthalpy, T is temperature and S is entropy. Temperature times entropy equals a free energy change, with the fixed temperature part of the state which defines constant entropy. This all adds to a constant; quantum state of entropy.
Post by: Bored chemist on 25/11/2021 15:26:51
It is weirdNot if you understand it.
Post by: hamdani yusuf on 05/12/2021 11:38:11
I think when silver is heated to 1000 Celcius, it loses most of its reflectiveness, which makes it similar to a black body. CMIIW.If I want to minimize heat loss from thermal radiation of a hot vessel, say 1000 °C, I must make the emissivity of its surface to near 0.
If the emissivity is near zero, that means (by Kirchhoff's law) that the absorptivity is also zero.
If the surface absorbs no light, then it must reflect it all.
That's why they silver the insides of thermos flasks.
Post by: Bored chemist on 05/12/2021 12:02:21
You can see a highlight and image reflected in this stream of meal here
https://videohive.net/item/pouring-molten-silver-into-a-mold/20499699
[ Invalid Attachment ]
Post by: hamdani yusuf on 05/12/2021 13:24:18
Post by: Bored chemist on 05/12/2021 13:40:55
But it emits light quite brightly in a dark room.No.
A black body at the same temperature would be much brighter.
Post by: hamdani yusuf on 05/12/2021 21:57:03
It's a grey body then. Is emissivity a constant, or it depends on temperature?But it emits light quite brightly in a dark room.No.
A black body at the same temperature would be much brighter.
Post by: Bored chemist on 06/12/2021 08:56:01
Post by: hamdani yusuf on 09/12/2021 04:02:41
It varies with temperature and wavelength.Is it possible for a material to have emissivity higher than black body for some specific range of frequency? (presumably lower for other frequency range)
Post by: Bored chemist on 09/12/2021 08:53:47
Probably not, though I wouldn't like to absolutely rule it out in the case of laser emission.It varies with temperature and wavelength.Is it possible for a material to have emissivity higher than black body for some specific range of frequency? (presumably lower for other frequency range)
Post by: hamdani yusuf on 17/03/2022 05:05:50
https://arxiv.org/abs/physics/0507007
https://www.researchgate.net/publication/26414792_An_Analysis_of_Universality_in_Blackbody_Radiation/link/0e5f9e2ff0c41c4932dc6612/download
Quote
An Analysis of Universality in Blackbody RadiationMy question is, how big is the required size of graphite to make a perfectly reflecting box act like a black body?
Pierre-Marie Robitaille, Ph.D.*
Chemical Physics Program
The Ohio State University, Columbus, Ohio 43210
Through the formulation of his law of thermal emission, Kirchhoff conferred upon blackbody radiation the
quality of universality [G. Kirchhoff, Annalen der Physik 109, 275 (1860)]. Consequently, modern physics holds that
such radiation is independent of the nature and shape of the emitting object. Recently, Kirchhoff’s experimental work
and theoretical conclusions have been reconsidered [P.M.L. Robitaille. IEEE Transactions on Plasma Science. 31(6),
1263 (2003)]. In this work, Einstein’s derivation of the Planckian relation is reexamined. It is demonstrated that claims
of universality in blackbody radiation are invalid.
From the onset, blackbody radiation was unique in possessing the virtue of universality [1,2]. The nature of the
emitting object was irrelevant to emission. Planck [3], as a student of Kirchhoff, adopted and promoted this concept
[4,5]. Nonetheless, he warned that objects sustaining convection currents should not be treated as blackbodies [5].
As previously discussed in detail [6], when Kirchhoff formulated his law of thermal emission [1,2], he utilized
two extremes: the perfect absorber and the perfect reflector. He had initially observed that all materials in his laboratory
displayed distinct emission spectra. Generally, these were not blackbody in appearance and were not simply related to
temperature changes. Graphite, however, was an anomaly, both for the smoothness of its spectrum and for its ability to
simply disclose its temperature. Eventually, graphite’s behavior became the basis of the laws of Stefan [7], Wien [8] and
Planck [3].
For completeness, the experimental basis for universality is recalled [1,2,5,6]. Kirchhoff first set forth to
manufacture a box from graphite plates. This enclosure was a near perfect absorber of light (ε =1, κ =1). The box had a
small hole, through which radiation escaped. Kirchhoff placed various objects in this device. The box would act as a
transformer of light [6]. From the graphitic light emitted, Kirchhoff was able to gather the temperature of the enclosed
object once thermal equilibrium had been achieved. A powerful device had been constructed to ascertain the
temperature of any object. However, this scenario was strictly dependent on the use of graphite.
Kirchhoff then sought to extend his findings [1,2,5]. He constructed a second box from metal, but this time the
enclosure had perfectly reflecting walls (ε =0, κ =0). Under this second scenario, Kirchhoff was never able to reproduce
the results he had obtained with the graphite box. No matter how long he waited, the emitted spectrum was always
dominated by the object enclosed in the metallic box. The second condition was unable to produce the desired spectrum.
As a result, Kirchhoff resorted to inserting a small piece of graphite into the perfectly reflecting enclosure [5].
Once the graphite particle was added, the spectrum changed to that of the classic blackbody. Kirchhoff believed he had
achieved universality. Both he, and later, Planck, viewed the piece of graphite as a "catalyst" which acted only to
increase the speed at which equilibrium was achieved [5]. If only time was being compressed, it would be
mathematically appropriate to remove the graphite particle and to assume that the perfect reflector was indeed a valid
condition for the generation of blackbody radiation.
However, given the nature of graphite, it is clear that the graphite particle was in fact acting as a perfect
absorber. Universality was based on the validity of the experiment with the perfect reflector, yet, in retrospect, and given
a modern day understanding of catalysis and of the speed of light, the position that the graphite particle acted as a
catalyst is untenable. In fact, by adding a perfect absorber to his perfectly reflecting box, it was as if Kirchhoff lined the
entire box with graphite. He had unknowingly returned to the first case. Consequently, universality remains without any
experimental basis.
Post by: Bored chemist on 17/03/2022 08:52:19
You need to give enough time for all the light bouncing round the box to hit the black object. (probably a few times because it's not actually a perfect absorber.
Another way to consider it is to imagine looking into the box- if it doesn't look black, it won't emit as if it is.
Post by: hamdani yusuf on 17/03/2022 09:36:21
In principle, it depends how long you are prepared to wait.Let's say the box is a cube 1 cubic meter, and the peep hole is on one of its corner. In a second, the light must have been reflected thousands of times.
You need to give enough time for all the light bouncing round the box to hit the black object. (probably a few times because it's not actually a perfect absorber.
Another way to consider it is to imagine looking into the box- if it doesn't look black, it won't emit as if it is.
Post by: Bored chemist on 17/03/2022 09:43:49
But imagine shining a narrow beam of light in through the hole.
Will it hit the black particle as it bounces round?
It's also important to remember that no surface is a truly perfect reflector.
So a small hole is quite a good black body, even if the box is polished.
Post by: hamdani yusuf on 17/03/2022 13:36:24
You will never get a perfect black hole.Eventually it will.
But imagine shining a narrow beam of light in through the hole.
Will it hit the black particle as it bounces round?
It's also important to remember that no surface is a truly perfect reflector.
So a small hole is quite a good black body, even if the box is polished.
As described by the research paper, the inside of the second box has an extremely low absorbance.
QuoteKirchhoff then sought to extend his findings [1,2,5]. He constructed a second box from metal, but this time the
enclosure had perfectly reflecting walls (ε =0, κ =0). Under this second scenario, Kirchhoff was never able to reproduce
the results he had obtained with the graphite box. No matter how long he waited, the emitted spectrum was always
dominated by the object enclosed in the metallic box. The second condition was unable to produce the desired spectrum.
Post by: hamdani yusuf on 17/03/2022 13:49:36
The temperature is 300K, just like the room temperature.
A 1kg Aluminum cube is placed right next to the first cube. Its initial temperature is 310K.
The Eddie current would increase the temperature of the aluminum cube, while reducing the rotation rate of the spinning magnet. Here we see electromagnetic energy transfer from lower temperature body to higher temperature body. Thus the radiation type can't be thermal, although it's surely electromagnetic in nature.
Post by: Bored chemist on 18/03/2022 13:24:36
Eventually it will.I am pleased to see that you recognise what I said earlier.
In principle, it depends how long you are prepared to wait.
I imagine a 1 liter plastic cube containing a 1 kg spinning bar magnet with negligible friction at 100 rotations per second.Energy is transferred to the aluminium, but it isn't thermal energy which the spinning magnet looses; but kinetic energy.
The temperature is 300K, just like the room temperature.
A 1kg Aluminum cube is placed right next to the first cube. Its initial temperature is 310K.
The Eddie current would increase the temperature of the aluminum cube, while reducing the rotation rate of the spinning magnet. Here we see electromagnetic energy transfer from lower temperature body to higher temperature body. Thus the radiation type can't be thermal, although it's surely electromagnetic in nature.
A cold bullet fired into hot water will heat the water.
Post by: hamdani yusuf on 19/03/2022 03:37:44
imagineThis video came to my mind.
Post by: hamdani yusuf on 19/03/2022 03:56:58
Energy is transferred to the aluminium, but it isn't thermal energy which the spinning magnet looses; but kinetic energy.Indeed. The question is, what distinguishes thermal energy from kinetic energy? What distinguishes thermal radiation from other kind of electromagnetic radiations?
Post by: hamdani yusuf on 19/03/2022 07:14:21
I imagine a 1 liter plastic cube containing a 1 kg spinning bar magnet with negligible friction at 100 rotations per second.Let's make the system more symmetrical. 1 kg aluminium bar is free to rotate in an axis, put inside a 1 liter plastic cube.
The temperature is 300K, just like the room temperature.
A 1kg Aluminum cube is placed right next to the first cube. Its initial temperature is 310K.
In case where those bars are coaxial, some kinetic energy will be transferred from the magnet to the aluminium bar. But if their axes are perpendicular to each other, the aluminium bar won't receive the kinetic energy.
Post by: Bored chemist on 19/03/2022 11:45:18
The question is, what distinguishes thermal energy from kinetic energy?The motion having some sort of structure, rather than being random. Essentially, it's an effect of entropy.
What distinguishes thermal radiation from other electromagnetic radiation?In the case of your experiment, the spectrum.
Post by: hamdani yusuf on 19/03/2022 12:42:52
Afaik, thermal energy has highest entropy among other kinds of energy, which makes it the most random form.The question is, what distinguishes thermal energy from kinetic energy?The motion having some sort of structure, rather than being random. Essentially, it's an effect of entropy.What distinguishes thermal radiation from other electromagnetic radiation?In the case of your experiment, the spectrum.
Black body radiation is the simplest spectral distribution of thermal electromagnetic radiation. The object's temperature can be calculated from the peak frequency. But most objects are not black body. Spectrum of low pressure gas has much different shape than black body radiation.
Post by: hamdani yusuf on 29/03/2022 04:14:47
For there to be an exchange of heat, the mean internal kinetic energy of one object must decrease and that of the other object must increase. We measure mean kinetic energy as temperature. If two objects are at the same temperature, their temperatures will not change by putting them in contact with one another. That's how temperature is derived from the zeroth law.https://en.wikipedia.org/wiki/Gravity_assist involves exchange of kinetic energy.
Quickly rotating magnets or electrets in a box have large kinetic energy, although they have ambient temperature.
We also have problems like https://en.wikipedia.org/wiki/Stellar_corona#Coronal_heating_problem.
And how https://en.wikipedia.org/wiki/Parker_Solar_Probe can survive high temperature environment.
Post by: hamdani yusuf on 29/03/2022 08:30:31
What seems more relevant is just to jump straight to a higher level answer:Do you have any idea why it's hard to define?
"Temperature", "thermal contact" and "heat" are all very difficult things to define. At least, they are difficult to define or explain at any microscopic scale (where you're trying to take a reductionist approach and break things down to the smallest indivisible units like particles with well defined properties). However, they can be easily defined (or really just decalred to exist) on a macroscopic scale. This is the usual development of the subject matter that is called "thermodynamics".
Post by: Bored chemist on 29/03/2022 08:37:53
Do you have any idea why it's hard to define?As he said; there's a difference between the macroscopic and microscopic pictures.
You also need to be aware of this
https://en.wikipedia.org/wiki/Equipartition_theorem
And this
https://en.wikipedia.org/wiki/Virial_theorem
Post by: hamdani yusuf on 29/03/2022 08:45:23
For example, if you try to take a microscopic definition for "heat" then it is not supposed to include a transfer of matter from one system to another BUT a transfer of photons such as infra-red radiation is something we would very much like to include in the definition of heat. What makes a photon different to some other particle with energy? If an electron and positron cross a barrier then that would seem to be a transfer of matter across the barrier and would not be considered as a heat transfer. However, if they annhilate on one side of the barrier, only some photons cross the barrier and then the photons interact with some nucleus on the other side of the barrier to reform particles and anti-particles - that would seem to be just fine.Is there any exclusion from following methods of heating water?
- Radio wave heating
- Microwave heating
- Infrared heating
- Visible Laser heating
- Induction heating
- Ohmic heating
Post by: hamdani yusuf on 29/03/2022 09:02:20
What makes it easier to define in macroscopic picture?Do you have any idea why it's hard to define?As he said; there's a difference between the macroscopic and microscopic pictures.
You also need to be aware of this
https://en.wikipedia.org/wiki/Equipartition_theorem
And this
https://en.wikipedia.org/wiki/Virial_theorem
Quote
https://en.wikipedia.org/wiki/Equipartition_theoremFor large molecules, it seems to be easier to vibrate than translate or rotate, especially when the shape is long like a needle, and the intermolecular space is limited.
In classical statistical mechanics, the equipartition theorem relates the temperature of a system to its average energies. The equipartition theorem is also known as the law of equipartition, equipartition of energy, or simply equipartition. The original idea of equipartition was that, in thermal equilibrium, energy is shared equally among all of its various forms; for example, the average kinetic energy per degree of freedom in translational motion of a molecule should equal that in rotational motion.
(https://upload.wikimedia.org/wikipedia/commons/2/23/Thermally_Agitated_Molecule.gif)
Post by: alancalverd on 29/03/2022 11:02:19
Post by: alancalverd on 29/03/2022 11:03:40
Quickly rotating magnets or electrets in a box have large kinetic energy,You ignored the word "internal" in my quote. It isn't there for padding!
Post by: hamdani yusuf on 29/03/2022 11:13:28
The magnets are inside a box.Quickly rotating magnets or electrets in a box have large kinetic energy,You ignored the word "internal" in my quote. It isn't there for padding!
IMO water molecules can be modeled as microscopic electrets.
Post by: hamdani yusuf on 29/03/2022 11:30:32
Temperature is a statistic of a very large ensemble so it is only defined macroscopically.How many molecules is the minimum limit for a system to have a defined temperature?
Post by: Bored chemist on 29/03/2022 13:08:16
Enough for the QM effects to be relatively small compared to the total energy.Temperature is a statistic of a very large ensemble so it is only defined macroscopically.How many molecules is the minimum limit for a system to have a defined temperature?
Post by: Bored chemist on 29/03/2022 13:09:58
seemsIf you are using that word in science, be very careful.
What "seems" to happen isn't reliably what does happen.
Post by: Bored chemist on 29/03/2022 13:11:41
For large molecules, it seems to be easier to vibrate than translate or rotate,How far do you think it has to translate or rotate?
Post by: Eternal Student on 29/03/2022 16:11:20
What makes it (temperature) easier to define in macroscopic picture?
As @alancalverd stated:
Temperature is a statistic of a very large ensemble so it is only defined macroscopically.Although I would have used a few more words and said something like this:
It has been observed that macroscopic systems seem to follow certain behaviours. Developing models that assume the system has quantities called state variables and there is a connection between those state variables called the equation(s) of state was extremely successful. This is thermodynamics. Different systems can have different state variables. Temperature and Pressure are just some common examples of state variables that some systems have and the ideal gas equation PV = kT is a good example of an equation of state that might apply.
So, "temperature" is just a state variable that some systems will have. This is an acceptable definition of temperature for thermodynamics. There is no requirement to attempt to explain temperature at any microscopic scale. It is simply a property that some systems will have.
You (Hamdani) might feel that a definition should really be an explanation for why something exists or what it is on some small scale. Most of us are biased and believe that a reductionist approach should be applied to science, i.e. that everything should be pulled apart and examined on the smallest and possibly most fundamental level. However, there may not be a more fundamental level for temperature. It does seem to be an emergent property that a whole system might have but not something that any small component like a particle in that system must have.
There are some objects and some systems that do not have a well defined temperature.
Simple examples: A small container of gas that has been left for days can be assumed to have a well defined temperature. A container of gas that has just had some high velocity gas particles added to one side of it does not have a well defined temperature until enough time has passed and an equilibrium and equi-partition of energy has been established.
Now, for some systems we can make additional assumptions. We can assume properties and relationships will hold above and beyond just having some state variables for the system and an equation of state. For example, an ideal gas can be assumed to consist of particles with properties you expect of particles like velocity and mass. We can then assume one state variable, Pressure, can be determined at the walls of the container as being the consequence of collisions and all the usual principles apply. (Note that it's not assumed pressure only exists at the walls, just that it is easily determined at the walls). Anyway, from these assumptions you can determine that the state variable "temperature" would be proportional to the average kinetic energy of the particles. This is a bonus, for some systems it does seem that temperature could have a microscopic explanation. It's a hint that there might be a good microscopic explanation for every real-world system but that's all - it's not a guarantee or an absolute requirement. It's sufficient that temperature is just a state variable of a system.
Best Wishes.
Post by: Eternal Student on 29/03/2022 18:49:56
Is there any exclusion from following methods of heating water?
- Radio wave heating
- Microwave heating
- Infrared heating
- Visible Laser heating
- Induction heating
- Ohmic heating
I'm not the definitive rule maker. These seem to be conventional forms of radiation and are very much the sort of energy transfer that you would want to consider as heat when you apply the existing and conventional concepts and models from thermodynamics to the problem you're considering. Ohmic heating is different but that is included in most definitions of heat. (See earlier posts and Wikipedia's definition of permitted heat transfer mechanisms).
You seem to be under the impression that there will always be one universally agreed upon definition of heat but I don't think there has to be one. Different systems only support a few mechanisms of heat transfer, they do not have to support them all.
It is optimistic to assume that if we keep taking a large enough picture, everything can be considered as a thermodynamic system and we would always be able to identify state variables like temperature for that system. It may actually be true but it's not required. Instead it's better to consider that thermodynamics is a simplification and idealisation, there are many systems to which thermodynamics can be applied. In each system there are different things that will be considered as a heat flow.
Best Wishes.
Post by: hamdani yusuf on 30/03/2022 14:50:42
Different systems can have different state variables. Temperature and Pressure are just some common examples of state variables that some systems have and the ideal gas equation PV = kT is a good example of an equation of state that might apply.I think this ideal gas definition is the simplest way (mathematically) to describe temperature. Practical methods to measure temperature, such as used in mercury and alcohol thermometer, bimetal, thermocouple, RTD, and infrared thermometer are less reliable, involve non-linearity, and have narrower range and use case.
Post by: hamdani yusuf on 31/03/2022 03:28:31
There are some objects and some systems that do not have a well defined temperature.Perhaps I can add another example. The air inside a running microwave oven, radio wave oven, induction cooker, laser oven, may show different temperature measurement value, depending on the thermometer type used.
Simple examples: A small container of gas that has been left for days can be assumed to have a well defined temperature. A container of gas that has just had some high velocity gas particles added to one side of it does not have a well defined temperature until enough time has passed and an equilibrium and equi-partition of energy has been established.
Post by: Eternal Student on 31/03/2022 04:24:07
I think this ideal gas definition is the simplest way (mathematically) to describe temperature.
Yes. I like a bit of theory.
In fairness, we should mention that it's a bit of shame that not everything is an ideal gas. People will still assign a temperature to these things and you can be pretty sure they will have used a hybrid of methods or approaches to defining temperature.
Best Wishes.
Post by: hamdani yusuf on 31/03/2022 07:16:43
Hi.For objects that are not ideal gas, their temperature can be defined as the same as the ideal gas which don't exchange energy with them when they are in contact, which is indicated by preservation of the ideal gas temperature where the pressure and volume are maintained. Of course, it's easier said than done. But at least, with a robust definition, the practical difficulties can be addressed and solved using engineering methods.I think this ideal gas definition is the simplest way (mathematically) to describe temperature.
Yes. I like a bit of theory.
In fairness, we should mention that it's a bit of shame that not everything is an ideal gas. People will still assign a temperature to these things and you can be pretty sure they will have used a hybrid of methods or approaches to defining temperature.
Best Wishes.
Post by: Bored chemist on 31/03/2022 08:56:42
Perhaps I can add another example. The air inside a running microwave oven, radio wave oven, induction cooker, laser oven, may show different temperature measurement value, depending on the thermometer type used.Temperature is still only meaningful if a system is at equilibrium.
For some systems- far from equilibrium- the temperatures can be different- or even negative.
Post by: hamdani yusuf on 31/03/2022 10:46:58
Temperature is still only meaningful if a system is at equilibrium.How do you define equilibrium?
Imagine a frozen pond in a winter. The depth is 10 meters. The air temperature above the surface is -10°C. One meter ice layer has been formed on the surface. The rest of the pond is still liquid water. This condition hasn't changed for a week. Is it in equilibrium?
Post by: Bored chemist on 31/03/2022 12:07:40
No. It can't be at eqm because it has a temperature gradient.Temperature is still only meaningful if a system is at equilibrium.How do you define equilibrium?
Imagine a frozen pond in a winter. The depth is 10 meters. The air temperature above the surface is -10°C. One meter ice layer has been formed on the surface. The rest of the pond is still liquid water. This condition hasn't changed for a week. Is it in equilibrium?
That's because heat is flowing from the Earth's core, into the water, up through the ice and out again.
Post by: alancalverd on 31/03/2022 18:29:32
The air inside a running microwave oven, radio wave oven, induction cooker, laser oven, may show different temperature measurement value, depending on the thermometer type used.No. The temperature of the air (assuming it is well stirred and thus in internal equilibrium) has a unique value. The practical problem is that the power source interacts differently with different types of thermometer and if you aren't careful you end up measuring the microwave absorbtivity of a thermocouple or whatever, or even generating a load of sparks and no data. An external thermistor bolometer is probably the simplest practical device for an industrial oven but even then you need to ensure that the emissivity of the air is greater than that of the oven itself - quite difficult to do. If the oven is ventilated, you could put a glass bottle gas thermometer inside and measure the pressure of the working volume of air, which will be close to that of its ambient. If it isn't ventilated, than a pressure gauge will give you the answer.
Post by: alancalverd on 31/03/2022 18:33:54
For some systems- far from equilibrium- the temperatures can be different- or even negative.The concept of negative temperature is interesting but AFAIK it can never be more than a concept. Something to do with Carnot, but then you can't trust the French.
Post by: Eternal Student on 31/03/2022 18:54:37
For objects that are not ideal gas, their temperature can be defined as the same as the ideal gas which don't exchange energy with them when they are in contactYes. That is one option and I'm sure it's the most common choice. This is often called a thermodynamic approach to defining temperature. Define two temperatures to be equivalent when heat doesn't flow from one body to the other when they are in thermal contact.
It involves macroscopic properties of the two bodies or two systems and some issues deciding what criteria must be met to say the two systems are in thermal contact. A lot of this has been discussed earlier.
There are alternative ways to define the temperature of a system that isn't an ideal gas which still try to connect it to temperature of an ideal gas, like sticking a thermometer into the test system and also into some samples of ideal gas held at known temperatures. Declare them to be at the same temperature if the thermometer reads the same.
Extending the definition of temperature to objects that aren't ideal gases would involve a hybrid of approaches to defining temperature (for example, the ideal gas law for the ideal gas + Thermodynamic approach for the test object transferring heat to the ideal gas ; or ideal gas law for the ideal gas + empirical temperature definition with a thermometer).
It's been said before but "temperature" is a difficult thing to define.
Wikipedia has a decent section describing various approaches for defining temperature which they describe as different "temperature scales" including:
Scales based on a thermodynamic approach; Empirical Scales based on physical properties that a thermometer device can have; Theoretical scales such as that obtained by using kinetic theory; Temperature scales just for an ideal gas obtained from the equation of state or ideal gas law.
See: https://en.wikipedia.org/wiki/Temperature#Classification_of_scales
There are few systems for which temperature, especially a definition based on kinetic theory of particles, is really well understood. I would say ideal gases are the only object for which it is well understood (but this is a simplification. The spirit of it is correct, kinetic theory and temperature for ideal gases is well understood and it isn't for much else. There are some theoretical constructions like crystal lattices for which some kinetic theory has been studied and possibly some other things in the literature which I haven't had the time to look at). Anyway, for an ideal gas, all the different definitions or approaches to determining temperature become identical.
Specifically,
1. two samples of ideal gas have the same temperature if and only if
2. there is no transfer heat from one system to the other when they are in thermal contact if and only if
3. their particles have the same average k.e. if and only if
4. a thermometer stuck into each sample reads the same if and only if
5. some other measure of temperature that I might have accidentally forgotten gave the same result.
For objects that aren't ideal gases it's not clear that all the definitions or approaches for determining temperature would give the same results.
- - - - - - - - - -
Practical methods to measure temperature, such as used in mercury and alcohol thermometer, bimetal, thermocouple, RTD, and infrared thermometer are less reliable, involve non-linearity, and have narrower range and use case.Yes. Although it's fairly arbitrary to say that these practical or thermometer based approaches to defining temperature show non-linearity. It implies that some other measure of temperature like that based on kinetic theory is better or more truthful. Perhaps the thermometer based approach is the right one and the absolute temperature that seems to have been standardized as the Kelvin scale shows non-linearity in comparison to that.
Historically, thermometers and/or the ideal gas law were used to measure and define temperature when thermodynamics was being developed. It wasn't until later that they realised it may not be measuring exactly what they wanted to measure for the most logical and streamlined formulation of thermodynamics and/or of microsocopic statistical mechanics.
- - - - - - - - - -
Perhaps I can add another example. The air inside a running microwave oven, radio wave oven, induction cooker, laser oven, may show different temperature measurement value, depending on the thermometer type used.Yes. See earlier discussion. Different thermometers and indeed different approaches to trying to define temperature can give different results for the temperature.
As @Bored chemist outlined: You have to be careful what you try and assign a temperature to. Is the air inside a running microwave just going to be considered as the air particles (like N2 molecules) and the application of kinetic theory to the motion of those particles OR as a composite object which is the entire contents of the oven and consists of air particles + the photons in it. If you were trying to determine the temperature of the composite object then the energy doesn't seem to have been equi-partioned yet: There is far more radiation in the microwave range then there should be for the natural radiation properties of the air. The contents of the oven isn't in a state of equilibrium yet. Give it more time and a well defined temperature should appear, of course that won't happen while the oven is left switched on and running, you're putting microwaves into it faster than the natural processes in the air and perhaps also in the walls of the oven can re-distribute and equi-partition the energy.
---Stopped writing, this is already too long and has overlapped with other replies---
Best Wishes.
Post by: Bored chemist on 31/03/2022 19:58:05
For some systems- far from equilibrium- the temperatures can be different- or even negative.The concept of negative temperature is interesting but AFAIK it can never be more than a concept.
The population inversion in a laser corresponds to a negative thermodynamic temperature.
It's a long way from equilibrium so a temperature isn't well defined. That's the point.
Post by: Bored chemist on 31/03/2022 20:00:31
No. The temperature of the air (assuming it is well stirred and thus in internal equilibrium) has a unique value.No.
It's possible for the rotational temperature of the water vapour to be different from the translational temperature or , especially if there's any ionisation, for the electron temperature to be different again.
Post by: Bored chemist on 31/03/2022 20:02:15
It isn't an ideal gas,
Post by: Eternal Student on 31/03/2022 21:41:21
We know, by definition, the temperature of water at its triple point.That's a definition of one value for a temperature for one type of macroscopic system. It doesn't provide a whole scale of temperature or any sense of how I can declare that another type of system (perhaps some liquid alcohol) is at the same temperature.
It isn't an ideal gas,
It is often extended to a whole scale of temperature such as the Celsius system, where the boiling point of water is used as the other fixed point of the scale and a thermometer device like a mercury thermometer has the difference in the height of the mercury column divided into 100 units (well it was 100 units when the freezing point of water was the lower fixed point, which is what they first used - but it's about 99.99 units if you use the triple point). The usual way of applying the same definitions of temperature to other systems (things that aren't just water) is to just stick the thermometer into that other system and take a reading of the height of the mercury column. Anyway, the whole system is then seen to be an empirical approach (it uses physical properties of a thermometer device) to define a temperature scale.
Best Wishes.
Post by: Bored chemist on 31/03/2022 23:16:13
That's a definition of one value for a temperature for one type of macroscopic system. It doesn't provide a whole scale of temperatureIt (together with the definition of absolute zero) is the only fixed point on the whole of the Kelvin temperature scale.
Last time I checked, they compared things to the water point, using a constant volume helium thermometer extrapolated to zero pressure.
That is, in effect, using the ideal gas laws.
This might be helpful.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4865254/
Post by: Eternal Student on 01/04/2022 01:46:55
This might be helpful.That is interesting and I've only scanned through it so far. I'll read more later.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4865254/
As far as I can see the latest revisions to the Kelvin temperature scale were made in 2019 and that paper or article was written in 2016. I think they (the article referenced) are talking about the modifications that were about to bring the standard to what it is now.
I think there's a mis-print in the article (first paragraph under the title Gas Thermometry) :
Gas thermometry relies on the statistical-mechanical connection between the three kinetic degrees of freedom of an ideal gas and thermodynamic temperature: ½ m〈v2〉 = 3kBT, where m is the mass of an atom and 〈v2〉 is the mean-square velocity of the atom.
I'm fairly sure that should be ½ m〈v2〉 = (3/2) . kBT.
Anyway, if you (@Hamdani) are really interested in the best way to define temperature it might be well worth having a look at that article and the simpler overview of the situation in Wikipedia.
It (the triple point of water) (together with the definition of absolute zero) is the only fixed point on the whole of the Kelvin temperature scale.The latest definition of the Kelvin scale for temperature won't require the triple point of water as a fixed point.
Since May 2019, that value (the triple point of water) has not been fixed by definition but is to be measured through microscopic phenomena
[Quote from Wikipedia]
Even absolute zero, 0 K, doesn't really fit the description of what we would have called a "fixed point" in the old days. No experiment has to be done or object set up to that temperature to calibrate the scale. That value just falls out naturally from the theoretical definition of temperature from statistical mechanics. You also have no choice about what numerical value you assign to this temperature, it couldn't be -10 or +10 because it's got to be proportional to the average kinetic energy of particles (which is supposed to be nothing). You don't really have any freedom to set any fixed points and corresponding numerical values at those fixed points for the temperature. For example, if you use the equation ½ m〈v2〉 = (3/2) . kBT, to define temperature, T, then the only way you can adjust the value of T for some pre-determined reference situation (like the average k.e. of the gas particles in an equilibrium mixture of ice, liquid and gas water, i.e. water at its triple point) would be by changing the Boltzman constant.
If I've read the article @Bored chemist presented correctly, the exact value of the Boltzman constant wasn't going to be fixed until 2018 (2 years after the paper was printed). With the new value fixed, the triple point of water would be close to 273.16 Kelvin but there was no expectation that it would be exactly that value.
- - - - - - -
There we go... . I had no idea the way we think about temperature has changed that much and this recently.
Thanks again for the article @Bored chemist .
Best Wishes.
Post by: hamdani yusuf on 01/04/2022 06:12:10
If I put thermometers at the bottom, middle, and top of the pond, they would show certain values. Are they meaningless?No. It can't be at eqm because it has a temperature gradient.Temperature is still only meaningful if a system is at equilibrium.How do you define equilibrium?
Imagine a frozen pond in a winter. The depth is 10 meters. The air temperature above the surface is -10°C. One meter ice layer has been formed on the surface. The rest of the pond is still liquid water. This condition hasn't changed for a week. Is it in equilibrium?
That's because heat is flowing from the Earth's core, into the water, up through the ice and out again.
Post by: hamdani yusuf on 01/04/2022 07:45:21
In a microwave oven, the thin metal in the bolometer can absorb the radiation and generate heat, which may exceed the air temperature inside the oven chamber.The air inside a running microwave oven, radio wave oven, induction cooker, laser oven, may show different temperature measurement value, depending on the thermometer type used.No. The temperature of the air (assuming it is well stirred and thus in internal equilibrium) has a unique value. The practical problem is that the power source interacts differently with different types of thermometer and if you aren't careful you end up measuring the microwave absorbtivity of a thermocouple or whatever, or even generating a load of sparks and no data. An external thermistor bolometer is probably the simplest practical device for an industrial oven but even then you need to ensure that the emissivity of the air is greater than that of the oven itself - quite difficult to do. If the oven is ventilated, you could put a glass bottle gas thermometer inside and measure the pressure of the working volume of air, which will be close to that of its ambient. If it isn't ventilated, than a pressure gauge will give you the answer.
Post by: Bored chemist on 01/04/2022 08:51:15
If I put thermometers at the bottom, middle, and top of the pond, they would show certain values. Are they meaningless?No, but "the temperature of the pond" is meaningless.
It has more than one temperature. A lot will be near 4 C, some will be between 4 and zero, some will be very near zero.
Post by: alancalverd on 01/04/2022 12:02:52
In a microwave oven, the thin metal in the bolometer can absorb the radiation and generate heat, which may exceed the air temperature inside the oven chamber.Only a fool would put anything but a gas thermometer inside a microwave oven to measure the air temperature. Dry air would work pretty well, but argon is even closer to an ideal gas.
The bolometer trick is to use a concave mirror to focus the radiation from the air onto the thermistor, all from outside the oven.
The engineer who lives inside my head wants to ask why you are interested in the air temperature inside an oven. Most people are more interested in the wall temperature, which determines the thermal radiative heating of whatever you want to cook, or the power input to the microwave or fan heater.
Post by: Bored chemist on 01/04/2022 13:20:42
Only a fool would put anything but a gas thermometer inside a microwave oven to measure the air temperature.Not really.
https://www.amazon.co.uk/Brannan-Microwave-Thermometer/dp/B005RDUSAW
Though it wouldn't achieve much to measure air temperature in a microwave.
Most people are more interested in the wall temperature,Not if it's a microwave oven.
The engineer who lives inside my head wants to ask why you are interested in the air temperature inside an oven.Because they know that the heat carried by that air in a conventional oven is a significant part of the cooking process.
That's why cooking times are reduced in fan ovens.
Most people are more interested in the wall temperatureI think more people are actually interested in how quickly stuff cooks.
That's related to wall temperature and air temperature in a conventional oven; and related to the power density in a microwave oven.
Post by: alancalverd on 01/04/2022 15:32:39
Bit of a sensitive point as, in a former incarnation, I was asked to review the draft European Directive on Physical Hazards which would have prevented the construction of any oven that could accommodate a human!
Not really.always read the small print:
https://www.amazon.co.uk/Brannan-Microwave-Thermometer/dp/B005RDUSAW
Quote
This a Meat thermometer , has metal skewer , there are no instructions. and look on Makers site , No trace of this product?
Answer:The metal skewer is to make a hole in meat etc to enable the thermometer to be inserted more easily. You don't put the thermometer IN the microwave - you simply use it to check the temperature of the foods you have cooked in there.
Which is just as well. If it's an alcohol-in-glass instrument, it's quite likely to boil and shatter in a microwave, even if the ambient air is cold. And I wouldn't shove a glass thermometer into a lump of dead meat that I was going to eat - the rectum of a dog is dodgy enough! - so it's probably plastic, and likely to melt in the cooker even in the absence of air.
Post by: Bored chemist on 01/04/2022 17:07:18
always read the small print:I did
http://taylor-enviro.com/media/IBs/514_ib.pdf
para 5
Post by: Bored chemist on 01/04/2022 17:08:39
Bit of a sensitive point as, in a former incarnation, I was asked to review the draft European Directive on Physical Hazards which would have prevented the construction of any oven that could accommodate a humanBull?
Post by: alancalverd on 01/04/2022 23:57:11
Quote from: alancalverd on Today at 15:32:39Sadly, no. The draft (compiled at your expense) contained a lot of figurative testes and bovine excrement including a ban on interventional use of MRI systems, which was the burden of my evidence to the Parliamentary scrutiny committee (at your expense). The draft was eventually rejected, to be replaced by something a little less absurd but even more expensive.QuoteBit of a sensitive point as, in a former incarnation, I was asked to review the draft European Directive on Physical Hazards which would have prevented the construction of any oven that could accommodate a humanBull?
Post by: hamdani yusuf on 02/04/2022 08:42:59
The engineer who lives inside my head wants to ask why you are interested in the air temperature inside an oven. Most people are more interested in the wall temperature, which determines the thermal radiative heating of whatever you want to cook, or the power input to the microwave or fan heater.Here's a tweet from @engineers_feed
"Any idiot can build a bridge that stands, but it takes an engineer to build a bridge that barely stands."
My intention when starting this thread is to find out the precise definition of temperature. We know that a system can have many forms of energy. They are often classified as kinetic and potential energy. Which category does temperature fall into?
If we put energy into a system, sometimes the system's temperature increases, sometimes it doesn't. What makes the difference?
Post by: hamdani yusuf on 02/04/2022 09:04:17
Not really.To measure food temperature, a non-contact infrared thermometer is safer and more convenience to use.
https://www.amazon.co.uk/Brannan-Microwave-Thermometer/dp/B005RDUSAW
Though it wouldn't achieve much to measure air temperature in a microwave.
Post by: hamdani yusuf on 02/04/2022 09:06:20
Which is just as well. If it's an alcohol-in-glass instrument, it's quite likely to boil and shatter in a microwave, even if the ambient air is cold.It might happen if you cook the thermometer alone, without being inserted into the food.
Post by: hamdani yusuf on 02/04/2022 09:18:37
I think more people are actually interested in how quickly stuff cooks.Agree.
Other things we usually care are:
Safety. We don't want harmful side effects, such as eye damage, hot splash, electrocution, carbon monoxide, NOx, radioactivity, etc.
Homogeneity of the heating. We don't want the food to be burnt into charcoal at one part while still being raw at other parts.
Energy bill. How much energy really goes to heating the food instead of being wasted away heating the container or exhaust air, or deforming the food.
Post by: Bored chemist on 02/04/2022 11:27:32
That's a great way of getting food poisoning.Not really.To measure food temperature, a non-contact infrared thermometer is safer and more convenience to use.
https://www.amazon.co.uk/Brannan-Microwave-Thermometer/dp/B005RDUSAW
Though it wouldn't achieve much to measure air temperature in a microwave.
Please don't make assertions like that.
If you really don't understand that food has to be cooked through, but an IR thermometer only measures surface temperature, then don't try giving advice.
Post by: Spring Theory on 02/04/2022 13:09:03
Absorbing a photon increases energy (and velocity) which results in more kinetic energy of the atom. As more and more photons are absorbed in longer and longer wavelengths, the total energy rises as well as the velocities at the outer orbitals. So think of the temperature of just one atom as the number (or concentration) of photons absorbed by the atom. Of course the longest wavelength photons absorbed are in the infrared range. These are also typically the first to be released by the atom.
If you combine many atoms together, the outer orbital electrons will interact with each other to transfer photons back and forth as well as momentum. The more the photons, the more the atoms bounce back and forth and the more kinetic energy of the group of atoms. This is the statistical link of temperature to kinetic energy.
Simplifying the description, you can define temperature as a scalar field (coordinates of position and time) where the temperature at each point (at the macro scale) is based on the concentration of photons (including the ones absorbed) at that point.
Post by: Bored chemist on 02/04/2022 13:13:49
This electron is traveling at 1/137 the speed of lightNo it isn't.
The uncertainty principle says you can't know exactly what its speed is.
Post by: Spring Theory on 02/04/2022 13:33:01
This electron is traveling at 1/137 the speed of lightNo it isn't.
The uncertainty principle says you can't know exactly what its speed is.
To be more exact, I have determined the velocity of the electron at the 1s orbital to be 2.1876912636431E+06 m/s.
Based on the uncertainty principle, you cannot disprove that.
Post by: hamdani yusuf on 02/04/2022 14:36:16
That's a great way of getting food poisoning.What makes it great?
Please don't make assertions like that.
If you want to know the inner temperature of the food, just cut it in half before measuring it using infrared thermometer. Why make it complicated?
Post by: hamdani yusuf on 02/04/2022 14:56:31
That's not what the uncertainty principle says.This electron is traveling at 1/137 the speed of lightNo it isn't.
The uncertainty principle says you can't know exactly what its speed is.
Post by: hamdani yusuf on 02/04/2022 15:07:53
The closest you can get to absolute zero is the ground state where the electron orbits the proton in the 1S orbital.What's the temperature corresponding to that state?
Post by: Origin on 02/04/2022 15:10:10
To be more exact, I have determined the velocity of the electron at the 1s orbital to be 2.1876912636431E+06 m/s.How did you determine that.
Post by: Bored chemist on 02/04/2022 15:18:43
That's not what the uncertainty principle says.In this context, it does.
Post by: hamdani yusuf on 02/04/2022 15:19:59
Absorbing a photon increases energy (and velocity) which results in more kinetic energy of the atom.How does it affect the potential energy?
For comparison, to send a satellite to geostationary orbit, more energy is required, compared to sending it to Low Earth Orbit, although the orbital speed is lower.
Post by: Bored chemist on 02/04/2022 15:21:24
What makes it great?Simplicity.
If you want to know the inner temperature of the food, just cut it in half before measuring it using infrared thermometer. Why make it complicated?So, it is useless for things like a burger which you want to cook whole.
Why not just accept that your idea was wrong?
Post by: Bored chemist on 02/04/2022 15:22:39
The temperature of a single atom is still not well defined.The closest you can get to absolute zero is the ground state where the electron orbits the proton in the 1S orbital.What's the temperature corresponding to that state?
Perhaps I can add another example. The air inside a running microwave oven, radio wave oven, induction cooker, laser oven, may show different temperature measurement value, depending on the thermometer type used.Temperature is still only meaningful if a system is at equilibrium.For some systems- far from equilibrium- the temperatures can be different- or even negative.
Post by: Eternal Student on 02/04/2022 16:23:00
I don't think I've spoken to you before, welcome to the forum.
You've said a lot of things that are interesting and quite reasonable. There are also a few things that might be a little bit dangerous or misleading.
Let's start by establishing the following:
1. I'm not the definitive expert on this.
2. As far as I'm concerned the purpose of a forum is to discuss things and ideally move people's undertsanding onwards and upwards. For example, my own understanding of temperature has had a bit of development since reading some articles about temperature.
So if I or other people disagree with some of the things you've said, don't worry about it too much. We need a few more users on this forum, don't disappear. You should be welcome to join the discussion.
Best Wishes.
Post by: Eternal Student on 02/04/2022 18:27:34
My intention when starting this thread is to find out the precise definition of temperature.
Then let's spend a moment and bring all the ideas that have already been mentioned in this thread together and up-to-date. It might also be useful for people joining the discussion who don't want to plough their way through the whole thread.
What is temperature?
There's been a lot of discussion already. Historically, there have been different ways of measuring temperature and therefore many different definitions of what temperature is. The earliest definitions were based on thermometer devices like mercury in glass thermometers. So temperature was just "that" which you can measure with a thermometer.
Later there were some empirical observations made on gases that seemed to behave a certain way. Some gases obeyed Boyles' Law and Charles' Law to a fair amount of accuracy. This was discovered prior to 1854. This started to give some reason to believe macroscopic properties of gases like their pressure and volume would be related to the temperature (the temperature determined by a thermometer) and opened the door to the possibility of defining temperature for some systems (like ideal gases) without reference to any thermometer device.
Thermodynamics and the formalisation of the modern ideal gas law started to be developed around 1854. After this, a different (let's say "better" and more usefull) understanding of what "temperature" might be and how it should be defined was available. Using thermodynamics you could define a temperature for various systems and not just ideal gases. For example, you can declare two systems to be at the same temperature if and only if no heat flows between them when they are in thermal contact. You can have an ideal gas as one of those systems and use the ideal gas law to put a numerical value on that temperature. You could also obtain definitions for temperature based on the operation of a Carnot engine.
Thermodynamic approaches to defining temperature have been discussed in this thread. These remained the most useful and commonly accepted definitions of temperature until May 2019.
Another area of science, "Kinetic Theory" and more generally microscopic statistical mechanics was also being developed alongside Thermodynamics. They (the scientific community) have really run away with that idea recently.....
As of May 2019, the Kelvin temperature scale has been formally re-defined. This is now an entirely theoretical approach to defining a temperature scale and therefore what "temperature in Kelvin" is understood to be.
It's still a bit arbitrary to suggest this definition of temperature is "better" than a thermodynamic approach or an empirical definition of temperature. Arguably it is "better" if you are trying to connect temperature to microscopic properties of particles, which is what you (Hamdani) seem to be seeking to do.
Best Wishes.
Post by: alancalverd on 02/04/2022 18:41:29
My intention when starting this thread is to find out the precise definition of temperature. We know that a system can have many forms of energy. They are often classified as kinetic and potential energy. Which category does temperature fall into?Temperature is a measure of the mean kinetic energy of the molecules inside an object. It is not a measure of the kinetic energy of the whole object, or the potential energy of any stresses within it. If you input energy in such a way as to increase the mean kinetic energy of the molecules inside an object, you will increase its temperature. If you do something else, you won't.
If we put energy into a system, sometimes the system's temperature increases, sometimes it doesn't. What makes the difference?
Post by: alancalverd on 02/04/2022 18:45:43
To be more exact, I have determined the velocity of the electron at the 1s orbital to be 2.1876912636431E+06 m/s.In fact the indeterminacy principle does exactly that. If you know its velocity then it must be travelling in a straight line (velocity being a vector) so it can't be located in an orbital.
Based on the uncertainty principle, you cannot disprove that.
Post by: Bored chemist on 02/04/2022 19:18:04
My intention when starting this thread is to find out the precise definition of temperature. We know that a system can have many forms of energy. They are often classified as kinetic and potential energy. Which category does temperature fall into?Neither.
That's why we call it thermal energy.
Post by: Spring Theory on 02/04/2022 21:39:25
Hi @Spring Theory ,
I don't think I've spoken to you before, welcome to the forum.
You've said a lot of things that are interesting and quite reasonable. There are also a few things that might be a little bit dangerous or misleading.
Let's start by establishing the following:
1. I'm not the definitive expert on this.
2. As far as I'm concerned the purpose of a forum is to discuss things and ideally move people's undertsanding onwards and upwards. For example, my own understanding of temperature has had a bit of development since reading some articles about temperature.
So if I or other people disagree with some of the things you've said, don't worry about it too much. We need a few more users on this forum, don't disappear. You should be welcome to join the discussion.
Best Wishes.
Dangerous is for Grizzly bears. Misleading implies intentional fraud. Neither of these terms should apply to a forum discussion.
Temperature usually refers to infrared spectrum radiation intensity. Temperature is also usually defined in terms of kinetic energy. I just tied these two together elegantly.
I will contribute when I can. Thanks.
Post by: Bored chemist on 02/04/2022 21:56:45
Misleading implies intentional fraud.Not really.
Temperature usually refers to infrared spectrum radiation intensity.Not really.
Temperature is also usually defined in terms of kinetic energy.Not really.
Post by: Origin on 02/04/2022 22:03:26
To be more exact, I have determined the velocity of the electron at the 1s orbital to be 2.1876912636431E+06 m/s.@Spring Theory, how did you determine that?
Post by: Eternal Student on 02/04/2022 22:30:34
Dangerous is for Grizzly bears. Misleading implies intentional fraud.It wasn't my intention to be offensive. Please don't assume it was. What you said might confuse and in that sense "mislead" others like the OP who asked the question "what is temperature?" I don't think you're trying to commit fraud or anything like that.
Best Wishes.
Post by: hamdani yusuf on 02/04/2022 23:09:21
So, it is useless for things like a burger which you want to cook whole.How thick is the burger?
It's still useful for many things else.
Quote
Why not just accept that your idea was wrong?
Post by: Eternal Student on 03/04/2022 01:07:34
I really don't know why burgers have become important. I was just going to back up to some earlier posts:
Originally a quote from @hamdani yusuf , re-used in a reply from Bored Chemist:
We know that a system can have many forms of energy. They are often classified as kinetic and potential energy. Which category does temperature fall into?
Bored Chemist replied:
Neither.That's old style. Presumably you went to school roughly when I did but you (bored chemist) presented the article about re-defining temperature, so you've got to play fair here.
That's why we call it thermal energy.
The modern definition of temperature (in Kelvin post 2019) is trying to remove the need for such oblique references as calling something "thermal energy". It does not step around declaring what that energy is at a microscopic scale and leave it open to all sorts of possible interpretations. Instead it embraces the notion of microscopic statistical mechanics head on.
Alancalverd replied:
Temperature is a measure of the mean kinetic energy of the molecules inside an object. It is not a measure of the kinetic energy of the whole object, or the potential energy of any stresses within it. If you input energy in such a way as to increase the mean kinetic energy of the molecules inside an object, you will increase its temperature. If you do something else, you won't.Which is much more in-line with the definition of temperature used in the modern Kelvin scale.
Temperature (in Kelvin post 2019) is very much meant to be a measure of the average kinetic energy of the particles of a system. (At least for simple systems it will be. Whether our Kinetic Theory is adequately developed to determine the behaviour of particles in all systems like solids, liquids or a gas of photons is a different question). If you put energy into a system that doesn't change the k.e.of the particles then, as Alancalverd stated, you won't change the temperature of the system.
However the issue remains murky: To the best of my knowledge, the requirement for a system to be in equilibrium hasn't disappeared in the new approach to defining temperature. If you put energy into a system (e.g. to raise electrons to an excited state or change something else about the system that might be considered as a potential energy change instead of kinetic energy change for the particles) then you must wait until an equilibrium is re-established before the system has a well defined temperature. When the energy of the system is re-distributed and the principles like the equi-partition of energy apply, it is very likely that the average k.e. of the particles will have increased. (I said "likely" not guaranteed to always happen, changes of state might be one example where you can put energy into a system but there is no change in temperature).
Speculation about changes of state:
The actual shared property (temperature) is the average energy per degree of freedom.
[Taken from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4865254/ . An article originally suggested by Bored Chemist ]
How they (the community of scientists using the new definitions of the Kelvin scale) consider or determine temperature for systems that can show a change of state (e.g. from gas to liquid) should be interesting to see. I haven't had time to look at this yet. However, if the kinetic theory they use allows the gas to have, lets say 5 degrees of freedom (I chose 5 because that's easily explained by the models we have for a diatomic gas), while the liquid state only supports 4 degrees of freedom (which is reasonable because we assume particles in a liquid state have less freedom of movement and therefore less parameters describing their energy) then you can see that is possible for the system to have more total energy in the gas phase than the liquid phase but the energy per degree of freedom, i.e. the "temperature" can remain the same. We have a possible explanation for latent heat, the system loses degrees of freedom during phase changes.
Let's make it clear that I haven't had time to look at this yet, it just seems reasonable. The new approach to defining a temperature scale (introduced since 2019) just wasn't in existence when I was studying thermodynamics.
Best Wishes.
Post by: Spring Theory on 03/04/2022 01:07:58
To be more exact, I have determined the velocity of the electron at the 1s orbital to be 2.1876912636431E+06 m/s.How did you determine that.
That is a discussion for a different thread. I don't want to detract from the point of this thread. Sufficient to say that it is generally accepted that the electron (or its wave function) has a velocity around the proton in a hydrogen atom.
Therefore, the smarty pants response to invalidate the actual value of the velocity is unproductive to the core goal here.
Post by: Spring Theory on 03/04/2022 01:18:54
The closest you can get to absolute zero is the ground state where the electron orbits the proton in the 1S orbital.What's the temperature corresponding to that state?
This is a point of debate and speculation (proper for this directory). I would consider the hydrogen atom at its ground state as absolute zero. The next step lower would be to knock the electron off the hydrogen atom, but you're adding energy to the system.
A proton by itself still has kinetic energy (spin) and the electron by itself still has kinetic energy (spin).
Really the only other option of absolute zero would be a universe without particles or photons.
Therefore, realistically, absolute zero is hydrogen at its ground state with no other photons nearby to interact with.
Post by: Origin on 03/04/2022 02:10:02
Therefore, the smarty pants response to invalidate the actual value of the velocity is unproductive to the core goal here.My question was how you came up with that number valid or not.
It seemed important to the the goal here since you brought it up. If you don't want to discuss it that's fine, no reason to get worked up.
Post by: hamdani yusuf on 03/04/2022 06:43:36
This is a point of debate and speculation (proper for this directory). I would consider the hydrogen atom at its ground state as absolute zero. The next step lower would be to knock the electron off the hydrogen atom, but you're adding energy to the system.Is temperature a quantized value?
Post by: Bored chemist on 03/04/2022 10:21:58
How thick is the burger?Thicker than the penetration depth of the IR the thermometer uses.
But, like the test tubes, the answer is that "any sensible thickness of burger" will do.
Post by: Bored chemist on 03/04/2022 10:24:13
Sufficient to say that it is generally accepted that the electron (or its wave function) has a velocity around the proton in a hydrogen atom.No.
That is not "generally accepted".
It doesn't have a velocity round the atom.
Do you realise that the Bohr model of an orbiting electron was discarded decades ago?
Post by: Bored chemist on 03/04/2022 10:26:49
The next step lower would be to knock the electron off the hydrogen atom, but you're adding energy to the system.Make up your mind.
Stripping the electrons off hydrogen atoms is easy; you just heat them to about 10,000 K
In what way is that closer to absolute zero?
Post by: Bored chemist on 03/04/2022 10:32:18
He multiplied c by the fine structure constant.To be more exact, I have determined the velocity of the electron at the 1s orbital to be 2.1876912636431E+06 m/s.@Spring Theory, how did you determine that?
In fairness, he nearly explained it earlier.
This electron is traveling at 1/137 the speed of light and thus a contributor to kinetic energy.
But it isn't the value that is under discussion.
It's the fact that the electron in a hydrogen atom doesn't have a velocity.
If it did, it would leave the atom.
Post by: Spring Theory on 03/04/2022 15:26:38
Absorbing a photon increases energy (and velocity) which results in more kinetic energy of the atom.How does it affect the potential energy?
For comparison, to send a satellite to geostationary orbit, more energy is required, compared to sending it to Low Earth Orbit, although the orbital speed is lower.
The potential energy would be affected by the distance from nuclear in the form of charge potential. The farther the distance, the lower the potential energy. Of course the higher velocity also means higher kinetic energy. This means energy has to be added in the form of a photon.
To understand potential energy vs kinetic energy best, the principle of least action is required. This principle is the basis for all generally accepted physics. It basically states that the difference between kinetic and potential energy (the action) is minimized.
Again, I don't want to get off topic with constructing a Lagrangian and differentiating it with respect to coordinates, etc but everyone should learn this powerful concept.
Post by: Bored chemist on 03/04/2022 16:05:48
everyone should learn this powerful concept.It certainly seems to improve self-confidence.
Post by: alancalverd on 03/04/2022 23:10:35
Is temperature a quantized value?No.
Post by: alancalverd on 03/04/2022 23:16:54
Sufficient to say that it is generally accepted that the electron (or its wave function) has a velocity around the proton in a hydrogen atom.Not since 1925. How old are you?
Quote
When describing what temperature is, it is easier to think of a single hydrogen atom.Beware! The journey to disaster begins with a single step in the wrong direction. Temperature is an ensemble statistic and is not defined for a single atom or particle. You'll add confusion by attempting to "describe" a mathematical function that has a clear definition. Two steps in the wrong direction.....
Quote
The farther the distance, the lower the potential energy.That's the inverse of the conventional description of potential, and rather surprising coming from a spring theorist.
Post by: Spring Theory on 04/04/2022 00:35:03
Sufficient to say that it is generally accepted that the electron (or its wave function) has a velocity around the proton in a hydrogen atom.Not since 1925. How old are you?QuoteWhen describing what temperature is, it is easier to think of a single hydrogen atom.Beware! The journey to disaster begins with a single step in the wrong direction. Temperature is an ensemble statistic and is not defined for a single atom or particle. You'll add confusion by attempting to "describe" a mathematical function that has a clear definition. Two steps in the wrong direction.....QuoteThe farther the distance, the lower the potential energy.That's the inverse of the conventional description of potential, and rather surprising coming from a spring theorist.
Actually you're right - the farther the distance the higher the electric charge potential energy. My bad.
I'm right on the other points though. How would you explain magnetic moments without a velocity?
Post by: Spring Theory on 04/04/2022 01:21:42
You hit the nail on the head BC...good one.everyone should learn this powerful concept.It certainly seems to improve self-confidence.
Post by: Bored chemist on 04/04/2022 08:45:55
I'm right on the other points though.No
You are not.
You are using an idea that died a hundred years ago.
How would you explain magnetic moments without a velocity?Good question; but the wrong one.
The right question is "how can magnetic moments be produced by velocity when, for an electron, teh velocity required would exceed the speed of light?".
The answer is "well- it can't be the velocity then.
And there is, as I pointed out, still the problem with the uncertainty principle.
Do the maths.
Calculate the uncertainty of the velocity of an electron which is confines within the radius of a hydrogen atom.
Post by: Spring Theory on 04/04/2022 13:13:49
I'm right on the other points though.No
You are not.
You are using an idea that died a hundred years ago.How would you explain magnetic moments without a velocity?Good question; but the wrong one.
The right question is "how can magnetic moments be produced by velocity when, for an electron, teh velocity required would exceed the speed of light?".
The answer is "well- it can't be the velocity then.
And there is, as I pointed out, still the problem with the uncertainty principle.
Do the maths.
Calculate the uncertainty of the velocity of an electron which is confines within the radius of a hydrogen atom.
You're referring to intrinsic spin.
Definition of "intrinsic spin": Let's give it a name that sounds smart but we really don't know what is going on here. Spin but not really spin.
How unsatisfying is that.
Charge with a velocity in a loop creates magnetic moments, not a pretend spin.
Post by: Bored chemist on 04/04/2022 16:10:54
Do the maths.
Calculate the uncertainty of the velocity of an electron which is confines within the radius of a hydrogen atom.
Post by: hamdani yusuf on 04/04/2022 23:50:54
The right question is "how can magnetic moments be produced by velocity when, for an electron, teh velocity required would exceed the speed of light?".What's the required velocity to produce the measured magnetic moment?
The answer is "well- it can't be the velocity then.
Post by: Bored chemist on 05/04/2022 10:45:26
https://www.physicsforums.com/threads/faster-than-light-subatomic-spin.661870/The right question is "how can magnetic moments be produced by velocity when, for an electron, teh velocity required would exceed the speed of light?".What's the required velocity to produce the measured magnetic moment?
The answer is "well- it can't be the velocity then.
Post by: alancalverd on 06/04/2022 23:05:05
How would you explain magnetic moments without a velocity?Assuming you are talking about nuclear and electron magnetic moments, they are associated with spin, not velocity.
Post by: hamdani yusuf on 09/04/2022 05:17:42
Quote
Laser cutting is a technology that uses a laser to vaporize materials, resulting in a cut edge.The object being cut by laser can reach temperature much higher than the gain medium of the laser itself. So we usually say that this energy transfer is not a thermal radiation, which raise the question, what is?
Post by: hamdani yusuf on 09/04/2022 05:18:44
Post by: Bored chemist on 09/04/2022 19:05:36
So we usually say that this energy transfer is not a thermal radiation, which raise the question, what is?Radiation.
Post by: hamdani yusuf on 09/04/2022 23:14:37
Non-thermal radiation.So we usually say that this energy transfer is not a thermal radiation, which raise the question, what is?Radiation.
What sets them apart?
Post by: Bored chemist on 10/04/2022 09:55:55
The spectrum.Non-thermal radiation.So we usually say that this energy transfer is not a thermal radiation, which raise the question, what is?Radiation.
What sets them apart?
Post by: alancalverd on 10/04/2022 16:53:10
Post by: Spring Theory on 10/04/2022 23:38:52
This is a point of debate and speculation (proper for this directory). I would consider the hydrogen atom at its ground state as absolute zero. The next step lower would be to knock the electron off the hydrogen atom, but you're adding energy to the system.Is temperature a quantized value?
Actually, yes. It could be quantized as the number of photons (and absorbed photons) in a volume of space.
Post by: Bored chemist on 11/04/2022 10:12:26
How "big" are the photons?This is a point of debate and speculation (proper for this directory). I would consider the hydrogen atom at its ground state as absolute zero. The next step lower would be to knock the electron off the hydrogen atom, but you're adding energy to the system.Is temperature a quantized value?
Actually, yes. It could be quantized as the number of photons (and absorbed photons) in a volume of space.
Post by: hamdani yusuf on 11/04/2022 11:41:49
Thermal radiation is the continuum spectrum of electromagnetic radiation emanating from the random movement of electrons in a hot body. Laser radiation is a narrow spectrum arising from coordinated quantum transitions.
(https://upload.wikimedia.org/wikipedia/commons/e/e7/Solar_spectrum_en.svg)
We can filter out some wavelengths from a black body radiation.
(https://image.made-in-china.com/43f34j00sdjEBAqJdwrM/25X25X25-Cube-Cross-Dichroic-RGB-Combiner-or-Splitter-X-Cube-Prism.webp)
We can combine lasers with different wavelengths into single light ray.
How much spectra can be removed from a continuous spectrum until it stops being a thermal radiation?
How much spectra can be added to a narrow laser spectrum until it starts being a thermal radiation?
Post by: Spring Theory on 11/04/2022 11:55:10
How "big" are the photons?This is a point of debate and speculation (proper for this directory). I would consider the hydrogen atom at its ground state as absolute zero. The next step lower would be to knock the electron off the hydrogen atom, but you're adding energy to the system.Is temperature a quantized value?
Actually, yes. It could be quantized as the number of photons (and absorbed photons) in a volume of space.
Big enough to fit in a volume of space.
Post by: Bored chemist on 11/04/2022 12:11:47
Did you not understand the quote marks?How "big" are the photons?This is a point of debate and speculation (proper for this directory). I would consider the hydrogen atom at its ground state as absolute zero. The next step lower would be to knock the electron off the hydrogen atom, but you're adding energy to the system.Is temperature a quantized value?
Actually, yes. It could be quantized as the number of photons (and absorbed photons) in a volume of space.
Big enough to fit in a volume of space.
If you have 2 eV of energy, do you consider it to be 1 visible photon, 2 IR ones or a whole lot of RF ones?
Incidentally, photons don't fill space.
Post by: Bored chemist on 11/04/2022 12:15:13
We can filter out some wavelengths from a black body radiation.And what is left isn't strictly "thermal", but it's close enough that we can ignore the difference.
How much spectra can be added to a narrow laser spectrum until it starts being a thermal radiation?Strictly, all of them.
However, it is reasonable to talk about "colour temperature" of sources that are not thermal.
The other thing that matters is, of course, the relative intensities.
If you have more blue light than red, the colour temperature can be very odd.
Post by: Eternal Student on 11/04/2022 12:56:44
You (Hamdani) seem to be looking for precise and absolute answers but I think the situation is generally left quite vague and much as suggested by @Bored chemist .
How much spectra can be added to a narrow laser spectrum until it starts being a thermal radiation?You could artificially re-create a black body spectrum and some might call this "thermal radiation". However, some people will reserve that name to radiation that was produced as a consequence of a body being at some well defined temperature and radiating naturally. You can re-create the same spectrum but it doesn't always acquire the name "thermal radiation". Sometimes the term "thermal radiation" describes the origin or cause of the radiation instead of the properties or spectrum that it has.
This is much like the term "gamma ray" being kept separate from the term "x-ray". You can have an X-ray of such high frequency that it is identical to a gamma ray but if it didn't originate from the nucleus of an atom then you just don't call it a gamma ray.
Here's the description from Wikipedia, just to illustrate the situation. In their definition, not all thermal radiation has a black body spectrum and not not all black body spectrums were produced in the process of thermal radiation. However, you should be aware that others will use the term "thermal radiation" and "black body radiation" much more interchangeably than this.
Thermal radiation is electromagnetic radiation generated by the thermal motion of particles in matter....
If a radiation object meets the physical characteristics of a black body in thermodynamic equilibrium, the radiation is called blackbody radiation.
https://en.wikipedia.org/wiki/Thermal_radiation
Best Wishes.
Post by: hamdani yusuf on 11/04/2022 13:02:44
And what is left isn't strictly "thermal", but it's close enough that we can ignore the difference.How much can we remove until the difference can no longer be ignored?
If you have more blue light than red, the colour temperature can be very odd.
It's actually quite common for hot enough objects.
Quote
https://en.wikipedia.org/wiki/Wien%27s_displacement_law#Discovery
The law is named for Wilhelm Wien, who derived it in 1893 based on a thermodynamic argument.[4] Wien considered adiabatic expansion of a cavity containing waves of light in thermal equilibrium. He showed that, under slow expansion or contraction, the energy of light reflecting off the walls changes in exactly the same way as the frequency. A general principle of thermodynamics is that a thermal equilibrium state, when expanded very slowly, stays in thermal equilibrium.
Wien himself deduced this law theoretically in 1893, following Boltzmann’s thermodynamic reasoning. It had previously been observed, at least semi-quantitatively, by an American astronomer, Langley. This upward shift in νmax with T is familiar to everyone—when an iron is heated in a fire, the first visible radiation (at around 900 K) is deep red, the lowest frequency visible light. Further increase in T causes the color to change to orange then yellow, and finally blue at very high temperatures (10,000 K or more) for which the peak in radiation intensity has moved beyond the visible into the ultraviolet.[5]
Post by: hamdani yusuf on 11/04/2022 13:06:21
Sometimes the term "thermal radiation" describes the origin or cause of the radiation instead of the properties or spectrum that it has.Or maybe it's based on the effect instead.
If a thermal radiation is filtered by a linear polarizer, is it still considered thermal radiation?
Post by: Bored chemist on 11/04/2022 13:12:17
How much can we remove until the difference can no longer be ignored?It depends what you are doing.
It's actually quite common for hot enough objects.Not many things are that hot.
The colour temperature of a blue LED is extremely high- which is absurd given that the emitter is near room temperature.
So, as I said...
If you have more blue light than red, the colour temperature can be very odd.
Post by: Bored chemist on 11/04/2022 13:12:55
If a thermal radiation is filtered by a linear polarizer, is it still considered thermal radiation?
It depends what you are doing.
Post by: hamdani yusuf on 11/04/2022 13:16:22
You (Hamdani) seem to be looking for precise and absolute answers but I think the situation is generally left quite vague and much as suggested by @Bored chemist .I'm fine with non-binary concepts and fuzzy variables. As long as it can be stated clearly what kind of changes can be made to shift the membership of "thermality" of a radiation.
Here's an example.
(https://upload.wikimedia.org/wikipedia/commons/thumb/6/61/Fuzzy_logic_temperature_en.svg/495px-Fuzzy_logic_temperature_en.svg.png)
https://en.wikipedia.org/wiki/Fuzzy_logic#Fuzzification
Post by: hamdani yusuf on 11/04/2022 13:24:34
Let's say I'm passing it through light filters.How much can we remove until the difference can no longer be ignored?It depends what you are doing.
It depends on how you count them.It's actually quite common for hot enough objects.Not many things are that hot.
Post by: hamdani yusuf on 11/04/2022 13:40:39
The colour temperature of a blue LED is extremely high- which is absurd given that the emitter is near room temperature.
Quote
https://en.wikipedia.org/wiki/Color_temperature
The color temperature of a light source is the temperature of an ideal black-body radiator that radiates light of a color comparable to that of the light source. Color temperature is a characteristic of visible light that has important applications in lighting, photography, videography, publishing, manufacturing, astrophysics, horticulture, and other fields. In practice, color temperature is meaningful only for light sources that do in fact correspond somewhat closely to the color of some black body, i.e., light in a range going from red to orange to yellow to white to blueish white; it does not make sense to speak of the color temperature of, e.g., a green or a purple light. Color temperature is conventionally expressed in kelvins, using the symbol K, a unit of measure for absolute temperature.
Color temperatures over 5000 K are called "cool colors" (bluish), while lower color temperatures (2700–3000 K) are called "warm colors" (yellowish). "Warm" in this context is an analogy to radiated heat flux of traditional incandescent lighting rather than temperature. The spectral peak of warm-colored light is closer to infrared, and most natural warm-colored light sources emit significant infrared radiation. The fact that "warm" lighting in this sense actually has a "cooler" color temperature often leads to confusion.[1]
Post by: Bored chemist on 11/04/2022 13:54:12
Let's say I'm passing it through light filters.Why?
Post by: Bored chemist on 11/04/2022 13:54:59
Thanks for posting the bit of wiki that says what I already said.The colour temperature of a blue LED is extremely high- which is absurd given that the emitter is near room temperature.Quotehttps://en.wikipedia.org/wiki/Color_temperature
The color temperature of a light source is the temperature of an ideal black-body radiator that radiates light of a color comparable to that of the light source. Color temperature is a characteristic of visible light that has important applications in lighting, photography, videography, publishing, manufacturing, astrophysics, horticulture, and other fields. In practice, color temperature is meaningful only for light sources that do in fact correspond somewhat closely to the color of some black body, i.e., light in a range going from red to orange to yellow to white to blueish white; it does not make sense to speak of the color temperature of, e.g., a green or a purple light. Color temperature is conventionally expressed in kelvins, using the symbol K, a unit of measure for absolute temperature.
Color temperatures over 5000 K are called "cool colors" (bluish), while lower color temperatures (2700–3000 K) are called "warm colors" (yellowish). "Warm" in this context is an analogy to radiated heat flux of traditional incandescent lighting rather than temperature. The spectral peak of warm-colored light is closer to infrared, and most natural warm-colored light sources emit significant infrared radiation. The fact that "warm" lighting in this sense actually has a "cooler" color temperature often leads to confusion.[1]
Post by: Bored chemist on 11/04/2022 13:59:13
I'm fine with non-binary concepts and fuzzy variables. As long as it can be stated clearly what kind of changes can be made to shift the membership of "thermality" of a radiation.Then you need to "clearly state" what you are talking about.
How many questions will you ask before you realise that all the answers are "It depends" because you are asking vague questions?
Post by: hamdani yusuf on 11/04/2022 15:35:50
Why?Because
It depends what you are doing.
Post by: hamdani yusuf on 11/04/2022 16:00:16
Then you need to "clearly state" what you are talking about.Which part hasn't been clear yet? I even put an illustration above.
Post by: hamdani yusuf on 11/04/2022 16:00:58
How many questions will you ask before you realise that all the answers are "It depends" because you are asking vague questions?It depends on what, exactly?
Post by: Origin on 11/04/2022 16:19:45
It depends on what, exactly?I am really curious, are you any clearer on what temperature is after these 10 pages or is temperature still as much a mystery as when you started this thread?
Post by: alancalverd on 11/04/2022 19:27:32
How much spectra can be removed from a continuous spectrum until it stops being a thermal radiation?Any or none. "Thermal" radiation merely denotes the source of the radiation, not the observed spectrum.
Post by: Bored chemist on 11/04/2022 20:17:30
Lots of things, for exampleHow many questions will you ask before you realise that all the answers are "It depends" because you are asking vague questions?It depends on what, exactly?
Let's say I'm passing it through light filters.Why?
Post by: hamdani yusuf on 12/04/2022 07:55:16
What are the requirements for a radiation source to be called thermal?How much spectra can be removed from a continuous spectrum until it stops being a thermal radiation?Any or none. "Thermal" radiation merely denotes the source of the radiation, not the observed spectrum.
Post by: hamdani yusuf on 12/04/2022 07:56:10
So you think you already understand it?It depends on what, exactly?I am really curious, are you any clearer on what temperature is after these 10 pages or is temperature still as much a mystery as when you started this thread?
Explain yourself.
Post by: Bored chemist on 12/04/2022 09:59:10
What are the requirements for a radiation source to be called thermal?
Quote from: Bored chemist on Yesterday at 13:12:17
It depends what you are doing.
Post by: Origin on 12/04/2022 12:37:45
So you think you already understand it?I asked if you are any clearer on what temperature is after these 10 pages of discussion. I didn't say anything about my understanding. So what do you think, any clearer?
Explain yourself.
Post by: Eternal Student on 12/04/2022 14:50:29
A few people, including myself, are just getting a bit uncertain what it is you ( @hamdani yusuf ) are trying to do.
As @Origin has mentioned there are 10 pages of stuff here now. If you (Hamdani) get an opportunity perhaps you could think about and write down a summary of the situation so far. You might include the following:
1. A short summary of what has been said or done so far and what you're happy with.
2. Some indication of what you're still uncertain of or would like to continue to discuss.
3. Some indication of your ultimate goal. For example, do you wish to present new ideas about what a temperature is?
You don't have to do this, you can obviously do whatever you want. It's just that several people (including me) aren't following the thread well, several things are getting repeated and future comments are at risk of becoming off-target.
Best Wishes.
Post by: hamdani yusuf on 13/04/2022 04:36:49
Looks like what we've got here is a failure to communicate.How much can we remove until the difference can no longer be ignored?It depends what you are doing.
Let's get this straight. If I do X, then a lot of frequency spectra can be removed without changing the status as thermal radiation. On the other hand, if I do Y, then a slight changes of frequency spectra will make the radiation no longer thermal. The question will be, what are the criteria for actions in X and Y category?
Post by: hamdani yusuf on 13/04/2022 04:53:32
Do you realize that this thread is placed in New Theories section?So you think you already understand it?I asked if you are any clearer on what temperature is after these 10 pages of discussion. I didn't say anything about my understanding. So what do you think, any clearer?
Explain yourself.
Post by: hamdani yusuf on 13/04/2022 05:58:39
Hi.For me, the point of this thread so far is to see if my idea is new, at least some part of it, and for forum members who follow this. To do that, I need to know how the concept of temperature is currently understood by most people.
A few people, including myself, are just getting a bit uncertain what it is you ( @hamdani yusuf ) are trying to do.
As @Origin has mentioned there are 10 pages of stuff here now. If you (Hamdani) get an opportunity perhaps you could think about and write down a summary of the situation so far. You might include the following:
1. A short summary of what has been said or done so far and what you're happy with.
2. Some indication of what you're still uncertain of or would like to continue to discuss.
3. Some indication of your ultimate goal. For example, do you wish to present new ideas about what a temperature is?
You don't have to do this, you can obviously do whatever you want. It's just that several people (including me) aren't following the thread well, several things are getting repeated and future comments are at risk of becoming off-target.
Best Wishes.
Here are some points I've learned.
- Temperature is mostly described as a measure of the kinetic energy of particles, which is related to their movements.
- But sometimes, temperature is also treated as potential energy, such as the case with Carnot engine and heat pump.
(https://upload.wikimedia.org/wikipedia/commons/thumb/2/22/Carnot_heat_engine_2.svg/450px-Carnot_heat_engine_2.svg.png)
- It's also realized that not all kinetic energy is classified as thermal. Synchronized particle movements are usually not considered as temperature, such as translation of the whole object.
Post by: Bored chemist on 13/04/2022 10:54:25
Looks like what we've got here is a failure to communicate.Yes.
We are not communicating because you keep ignoring the reply.
Let's get this straight. If I do X, then a lot of frequency spectra can be removed without changing the status as thermal radiation. On the other hand, if I do Y, then a slight changes of frequency spectra will make the radiation no longer thermal. The question will be, what are the criteria for actions in X and Y category?
Quote from: Bored chemist on 11/04/2022 13:12:55
Quote from: Bored chemist on Yesterday at 13:12:17
It depends what you are doing.
Whether or not a particular spectrum would be considered "thermal" or not, depends on the context.
Temperature is mostly described as a measure of the kinetic energy of particles, which is related to their movements.
Not really.
Thermal energy is any sort of energy.
So, the random movement of the particles of a gas is thermal energy
But so is the energy of vibrations of the molecules of the gas.
And half the time, that energy is potential energy stored in the springiness of the molecules.
The rotational energy is considered separately- because it doesn't exist for single atoms, only molecules.
And the electronic energy is also part of thermal energy- so the energy in oxygen carried by the excited singlet state of O2 is also thermal energy (Though that's not a big contributor at room temperature).
And in principle, you need to consider the potential energy due to magnetic moments
https://en.wikipedia.org/wiki/Magnetic_refrigeration
And it's only "thermal" energy if the energy is (ideally) equally shared out between all these various forms.
So, did you not read the stuff about equipartition, or did you not understand it?
Post by: Origin on 13/04/2022 13:19:32
Post by: hamdani yusuf on 13/04/2022 22:54:05
But so is the energy of vibrations of the molecules of the gas.Isn't vibration a kind of movement?
Post by: hamdani yusuf on 13/04/2022 22:56:32
Whether or not a particular spectrum would be considered "thermal" or not, depends on the context.What's an example where a particular spectrum would be considered "thermal" in one context, but not in another context?
Post by: Bored chemist on 13/04/2022 22:58:45
But so is the energy of vibrations of the molecules of the gas.Isn't vibration a kind of movement?
And half the time, that energy is potential energy stored in the springiness of the molecules.
OK what I should have said is that half the energy (on average) is not kinetic energy but potential energy.
Post by: hamdani yusuf on 13/04/2022 23:01:06
And it's only "thermal" energy if the energy is (ideally) equally shared out between all these various forms.Since the ideal conditions are not usually met, then most of the time it's not considered as thermal?
Post by: Bored chemist on 13/04/2022 23:07:45
A xenon arc lamp gives a fairly good approximation to thermal spectrum- unless you look near the emission lines at the right hand endWhether or not a particular spectrum would be considered "thermal" or not, depends on the context.What's an example where a particular spectrum would be considered "thermal" in one context, but not in another context?
https://en.wikipedia.org/wiki/Xenon_arc_lamp#/media/File:Xenon_arc_lamp_profile.png
The visible radiation from the sun is close enough to a thermal spectrum to let us estimate the temperature of the sun's surface.
But the emission lines were what allowed someone to demonstrate the existence of helium
Post by: Bored chemist on 13/04/2022 23:09:20
The equipartition condition is usually met - at least to a very high degree of precision.And it's only "thermal" energy if the energy is (ideally) equally shared out between all these various forms.Since the ideal conditions are not usually met, then most of the time it's not considered as thermal?
So , for example, a tungsten light bulb, a candle , or sunlight is a pretty good approximation to a thermal spectrum.
Post by: hamdani yusuf on 16/04/2022 04:31:42
A bit of information reduces uncertainty by a half.Whether or not a particular spectrum would be considered "thermal" or not, depends on the context.What's an example where a particular spectrum would be considered "thermal" in one context, but not in another context?
Your statement contains one bit of information, regardless of its correctness. Namely, thermality of a spectrum is not solely determined by its spectral distribution. There's at least another factor to consider in determining thermality, which you called context. The same exact spectrum can have different thermal status depending on its context. Is there any additional information on the context which can reduce the uncertainty, and make our knowledge more precise?
Post by: Bored chemist on 16/04/2022 11:33:56
Yes there is.A bit of information reduces uncertainty by a half.Whether or not a particular spectrum would be considered "thermal" or not, depends on the context.What's an example where a particular spectrum would be considered "thermal" in one context, but not in another context?
Your statement contains one bit of information, regardless of its correctness. Namely, thermality of a spectrum is not solely determined by its spectral distribution. There's at least another factor to consider in determining thermality, which you called context. The same exact spectrum can have different thermal status depending on its context. Is there any additional information on the context which can reduce the uncertainty, and make our knowledge more precise?
That is why I provided it.
Did you somehow miss this bit?
A xenon arc lamp gives a fairly good approximation to thermal spectrum- unless you look near the emission lines at the right hand end
https://en.wikipedia.org/wiki/Xenon_arc_lamp#/media/File:Xenon_arc_lamp_profile.png
The visible radiation from the sun is close enough to a thermal spectrum to let us estimate the temperature of the sun's surface.
But the emission lines were what allowed someone to demonstrate the existence of helium
Post by: hamdani yusuf on 16/04/2022 22:22:21
A xenon arc lamp gives a fairly good approximation to thermal spectrum- unless you look near the emission lines at the right hand endIt's good approximation where it is, and bad where it is not.
What's the context or factor we need to consider in determining if a spectrum is thermal radiation or not?
Post by: alancalverd on 17/04/2022 17:24:28
Post by: alancalverd on 17/04/2022 17:25:46
But sometimes, temperature is also treated as potential energy,No. It is a measure of potential, not energy.
Post by: hamdani yusuf on 18/04/2022 09:39:07
You're right. I should say that objects with higher temperature are some times treated like they have higher potential energy compared to the same/similar objects but at lower temperature.But sometimes, temperature is also treated as potential energy,No. It is a measure of potential, not energy.
Post by: hamdani yusuf on 18/04/2022 09:44:05
A thermal spectrum is a continuum. An actual spectrum may have discrete bits added or subtracted, or may be entirely non-thermal.Take a continuous spectrum, but has flat distribution (up to certain frequency), instead of black body radiation. Is it considered thermal?
What do you mean by entirely non-thermal? Do you have an example?
Post by: Annieclo on 19/04/2022 02:09:08
Quote
This a Meat thermometer , has metal skewer , there are no instructions. and look on Makers site , No trace of this product?
Answer:The metal skewer is to make a hole in meat etc to enable the thermometer to be inserted more easily. You don't put the thermometer IN the microwave - you simply use it to check the temperature of the foods you have cooked in there.
Absolutely! I Use it to make sure that the center of the meat is at a temperature that renders it safe. In particular useful for grilled chicken thighs and hamburgers.
By doing this I have found that chicken is moist but safe, same for hamburgers.
It's good for those who try different meat recipes.
Post by: hamdani yusuf on 19/04/2022 05:09:29
Post by: Bored chemist on 19/04/2022 18:17:58
Another important factor in thermal radiation is emissivity. Is radiation by an object with extremely low emissivity considered thermal?It still depends.
Post by: alancalverd on 19/04/2022 19:55:15
I should say that objects with higher temperature are some times treated like they have higher potential energy compared to the same/similar objects but at lower temperature.Same object, yes. Similar object, no. The thermal energy (i.e. heat content) of a body of mass M, specific heat capacity S and temperature T is MST.
Post by: alancalverd on 19/04/2022 19:57:16
Take a continuous spectrum, but has flat distribution (up to certain frequency), instead of black body radiation. Is it considered thermal?If it is due to heat, yes.
Post by: Bored chemist on 19/04/2022 20:43:13
But heat wouldn't give a flat spectrum so it can't be thermal- though it might be close enough.Take a continuous spectrum, but has flat distribution (up to certain frequency), instead of black body radiation. Is it considered thermal?If it is due to heat, yes.
I think we have an example of the "all Dalmatians are dogs, but not all dogs are Dalmatians" issue here.
A thermal spectrum is a continuum.All thermal spectra are continuous, but not all continuous spectra are thermal.
Post by: Bored chemist on 19/04/2022 20:44:17
Except that S varies with T.I should say that objects with higher temperature are some times treated like they have higher potential energy compared to the same/similar objects but at lower temperature.Same object, yes. Similar object, no. The thermal energy (i.e. heat content) of a body of mass M, specific heat capacity S and temperature T is MST.
Post by: hamdani yusuf on 20/04/2022 09:09:00
Sunlight is filtered to produce a narrow spectrum radiation. Is it considered thermal?Take a continuous spectrum, but has flat distribution (up to certain frequency), instead of black body radiation. Is it considered thermal?If it is due to heat, yes.
Post by: hamdani yusuf on 20/04/2022 09:27:15
All thermal spectra are continuous, but not all continuous spectra are thermal.Sunlight is filtered by a band stop filter which absorbs a narrow spectrum radiation at 500 nm wavelength. Is it still considered a thermal spectrum even though it's no longer continuous?
Post by: alancalverd on 20/04/2022 16:16:07
But at any particular value of T (except at critical temperatures between phase changes) the heat content is MST. IN a phase change it is T(ΣMxSx).Except that S varies with T.I should say that objects with higher temperature are some times treated like they have higher potential energy compared to the same/similar objects but at lower temperature.Same object, yes. Similar object, no. The thermal energy (i.e. heat content) of a body of mass M, specific heat capacity S and temperature T is MST.
Post by: Bored chemist on 20/04/2022 20:11:10
But at any particular value of T (except at critical temperatures between phase changes) the heat content is MST. IN a phase change it is T(ΣMxSx).No
As discussed earlier.
If there's not enough thermal energy to excite vibrations then the vibrational part of the heat capacity is zero, but at higher temperatures it's significant.
So S varies with T even though there is no phase change.
https://www.engineersedge.com/thermodynamics/specific_heat_common_gases__13695.htm
Post by: Bored chemist on 20/04/2022 20:12:16
Sunlight is filtered to produce a narrow spectrum radiation. Is it considered thermal?Take a continuous spectrum, but has flat distribution (up to certain frequency), instead of black body radiation. Is it considered thermal?If it is due to heat, yes.
Another important factor in thermal radiation is emissivity. Is radiation by an object with extremely low emissivity considered thermal?It still depends.
All thermal spectra are continuous, but not all continuous spectra are thermal.Sunlight is filtered by a band stop filter which absorbs a narrow spectrum radiation at 500 nm wavelength. Is it still considered a thermal spectrum even though it's no longer continuous?
Another important factor in thermal radiation is emissivity. Is radiation by an object with extremely low emissivity considered thermal?It still depends.
Post by: hamdani yusuf on 21/04/2022 02:54:20
It still depends.Is it supposed to be an answer?
Let's agree that it depends on something.
What does it depend on?
Post by: Bored chemist on 21/04/2022 08:48:09
Is it supposed to be an answer?It's the truth.
Let's agree that it depends on something.Good
What does it depend on?I already told you twice.
Did you not understand?
Post by: hamdani yusuf on 21/04/2022 11:35:07
I already told you twice.Which one?
Post by: alancalverd on 21/04/2022 11:44:50
NoI think you mean yes. Everyone knows that S varies with T, but at any particular value of T, S will have a value.
Post by: Bored chemist on 21/04/2022 12:54:26
Yes, and the clever ones know that you need to integrate the energy added over the temperature rise.NoI think you mean yes. Everyone knows that S varies with T, but at any particular value of T, S will have a value.
Your view is like saying that the top of a hill is flat and the bottom of the hill is flat so you can calculate the potential energy of an object at the top of the hill by multiplying the weight by zero.
Post by: Bored chemist on 21/04/2022 13:19:47
https://www.engineeringtoolbox.com/hydrogen-d_976.html
And I copied some of it into a spreadsheet
The first row says
175 13.12
At 175K the (constant pressure) heat capacity of 1 Kg of H2 is 13.12 KJ per kelvin
So, raising the temperature of that 1 Kg to 176 K would take 13.12 KJ
And that's where Alan gets his wrong number from.
There's no data in the table for 176K, or 177K.
But there's data for 200K (where Cp is 13.53) and we can assume it's close to a linear change with temperature.
So, if we want to heat the gas to 200K we multiply the average heat capacity by the temperature rise.
And we can keep on doing that, and add up the heat at each step to get the total heat added to the gas as a function of temperature.
I'm lazy enough to use a spreadsheet to do that, and here's the result. I have included Alan's number - the product of the heat capacity and the temperature, for comparison. (I'm talking about 1Kg of H2 so the mass (M) is 1.
[ Invalid Attachment ]
Post by: Bored chemist on 21/04/2022 13:23:10
I already told you twice.Which one?
Yes there is.
That is why I provided it.
Did you somehow miss this bit?
Quote from: Bored chemist on 13/04/2022 23:07:45
A xenon arc lamp gives a fairly good approximation to thermal spectrum- unless you look near the emission lines at the right hand end
https://en.wikipedia.org/wiki/Xenon_arc_lamp#/media/File:Xenon_arc_lamp_profile.png
The visible radiation from the sun is close enough to a thermal spectrum to let us estimate the temperature of the sun's surface.
But the emission lines were what allowed someone to demonstrate the existence of helium
Post by: hamdani yusuf on 21/04/2022 13:36:19
Alan's previous post is more like using average value of S in the range of temperature under consideration. No multiplication by zero is involved.Yes, and the clever ones know that you need to integrate the energy added over the temperature rise.NoI think you mean yes. Everyone knows that S varies with T, but at any particular value of T, S will have a value.
Your view is like saying that the top of a hill is flat and the bottom of the hill is flat so you can calculate the potential energy of an object at the top of the hill by multiplying the weight by zero.
Post by: Bored chemist on 21/04/2022 13:37:21
No multiplication by zero is involved.Nobody said it was.
Did you notice that Alan got completely the wrong answer?
Do you consider that to be important?
Post by: hamdani yusuf on 21/04/2022 13:46:01
I think that something should be a noun.I already told you twice.Which one?Yes there is.
That is why I provided it.
Did you somehow miss this bit?
Quote from: Bored chemist on 13/04/2022 23:07:45
A xenon arc lamp gives a fairly good approximation to thermal spectrum- unless you look near the emission lines at the right hand end
https://en.wikipedia.org/wiki/Xenon_arc_lamp#/media/File:Xenon_arc_lamp_profile.png
The visible radiation from the sun is close enough to a thermal spectrum to let us estimate the temperature of the sun's surface.
But the emission lines were what allowed someone to demonstrate the existence of helium
Post by: hamdani yusuf on 21/04/2022 13:49:24
No multiplication by zero is involved.Nobody said it was.
Your view is like saying that the top of a hill is flat and the bottom of the hill is flat so you can calculate the potential energy of an object at the top of the hill by multiplying the weight by zero.
Post by: Bored chemist on 21/04/2022 15:05:47
You highlighted the wrong bitNo multiplication by zero is involved.Nobody said it was.Your view is like saying that the top of a hill is flat and the bottom of the hill is flat so you can calculate the potential energy of an object at the top of the hill by multiplying the weight by zero.
Your view is like saying that the top of a hill is flat and the bottom of the hill is flat so you can calculate the potential energy of an object at the top of the hill by multiplying the weight by zero.
Nobody said it was actually doing it.
Post by: Bored chemist on 21/04/2022 15:07:58
OK, fair enough.I think that something should be a noun.I already told you twice.Which one?Yes there is.
That is why I provided it.
Did you somehow miss this bit?
Quote from: Bored chemist on 13/04/2022 23:07:45
A xenon arc lamp gives a fairly good approximation to thermal spectrum- unless you look near the emission lines at the right hand end
https://en.wikipedia.org/wiki/Xenon_arc_lamp#/media/File:Xenon_arc_lamp_profile.png
The visible radiation from the sun is close enough to a thermal spectrum to let us estimate the temperature of the sun's surface.
But the emission lines were what allowed someone to demonstrate the existence of helium
It depends on your intention.
Intention is a noun.
It's like saying "is a knife considered sharp?", without saying what you are going to do with it.
Post by: alancalverd on 21/04/2022 15:58:44
Clearly the heat energy of a kilogram of hydrogen is not zero at 175K - that's just from the definition of the Kelvin scale!
And the boiling point of hydrogen is about 25K, so to be really fastidious we need to allow for the phase change.
Fortunately Sliq is pretty close to 14.4 so we can approximate the heat energy at 175K as around 14 x 175 = 2450. If you add that to BC's number you end up pretty close to mine.
As a nod to other pedants, yes, if you know S is a function of T you must integrate ∫SdT rather than just multiply by the final temperature. And I've ignored the latent heat of melting and vaporisation too!
Post by: Bored chemist on 21/04/2022 19:35:43
As a nod to other pedants, yes, if you know S is a function of T you must integrate ∫SdT rather than just multiply by the final temperature.So...
You are actually saying I was right all along...
An odd way to put it but... better late than never.
Except that S varies with T.I should say that objects with higher temperature are some times treated like they have higher potential energy compared to the same/similar objects but at lower temperature.Same object, yes. Similar object, no. The thermal energy (i.e. heat content) of a body of mass M, specific heat capacity S and temperature T is MST.
Post by: Bored chemist on 21/04/2022 19:47:33
[ Invalid Attachment ]
But... whatever... the fundamental issue remains.
You can't just multiply T by Cp because Cp isn't a constant, so you don't know which value to pick.
Post by: alancalverd on 21/04/2022 21:25:43
At 175K the (constant pressure) heat capacity of 1 Kg of H2 is 13.12 KJ per kelvinWhich, multiplied by 175, gives you (to a first approximation) 2310 kJ, not 0.
Just because 175K is a bit chilly doesn't mean you can't extract some heat energy from your kilogram of hydrogen. You could get about 1300 kJ from it by constructing a Stirling engine with a liquid nitrogen cold source - practicable if pointless.
Post by: Bored chemist on 21/04/2022 21:31:10
Did you notice this bit?At 175K the (constant pressure) heat capacity of 1 Kg of H2 is 13.12 KJ per kelvinWhich, multiplied by 175, gives you (to a first approximation) 2310 kJ, not 0.
Just because 175K is a bit chilly doesn't mean you can't extract some heat energy from your kilogram of hydrogen. You could get about 1300 kJ from it by constructing a Stirling engine with a liquid nitrogen cold source - practicable if pointless.
then add the 2450 that Alan talked about
It's the bit where, even if we allow for that you are still wrong.
(and you seem to be repeating yourself.)
It would be interesting if we had data for other gases.
Post by: hamdani yusuf on 05/05/2022 04:13:08
and thermal radiation
Are there something that you disagree with them?
Post by: Bored chemist on 05/05/2022 08:31:51
It can't be a very good attempt; it shows the solid as full of springs, and then it ignores potential energy.
Post by: alancalverd on 05/05/2022 10:19:28
Post by: Bored chemist on 05/05/2022 11:41:01
You supply mechanical energy.
So, the first sentence of the vid is wrong.
And then it makes the flat out false assertion that rotation doesn't contribute to temperature.
It does.
Why does the video get so many things wrong?
Post by: Eternal Student on 05/05/2022 14:36:03
One of the issues with the the videos is that they are very long, so I've only skimmed through each one.
I also don't know who produced or created them, so there's no guide as to their reliability or accuracy.
The first video looks like they have made some simplifications.
Their phrase " temperature is only a measure of the average translational k.e. of a molecule, not the rotational k.e." cannot be taken in isolation from their mention of the equi-partition of energy among all the degrees of freedom.
So if you have equi-partition of energy, then increasing the rotational k.e. will increase the translational k.e. and vice versa, or to say that another way, it's a bit arbitrary to say that rotational k.e. of the molecules doesn't contribute to temperature. Temperature is proportional to the energy in any one degree of freedom you choose to use. By some vector algebra, it would be proportional to the overall translational k.e. if you prefer to consider that.
I skipped through most of the second video. They seemed to be suggesting ways to work with realistic thermal emissions that aren't exactly black bodies. In the first few minutes there was some mention of "radiation" as one of the main mechanisms for heat transfer along with conduction and convection. Did they define "thermal radiation" as radiation that arises from a body naturally whenever it's temperature is above 0 Kelvin - that would be OK as a definition. I don't think they worried about explaining the mechanism for the creation of that radiation or any other fine details, just noted that a hot body would radiate and approximate a black body.
Best Wishes.
Post by: hamdani yusuf on 06/05/2022 11:10:07
It can't be a very good attempt; it shows the solid as full of springs, and then it ignores potential energy.Perhaps the potential energy isn't counted as (doesn't contribute to) temperature.
Post by: Bored chemist on 06/05/2022 12:58:48
It is counted and does contribute.It can't be a very good attempt; it shows the solid as full of springs, and then it ignores potential energy.Perhaps the potential energy isn't counted as (doesn't contribute to) temperature.
Why choose to be wrong about that?
Post by: Eternal Student on 06/05/2022 13:34:18
Perhaps the potential energy isn't counted as (doesn't contribute to) temperature.Do you mean you haven't actually spent your ( @hamdani yusuf ) own time watching those videos? Their answer is in the video.
Best Wishes.
Post by: hamdani yusuf on 06/05/2022 22:34:06
Then the definition would be false.It is counted and does contribute.It can't be a very good attempt; it shows the solid as full of springs, and then it ignores potential energy.Perhaps the potential energy isn't counted as (doesn't contribute to) temperature.
Why choose to be wrong about that?
What's to explain? The caption statement is almost correct: the definition of temperature is the average internal kinetic energy of a body. You can't explain a definition!
Post by: hamdani yusuf on 06/05/2022 22:44:15
Hi.You don't have to agree with everything presented in a video.Perhaps the potential energy isn't counted as (doesn't contribute to) temperature.Do you mean you haven't actually spent your ( @hamdani yusuf ) own time watching those videos? Their answer is in the video.
Best Wishes.
Post by: Bored chemist on 07/05/2022 01:16:21
Alan is wrong. That's not news.Then the definition would be false.It is counted and does contribute.It can't be a very good attempt; it shows the solid as full of springs, and then it ignores potential energy.Perhaps the potential energy isn't counted as (doesn't contribute to) temperature.
Why choose to be wrong about that?What's to explain? The caption statement is almost correct: the definition of temperature is the average internal kinetic energy of a body. You can't explain a definition!
Post by: hamdani yusuf on 07/05/2022 03:38:11
What's to explain? The caption statement is almost correct: the definition of temperature is the average internal kinetic energy of a body. You can't explain a definition!How do you distinguish between internal and external energy?
What do you think about a resonating tuning fork?
What do you think about a resonating LC circuit?
Electrical current circulating indefinitely in a superconducting ring?
Post by: hamdani yusuf on 07/05/2022 04:13:59
It is counted and does contribute.As an example, an object absorbs 2 Joule of energy. 1 Joule is converted to potential energy, and 1 Joule is converted to kinetic energy.
Another object with same mass absorbs 2 Joule of energy. 0 Joule is converted to potential energy, and 2 Joules is converted to kinetic energy.
According to the definition above, the temperature of the object increases corresponding to the increase of kinetic energy. Hence the second object increases its temperature twice as much as the first object.
Post by: Eternal Student on 07/05/2022 12:51:36
...an object absorbs 2 Joule of energy. 1 Joule is converted to potential energy, and 1 Joule is converted to kinetic energy. Another object with same mass absorbs 2 Joule of energy. 0 Joule is converted to potential energy, and 2 Joules is converted to kinetic energy....... the second object increases its temperature twice as much as the first object...
Not quite. In the general case, one of the following applies:
(i) The second object is not yet in thermal equillibrium. There isn't a equal partition of energy between the various modes or degrees of freedom it can support as forms of internal energy. As such its temperature is not well defined yet.
(ii) The "potential energy" you were considering was never one of the ways in which it can support internal energy, for example it might be gravitational potential energy due to lifting the object up higher. It had no relevance for temperature. (In which case, you would be right, gaining that sort of potential energy didn't make the first object hotter, it was 1 J of energy in some form that didn't change its temperature).
Best Wishes.
Post by: hamdani yusuf on 07/05/2022 13:56:04
(i) The second object is not yet in thermal equillibrium. There isn't a equal partition of energy between the various modes or degrees of freedom it can support as forms of internal energy. As such its temperature is not well defined yet.Then wait until it is well defined.
(ii) The "potential energy" you were considering was never one of the ways in which it can support internal energy, for example it might be gravitational potential energy due to lifting the object up higher. It had no relevance for temperature. (In which case, you would be right, gaining that sort of potential energy didn't make the first object hotter, it was 1 J of energy in some form that didn't change its temperature).If we add thermal energy to an object but its temperature doesn't change, then according to the definition above, its internal kinetic energy doesn't change. Hence, the energy should be converted into something else, which can be external kinetic energy, external potential energy, internal potential energy, or combinations of those kinds of energy.
Melting ice may cross our minds as an example.
Post by: Eternal Student on 07/05/2022 15:53:33
Then wait until it is well defined.The fundamental idea is that the energy will automatically be re-distributed throughout the system, so that you have an equi-partition of energy in all the modes or degrees of freedom that the system can support. So, if you do wait, then some of the energy, the 2 J of internal kinetic energy will be passed to other modes of storing energy. Taking a generalised example, if your system supports kinetic energy of the particles and also potential energy in the bonds (or springs) between atoms then you end up with 1 J remaining as kinetic and 1 J being potential energy. i.e. The second object becomes exactly like the first object in your example.
If we add thermal energy to an object but its temperature doesn't change, then according to the definition above, its internal kinetic energy doesn't change. Hence, the energy should be converted into something else, which can be external kinetic energy, external potential energy, internal potential energy, or combinations of those kinds of energy.As mentioned before, Temperature is the measure of energy in ANY one degree of freedom or mode of supporting internal energy that the system has. Vector geometry is on our side, so the average overall translational kinetic energy, ½mv2, of molecules (in something like a simple gas) can also be considered as something proportional to temperature.
Melting ice may cross our minds as an example.
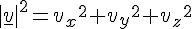 [By vector geometry, vx = x component of v etc.]
[By vector geometry, vx = x component of v etc.]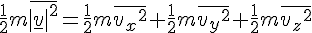 [Bar over = average over all particles. Note that it is the mean square not the square of the mean.]
[Bar over = average over all particles. Note that it is the mean square not the square of the mean.]But due to equi-partition,
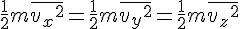 = the (average) energy of a particle in any one mode =
= the (average) energy of a particle in any one mode = 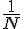 . (Total Energy in one mode) (where N = number of particles in the substance).
. (Total Energy in one mode) (where N = number of particles in the substance).So the average overall translational k.e. = 3 times average k.e. in one mode = something proportional to Temperature. Both the overall translational k.e. AND the energy in any one mode are proportional to temperature, just with different constants of proportionality. In simple kinetic theory of gases we have:
(Total Energy in any one mode) = ½ KB . T with T = temperature, KB = Boltzmann constant.
Now we can consider what might be happening when there is a change of state:
Sometimes when there is a phase change (let's say solid to gas), the new system (the gas) supports more modes of storing energy. As a simple example consider a diatomic gas. While in the gas state, it can support 2 different rotations that were not possible while the molecules were bound in the solid state. Temperature is proportional to the energy in ANY one degree of freedom you choose to use.
So one thing that can happen when there is a change of state is that as parts of the system break off from the solid state and form the next state, there simply are more modes of storing energy. So the energy in any ONE mode can remain constant (the temperature doesn't change) but the total internal energy stored in the substance does increase. So in answering "where does the latent heat go?", we can say that it is stored internally in the substance, it doesn't result in any increase in the translational k.e. of the particles (and hence no change in temperature) because there is a whole new mode of internal energy now available and energy has to be re-distributed to that new mode ("fill it up") to maintain an equi-partition of energy for the system.
The new system of defining temperature (since May 2019) bases everything on kinetic theory. It's very new and not something I'm very familiar with. There does appear to be a change in the number of modes for supporting internal energy when a substance changes state but the discussion above is a simplification of the situation.
References:
https://en.wikipedia.org/wiki/Temperature#Kinetic_theory_approach
(and more generally the entire article about Temperature, where the very new adjustments as of May 2019 are still only just being introduced, edited and corrected even now).
Best Wishes.
Post by: alancalverd on 07/05/2022 16:23:26
How do you distinguish between internal and external energy?It is the average kinetic energy of the particles inside the body, regardless of how fast the body is moving or what its gravitational potential may be.
Post by: alancalverd on 07/05/2022 16:51:41
As an example, an object absorbs 2 Joule of energy. 1 Joule is converted to potential energy, and 1 Joule is converted to kinetic energy.Correct.
Another object with same mass absorbs 2 Joule of energy. 0 Joule is converted to potential energy, and 2 Joules is converted to kinetic energy.
According to the definition above, the temperature of the object increases corresponding to the increase of kinetic energy. Hence the second object increases its temperature twice as much as the first object.
An increase of internal potential energy would correspond to a partial or total change of state within the body.
I encountered this when measuring radiation dose with a calorimeter. Dose is defined as energy aborbed per unit mass, and the principal concern for radiation protection and radiotherapy is the measurement of dose to water. For practical simplicity most primary standard calorimeters use graphite as the absorber because it is mechanically stable and has about a tenth of the specific heat capacity of water so undergoes a larger temperature change (a lethal dose of ionising radiation raises your body temperature by about 0.001 degree - my task was to measure that to ± 10-6K). One of my colleagues built a water calorimeter - rather less portable device but clearly worth directly measuring the quantity of interest rather than trying to derive it. Problem was that the water calorimeter generally measured about 3% less than the graphite calorimeter, though both were calibrated to ± 0.01%. I thought the difference was due to "virgin" water forming metastable polymers when irradiated, because the defect gradually decreased with extended irradiation to high doses but later work has revealed all sorts of complex chemistry possible with just H and O atoms and plenty of energetic photons.
Post by: Bored chemist on 07/05/2022 18:16:09
You should have asked a chemist.As an example, an object absorbs 2 Joule of energy. 1 Joule is converted to potential energy, and 1 Joule is converted to kinetic energy.Correct.
Another object with same mass absorbs 2 Joule of energy. 0 Joule is converted to potential energy, and 2 Joules is converted to kinetic energy.
According to the definition above, the temperature of the object increases corresponding to the increase of kinetic energy. Hence the second object increases its temperature twice as much as the first object.
An increase of internal potential energy would correspond to a partial or total change of state within the body.
I encountered this when measuring radiation dose with a calorimeter. Dose is defined as energy aborbed per unit mass, and the principal concern for radiation protection and radiotherapy is the measurement of dose to water. For practical simplicity most primary standard calorimeters use graphite as the absorber because it is mechanically stable and has about a tenth of the specific heat capacity of water so undergoes a larger temperature change (a lethal dose of ionising radiation raises your body temperature by about 0.001 degree - my task was to measure that to ± 10-6K). One of my colleagues built a water calorimeter - rather less portable device but clearly worth directly measuring the quantity of interest rather than trying to derive it. Problem was that the water calorimeter generally measured about 3% less than the graphite calorimeter, though both were calibrated to ± 0.01%. I thought the difference was due to "virgin" water forming metastable polymers when irradiated, because the defect gradually decreased with extended irradiation to high doses but later work has revealed all sorts of complex chemistry possible with just H and O atoms and plenty of energetic photons.
We know that irradiating water makes things like hydrogen peroxide and we know that making rocket fuel takes energy.
Post by: alancalverd on 07/05/2022 22:45:24
Post by: Bored chemist on 07/05/2022 22:53:31
- we'd expected everything to recombine in microseconds.You do know that you can just buy hydrogen peroxide as a solution in water, don't you?
Post by: hamdani yusuf on 08/05/2022 04:50:14
The fundamental idea is that the energy will automatically be re-distributed throughout the system, so that you have an equi-partition of energy in all the modes or degrees of freedom that the system can support. So, if you do wait, then some of the energy, the 2 J of internal kinetic energy will be passed to other modes of storing energy. Taking a generalised example, if your system supports kinetic energy of the particles and also potential energy in the bonds (or springs) between atoms then you end up with 1 J remaining as kinetic and 1 J being potential energy. i.e. The second object becomes exactly like the first object in your example.Let's put your analysis to a concrete example. The first object is a diatomic gas, such as N2, while the second object is a monoatomic gas, such as Neon. Is it still valid?
Post by: Bored chemist on 08/05/2022 09:58:20
Take the same number of molecules of each gas starting from very cold, and add heat slowly.
In order to get cold enough, you might need to work at low pressure to stop them turning to liquid or solid,but that's a different issue.
And we can use a large box, to address a different issue, but I will come back to that.
At low enough temperatures, the heat capacities of the gases are the same- the heat that you add goes into making the molecules translate.
So rises in the temperatures of the two gases are both the same.
But there comes a point (A few K, I think) where there is enough energy to make the N2 molecules rotate.
(Not many people think about this, but rotational energy is quantised).
So the heat that you add to the neon all goes into increasing the translational energy of the molecules.
But some of the energy you add to the nitrogen goes into making the nitrogen molecules rotate.
So now you have the same amount of heat added to both gases, but in the case of the nitrogen it is shared out amend 5 degrees of freedom, but with the neon it is only split between 3 degrees of freedom.
So the temperature rise- the average energy per degree of freedom- is smaller for the nitrogen.
For a given amount of heat, the neon gets hotter than the nitrogen.
Eventually, you get the gas hot enough that you start to excite the vibration of the nitrogen molecule as well, and its heat capacity rises again.
And then as you continue to heat both gases, the rates of change of temperature become even more different.
So, it is simply not true to say you can ignore everything but translational KE.
For what it's worth, the translational KE is also quantise and, for an atom in a small enough box, the temperature is rather poorly defined but that's seldom a practical issue.
The important thing is that what the video says is simply wrong.
Post by: Eternal Student on 08/05/2022 23:07:28
I don't have much disagreement with what @Bored chemist has said. That's a very detailed examination of what might happen as you start to heat N2. In particular, experimental measurements of the specific heat capacity does show slight variation with temperature (at least old definition temperature).
The only issue I would have is that is that I don't think we should use any Quantum Mechanics or attempt to quantise everything. Temperature (modern temperature) does not concern itself with a precise description of anything, it's just a useful concept in statistical mechanics which relies very much on classical mechanics being applied to the microscopic particles instead of quantum mechanics.
.....Since May, 2019, its degrees (they meant degrees Kelvin) have been defined through particle kinetic theory, and statistical mechanics....
[From Wikipedia: under sub-category "International Kelvin Scale" of this page https://en.wikipedia.org/wiki/Temperature]
.....Kinetic theory provides a microscopic account of temperature for some bodies of material, especially gases, based on macroscopic systems' being composed of many microscopic particles, such as molecules and ions of various species, the particles of a species being all alike. It explains macroscopic phenomena through the classical mechanics of the microscopic particles.
[Taken from the "Kinetic theory approach" sub-section of the above article in Wikipedia.]
What does this mean in practical terms? Rotational k.e. should, in reality be quantised, as discussed by @Bored chemist , however the very latest (since 2019) definition of temperature for the N2 simply doesn't care.
I'm not responsible - I didn't change the way temperature is defined or thought about. Personally, I think the decision to use classical mechanics in their models has seriously limited the expected lifetime of this new approach to defining temperature. We'll have a new approach in 20 years, which probably will start using Quantum Mechanics or else return to using a Thermodynamic temperature definition.
Best Wishes.
Post by: hamdani yusuf on 09/05/2022 10:09:39
At low enough temperatures, the heat capacities of the gases are the same- the heat that you add goes into making the molecules translate.Why don't polyatomic gases rotate nor vibrate at low temperature?
So rises in the temperatures of the two gases are both the same.
Post by: hamdani yusuf on 09/05/2022 10:12:32
But there comes a point (A few K, I think) where there is enough energy to make the N2 molecules rotate.What's the minimum non-zero quantity of rotational energy?
(Not many people think about this, but rotational energy is quantised).
Is vibrational energy also quantized?
Post by: Eternal Student on 09/05/2022 12:18:56
Why don't polyatomic gases rotate nor vibrate at low temperature?In the models they do rotate slowly at any temperature above 0 Kelvin. QM is not important. There would be a continuous probability distribution function to describe the probability of any particle having a given rotation.
In reality they may or may not because angular momentum should be quantised according to QM. If you had a large number of N2 molecules you may find a discrete distribution for their rotations. Some have 0 angular momentum, others should have angular momentum = n . ħ/2 for some positive integer n.
Best Wishes.
Post by: Bored chemist on 09/05/2022 13:00:33
Why don't polyatomic gases rotate nor vibrate at low temperature?Because the energy available to them is less than that required to get them to rotate or vibrate.
Is vibrational energy also quantized?Yes.
QM is not important.Yes it is.
What's the minimum non-zero quantity of of rotational energy?It depends on the molecule.
For N2 I think it's about 10^-4 eV.
Post by: Eternal Student on 09/05/2022 15:27:33
QM is important in reality. That's not in dispute.
However, it does not appear in the classical microscopic mechanical models that are used to define temperature since May 2019.
Temperature (in kelvin, since 2019) is NOT telling you something about the actual kinetic energy of a particle you can observe in reality. It is much more abstract than that, it is the kinetic energy a particle would have in the model.
Let's not lose sight of the idea that particles might not even exist in reality, everything might be waves or some such thing. However, there are always particles in the model, they have kinetic energy and classical mechanics is used.
This is actually a fairly small and unimportant issue and it's hardly worth distracting @hamdani yusuf from whatever the main topic was originally hoping to cover. However, it's interesting, i.m.o. so I'll spend another moment discussing it.
In practice it's not like the shift in considering what temperature is supposed to be maters a lot. At temperatures above a few degrees kelvin and for a large number of particles you are out of the regime of Q.M. and there is good agreement between a classical microscopic model and properties you might actually observe in reality. However, just for the sake of completion, it must be noted that "the temperature" of a body, under the latest definitions and international agreements for the kelvin scale has an entirely theoretical basis....
There is a paradigm shift in what "temperature" is supposed to be. It is not a description of some real physical property like kinetic energy that particles actually have in reality. Instead it is a description of the average kinetic energy that a particle would have in the model due to the energy that has been transferred to it. The numerical value of the temperature in kelvin (since May 2019) is only telling you something that is easily identified in the model and may be quite impossible to measure or observe in reality. It just turns out that for a macroscopic object (and above extremely low energies like a few degrees kelvin) classical microscopic mechanics does describe the situation well and a property emerges in the macroscopic object that behaves as we would hope and has the properties we would want to describe as the "temperature" of that real life macroscopic body.
As outlined earlier, for right or wrong, the scientists have used classical mechanics and not QM in constructing their models for the microscopic thermal behaviour modelling of substances. That's not my fault, that's just what they've done.
--------------
Returning to the original question of the OP:
What is temperature?
Reasonable answers and discussions have been made in countless earlier posts. It might be best to say there are now several different approaches to defining temperature.
Wikipedia describes some of these approaches .... empirical, theoretical, thermodynamic, ... etc.
Each of these approaches produces a different idea of what temperature is. So "temperature" as the term is used by scientists is one of several different things. The only good news is that for most macroscopic objects and applications in most situations, they all behave similarly and it's even possible to identify that any one scale is measuring almost exactly the same sort of thing as another scale. Note that we tend to say "scale" and it sounds like the only difference is in the choice of units for measurement but that's not what is actually happening. The true situation is that slightly different things or different properties are actually being quantified by each scale. For example, one scientist is measuring the height of a person, another scientist is measuring the length of their shadow, outside of the standard regime or situation (so that's a macroscopic object with millions of particles and temperatures high enough so that QM is unimportant) one measurement is not just a fixed conversion factor times by the other measurement, they just are very different and non-comparable things.
In the post 2019 definition of temperature in kelvin, the temperature of an object is not required to be a measure of any physical thing you could actually observe. Instead it is a much more abstract quantity, it is the average k.e. that a particle would have in the model. It doesn't matter whether you could actually observe particles and measure them to have kinetic energies like that. Whatetver the situation, it is understood that temperature is describing something - an average kinetic energy - that might exist only in theory, in the model.
However, this ("temperature" quantity) emerges as a real physical property or characteristic of a macroscopic object, statistical averages hold, all of statistical mechanics and thermodynamics will hold well for that object etc. etc.
Overall then, it's possible to say "temperature (as defined by the modern kelvin scale)" is an entirely theoretical quantity, however it becomes a real emergent property which can be assigned to macroscopic objects. That is to say, at least to a high level of accuracy, it can be considered and modelled as a continuous parameter, T, that a macroscopic body has.
Best Wishes.
Post by: Bored chemist on 09/05/2022 16:10:07
And that means that heat capacities really do very with temperature.
The QM effects of rotation are usually safely ignored (maybe not for H2 at low temps- which is becoming increasingly important)- but the QM effects are also manifest in the variations of heat capacity with temperature.
The vibrational effects are not something you can ignore.
There's currently a thread on this site asking about the maximum temperature you can reach with a charcoal fire.
If you ignore the (quantised) vibrational heat capacity in that calculation, you get a seriously wrong answer.
I'd have to check, but I think you need to start looking at the electronic transitions too.
Post by: hamdani yusuf on 10/05/2022 14:13:37
Because the energy available to them is less than that required to get them to rotate or vibrate.Is translational energy also quantized?
If not, what makes the difference?
Post by: Bored chemist on 10/05/2022 18:32:35
Is translational energy also quantized?
For what it's worth, the translational KE is also quantised
If not, what makes the difference?Good question.
I'm saying that you consider all the ways in which the molecule can carry energy when you consider temperature.
So I'm not saying that translational energy is "special" and it's the only one which counts.
So there isn't a difference (apart from a practical one- unless the box is very small, the translational energy levels are very close together and the effect is not measurable).
You need to ask the people who think that vibrational energy doesn't count why they think it's different.
Post by: hamdani yusuf on 10/05/2022 23:19:28
I think that the experimental plot above plays important role in the development of equipartition theory, also the concept of degree of freedom. But the difference in the gradient of the curve shows that at least at some points, the energy distribution among different degrees of freedoms are not equal.
Post by: alancalverd on 11/05/2022 00:04:20
Temperature (in kelvin, since 2019) is NOT telling you something about the actual kinetic energy of a particle you can observe in reality. It is much more abstract than that, it is the kinetic energy a particle would have in the model.Pedant mode engaged.
A single particle cannot have a temperature.Temperature is only defined as a mesoscopic parameter, or, if you prefer, an emergent property of an ensemble.
Post by: Eternal Student on 11/05/2022 00:50:12
A single particle cannot have a temperature.Temperature is only defined as a mesoscopic parameter, or, if you prefer, an emergent property of an ensemble.Yes, absolutely true. Did I undermine that? Sorry if I did.
Also "mesoscopic"? Did you really mean middle-sized? I'd be alright with a macroscopic property myself.
I think the flow of the original statement(s) were already becoming excessively cumbersome. It certainly wasn't that a particle had a temperature, it doesn't but it does have a kinetic energy. Or at the very least, it does in the model.
Temperature is telling you about the average kinetic energy of a particle among a myriad of otherwise identical particles that make up the whole macroscopic object (and in the case of a theoretical temperature scale, it's still only information for the average k.e. of a particle in the model ).
Best Wishes.
Post by: Bored chemist on 11/05/2022 08:45:15
(https://upload.wikimedia.org/wikipedia/commons/thumb/0/07/DiatomicSpecHeat1.png/300px-DiatomicSpecHeat1.png)Or it shows that the number of degrees of freedom isn't an integer.
I think that the experimental plot above plays important role in the development of equipartition theory, also the concept of degree of freedom. But the difference in the gradient of the curve shows that at least at some points, the energy distribution among different degrees of freedoms are not equal.
Post by: hamdani yusuf on 11/05/2022 12:47:50
Or it shows that the number of degrees of freedom isn't an integer.That's new to me. Do you have supporting evidence for your hypothesis?
Quote
https://en.m.wikipedia.org/wiki/Degrees_of_freedom_(physics_and_chemistry)The article doesn't mention about quantization of translational energy. But the previous plot shows that it's already activated at 0 K.
Both the rotational and vibrational modes are quantized, requiring a minimum temperature to be activated.[7] The "rotational temperature" to activate the rotational degrees of freedom is less than 100 K for many gases. For N2 and O2, it is less than 3 K.[1] The "vibrational temperature" necessary for substantial vibration is between 103 K and 104 K, 3521 K for N2 and 2156 K for O2.[1] Typical atmospheric temperatures are not high enough to activate vibration in N2 and O2, which comprise most of the atmosphere.
Post by: Bored chemist on 11/05/2022 13:29:14
Do you have supporting evidence for your hypothesis?Yes; your graph.
Since the heat capacity is 1/2 R per degree of freedom, that graph is effectively a graph of "number of degrees of freedom per molecule" vs temperature.
The sloping bits correspond to non integer values of the DoF.
But the previous plot shows that it's already activated at 0 K.
The heat capacity at 0 K is zero.
"So the heat capacity must go to zero at absolute zero"
from
https://en.wikipedia.org/wiki/Third_law_of_thermodynamics
So, we know that it isn't.
We know that the graph is wrong.
It would be quicker if you started by learning science from the bottom up, rather than trying to understand the complicated bits before you understand the underlying principles.
Post by: hamdani yusuf on 12/05/2022 03:09:14
Yes; your graph.What's your sources?
Since the heat capacity is 1/2 R per degree of freedom, that graph is effectively a graph of "number of degrees of freedom per molecule" vs temperature.
The sloping bits correspond to non integer values of the DoF.
Post by: hamdani yusuf on 12/05/2022 03:16:09
The heat capacity at 0 K is zero.Where's the switching point from 0 to 3R/2 ?
"So the heat capacity must go to zero at absolute zero"
from
https://en.wikipedia.org/wiki/Third_law_of_thermodynamics
So, we know that it isn't.
We know that the graph is wrong.
What's the slope of the change?
Post by: Bored chemist on 12/05/2022 08:43:38
What's the slope of the change?Upwards to the right.
Obviously the gradient changes with temperature.
Last time I saw it modeled, they fitted the hyperbolic tangent function to the data.
I'm not sure if that was on theoretical grounds, or just because it's the right shape.
What's your sources?56 years of acquired experience.
Post by: hamdani yusuf on 12/05/2022 15:20:50
Where's the switching point from 0 to 3R/2 ?I'll rephrase it to make it more precise.
What's the minimum temperature where the specific heat of gas is 3R/2?
Post by: Bored chemist on 12/05/2022 17:59:14
What's the minimum temperature where the specific heat of gas is 3R/2?
It depends.
What would you actually do with the information if I told you?
Why not study science?
Then you would be able to work it out rather than asking pointless questions.
Post by: hamdani yusuf on 13/05/2022 05:40:51
What does it mean when a system has 3.4 degrees of freedom?What's the slope of the change?Upwards to the right.
Obviously the gradient changes with temperature.
Last time I saw it modeled, they fitted the hyperbolic tangent function to the data.
I'm not sure if that was on theoretical grounds, or just because it's the right shape.What's your sources?56 years of acquired experience.
Post by: hamdani yusuf on 13/05/2022 05:41:47
Don't you think that science should be objective?What's the minimum temperature where the specific heat of gas is 3R/2?
It depends.
What would you actually do with the information if I told you?
Why not study science?
Then you would be able to work it out rather than asking pointless questions.
Post by: Bored chemist on 13/05/2022 18:12:23
Don't you think that science should be objective?I never suggested otherwise.
Incidentally, you forgot to answer my questions.
Please do so.
What does it mean when a system has 3.4 degrees of freedom?That you are a little less than half way between the flat bits of the graph at 3 and 4.
Post by: alancalverd on 13/05/2022 23:58:56
Also "mesoscopic"? Did you really mean middle-sized?somewhere between microscopic (where quantum effects add significant noise to your measurement) and astronomical (where simultaneity cannot be assumed). Usually refers to manageable things like bricks and cows.
Temperature is telling you about the average kinetic energy of a particle among a myriad of otherwise identical particlesThey don't need to be identical. Porridge is inhomogeneous, but Goldilocks was able to measure its temperature. Temperature is the mean kinetic energy of all the particles in a sample.
Post by: Eternal Student on 14/05/2022 02:19:54
Thanks for your comments @alancalverd .
They don't need to be identical. Porridge is inhomogeneous, but Goldilocks was able to measure its temperature. Temperature is the mean kinetic energy of all the particles in a sample.The original statement(s) I made (pages back, for example post #278) concerns the modern definition of temperature in kelvin since May 2019. In the models they use for that theoretical temperature scale, all the particles of one species are assumed to be identical to each other (apart from having their own velocity which follows a Boltzmann distribution). There's just no way I could have all the i dotted and all the t crossed.
Kinetic theory provides a microscopic account of temperature for some bodies of material, especially gases, based on macroscopic systems' being composed of many microscopic particles, such as molecules and ions of various species, the particles of a species being all alike.
https://en.wikipedia.org/wiki/Temperature#Kinetic_theory_of_gases
Anyway, the particles of a species in the porridge are identical (in the model) but the porridge can have more than one species of particle.
- - - - - - - - - -
The first bowl was <unknown>, the second bowl was <unknown> but the last was just <unknown>
As it happens I don't think there is a good theoretical model for porridge. As such Goldilocks probably can't determine the temperature of her porridge on the modern (post 2019) kelvin scale. That temperature is simply "unknown" or "undetermined" at this time.
....In an ideal gas, and in other theoretically understood bodies, the Kelvin temperature is defined to be proportional to the average kinetic energy of non-interactively moving microscopic particles, which can be measured by suitable techniques.... [ https://en.wikipedia.org/wiki/Temperature#Theoretical_scales ]
... making the clear implication that if the porridge is not theoretically understood, specifically that we don't have a good model for it right now, then .... who knows, best not give it a temperature in kelvin (kelvin post 2019)....
It would be worth me saying this again: The very latest entirely theoretical approach to defining temperature came in from May 2019 and Wikipedia articles on temperature are getting edited every week trying to bring them inline with these changes. I'm not claiming to be an expert on the new approach.
Best Wishes.
Post by: hamdani yusuf on 14/05/2022 03:47:52
I never suggested otherwise.It seems you did.
It depends.
What would you actually do with the information if I told you?
Incidentally, you forgot to answer my questions.Which one?
Please do so.
Post by: hamdani yusuf on 14/05/2022 03:53:12
That you are a little less than half way between the flat bits of the graph at 3 and 4.What's the physical interpretation of that?
What makes it better than interpreting that rotational energy hasn't been fully activated?
Post by: Bored chemist on 14/05/2022 10:44:00
https://en.wikipedia.org/wiki/Partition_function_(statistical_mechanics)
Post by: Eternal Student on 14/05/2022 14:13:02
This thread has got quite unpleasant, hasn't it? It's not my forum and there's no reason my opinion is worth writing down or reading. However, I don't think I'll be following this thread anymore. My apologies if I don't reply to someone who has quoted or tagged me ( @Eternal Student ) etc.
Best Wishes.
Post by: alancalverd on 16/05/2022 00:18:15
As it happens I don't think there is a good theoretical model for porridge. As such Goldilocks probably can't determine the temperature of her porridge on the modern (post 2019) kelvin scale. That temperature is simply "unknown" or "undetermined" at this time.Things aren't quite that bad. She may not find it easy to calculate, but it's very easy to measure with a thermometer calibrated in K.
Post by: Rodneyhhernandez on 16/05/2022 13:27:20
Do I understand?
Post by: Bored chemist on 16/05/2022 13:29:43
Depending on the temperature molecules move faster or slower?Yes.
Do I understand?
Hotter molecules move faster.
But, as is often the case, the detail is more complicated than that
Post by: hamdani yusuf on 21/05/2022 11:29:58
Things aren't quite that bad. She may not find it easy to calculate, but it's very easy to measure with a thermometer calibrated in K.Different types of thermometers have their limits in range and linearities. How would you calibrate them against each other, if temperature is not well defined in standardized definition?
Post by: Bored chemist on 21/05/2022 12:16:25
How would you calibrate them against each other,Practically speaking, like this.
https://en.wikipedia.org/wiki/International_Temperature_Scale_of_1990
Theoretically, like this
https://en.wikipedia.org/wiki/2019_redefinition_of_the_SI_base_units#Kelvin
Post by: Origin on 21/05/2022 14:46:59
Different types of thermometers have their limits in range and linearities. How would you calibrate them against each other, if temperature is not well defined in standardized definition?After 16 pages you sound more confused about temperature than when you started. :o
Post by: alancalverd on 21/05/2022 22:21:46
Temperature is fully defined, and the fixed points on the Celsius scale are entirely adequate for determining the temperature of porridge since it consists mostly of liquid water.Things aren't quite that bad. She may not find it easy to calculate, but it's very easy to measure with a thermometer calibrated in K.Different types of thermometers have their limits in range and linearities. How would you calibrate them against each other, if temperature is not well defined in standardized definition?
Post by: hamdani yusuf on 23/05/2022 03:43:10
After 16 pages you sound more confused about temperature than when you started.So, what's your answer to this question : what is temperature?
Post by: hamdani yusuf on 23/05/2022 03:48:08
How can we make sure that those defining points conform to the definition of temperature?How would you calibrate them against each other,Practically speaking, like this.
https://en.wikipedia.org/wiki/International_Temperature_Scale_of_1990
Theoretically, like this
https://en.wikipedia.org/wiki/2019_redefinition_of_the_SI_base_units#Kelvin
Quote
https://en.wikipedia.org/wiki/International_Temperature_Scale_of_1990#Defining_points
Substance and its state Defining point (range)
K °C °R °F
Triple point of hydrogen 13.8033 −259.3467 24.8459 −434.8241
Triple point of neon 24.5561 −248.5939 44.2010 −415.4690
Triple point of oxygen 54.3584 −218.7916 97.8451 −361.8249
Triple point of argon 83.8058 −189.3442 150.8504 −308.8196
Triple point of mercury 234.3156 −38.8344 421.7681 −37.9019
Triple point of water[note 1] 273.16 0.01 491.69 32.02
Melting point[note 2] of gallium 302.9146 29.7646 545.2463 85.5763
Freezing point[note 2] of indium 429.7485 156.5985 773.5473 313.8773
Freezing point[note 2] of tin 505.078 231.928 909.140 449.470
Freezing point[note 2] of zinc 692.677 419.527 1,246.819 787.149
Freezing point[note 2] of aluminium 933.473 660.323 1,680.251 1,220.581
Freezing point[note 2] of silver 1,234.93 961.78 2,222.87 1,763.20
Freezing point[note 2] of gold 1,337.33 1,064.18 2,407.19 1,947.52
Freezing point[note 2] of copper 1,357.77 1,084.62 2,443.99 1,984.32
Post by: Bored chemist on 23/05/2022 12:07:53
How can we make sure that those defining points conform to the definition of temperature?By doing science.
You should try it some time.
Post by: hamdani yusuf on 23/05/2022 14:09:10
Are they necessary to be defined besides of practical purpose?How can we make sure that those defining points conform to the definition of temperature?By doing science.
You should try it some time.
Can they be derived purely theoretically?
How do you calibrate temperature far from those defining points, such as 1 milli Kelvin, or 1 million Kelvin?
Post by: Spring Theory on 23/05/2022 14:10:06
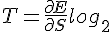
Where T is temperature, E is the energy of the system and S is the entropy of the system.
Temperature is the amount of energy required to change the entropy by one bit.
Post by: hamdani yusuf on 23/05/2022 15:06:41
I think you guys are getting very unproductive. My elegant description of temperature in the form of concentration of photons gives the concept. In statistical mechanics temperature is defined starting with Entropy (setting Boltzmann's constant to 1):What does the log2 mean? Is it a constant?
Where T is temperature, E is the energy of the system and S is the entropy of the system.
Temperature is the amount of energy required to change the entropy by one bit.
How do you explain that melting ice doesn't change its temperature while absorbing heat energy?
Post by: Bored chemist on 23/05/2022 18:31:36
My elegant description of temperatureIt may be elegant, but it isn't much use.
Do you think that you can clearly explain the concept of entropy changes without using temperature in your explanation?
Post by: alancalverd on 23/05/2022 18:41:38
Temperature is the amount of energy required to change the entropy by one bit.So the entropy of an object at constant temperature is continually changing? Welcome to the world of Hamdani Yusuf!
Post by: alancalverd on 23/05/2022 18:42:54
So, what's your answer to this question : what is temperature?A measure of the internal kinetic energy of a body.
Post by: alancalverd on 23/05/2022 18:48:53
How do you calibrate temperature far from those defining points, such as 1 milli Kelvin, or 1 million Kelvin?You can only calibrate an instrument at the agreed fixed points. You can interpolate or extrapolate other values.
I measured microdegfree temperature changes with a thermistor, whilst my good buddy (also named Alan) in the next lab measured plasma temperatures of 100 MK by studying the electron resonance spectrum. Herr Boltzmann's Konstant was very helpful in both cases.
Post by: Spring Theory on 23/05/2022 20:06:01
I think you guys are getting very unproductive. My elegant description of temperature in the form of concentration of photons gives the concept. In statistical mechanics temperature is defined starting with Entropy (setting Boltzmann's constant to 1):What does the log2 mean? Is it a constant?
Where T is temperature, E is the energy of the system and S is the entropy of the system.
Temperature is the amount of energy required to change the entropy by one bit.
How do you explain that melting ice doesn't change its temperature while absorbing heat energy?
Yes log2 is one bit, a constant.
Melting ice is doing work on the molecular structure.
Post by: alancalverd on 23/05/2022 23:06:14
Post by: Spring Theory on 24/05/2022 00:28:23
Would that be 0.3010 or 0.6931? Or does it mean log2 of any number you can think of?
0.3010
Post by: Spring Theory on 24/05/2022 00:38:00
My elegant description of temperatureIt may be elegant, but it isn't much use.
Do you think that you can clearly explain the concept of entropy changes without using temperature in your explanation?
Entropy is the probability function of the potential states of a system. It directly relates to the information you know about a system.
If you know everything or the exact state of the system, then it has zero entropy. If you only know there are a potential number of states, n, and there is an equal probability of each state, then the entropy is proportional to n.
Essentially, entropy increases with your ignorance of the state of a system, but is usually used in calculations as a property of the system.
Post by: hamdani yusuf on 24/05/2022 03:14:49
Why do you use base 10 instead of e?Would that be 0.3010 or 0.6931? Or does it mean log2 of any number you can think of?
0.3010
What's your dimensional analysis?
Post by: hamdani yusuf on 24/05/2022 03:26:02
I disagree with the statements above. But of course you are free to put everyone who disagree with you into the same category, but the more appropriate name would be the world of non-Alan Calverd.Temperature is the amount of energy required to change the entropy by one bit.So the entropy of an object at constant temperature is continually changing? Welcome to the world of Hamdani Yusuf!
Post by: hamdani yusuf on 24/05/2022 03:28:34
You need to specify what you mean with internal and kinetic, in contrast to external and potential.So, what's your answer to this question : what is temperature?A measure of the internal kinetic energy of a body.
Post by: Bored chemist on 24/05/2022 08:57:08
You need to stop repeating the error that it's kinetic energy that counts.So, what's your answer to this question : what is temperature?A measure of the internal kinetic energy of a body.
It's any form of energy.
However, when a body has a well defined temperature the energy per degree of freedom will be the same anyway.
Post by: Bored chemist on 24/05/2022 09:00:37
The gradient is d height/ d distance .Temperature is the amount of energy required to change the entropy by one bit.So the entropy of an object at constant temperature is continually changing? Welcome to the world of Hamdani Yusuf!
That doesn't mean that all hills are moving.
That's not H Y's world, nor S T's world.
It's only in your world.
Post by: Bored chemist on 24/05/2022 09:02:44
Why do you use base 10 instead of e?That might be the best question H Y has ever asked.
Post by: alancalverd on 24/05/2022 10:20:34
It's any form of energy.Er, no. The potential energy of a strained lattice may be enormous, but as that can't be transferred by thermal conduction to another body, it doesn't affect its temperature.
There's a discussion elsewhere about dissolving a stressed spring.
Post by: alancalverd on 24/05/2022 10:23:02
So if T>0, by ST's statement there is plenty of available energy in the system to change its entropy, and nothing to stop it.The gradient is d height/ d distance .Temperature is the amount of energy required to change the entropy by one bit.So the entropy of an object at constant temperature is continually changing? Welcome to the world of Hamdani Yusuf!
That doesn't mean that all hills are moving.
That's not H Y's world, nor S T's world.
It's only in your world.
Post by: Spring Theory on 24/05/2022 12:15:19
Why do you use base 10 instead of e?Would that be 0.3010 or 0.6931? Or does it mean log2 of any number you can think of?
0.3010
What's your dimensional analysis?
Entropy is dimensionless. This is why temperature is in units of energy.
Information theory uses base 2, some physics use natural log. I just picked base 10. You can pick what ever you want to be defined as one bit. Any conversion factor can be absorbed by the constant I set to 1. That's not the important part.
Entropy is the average of the logarithm of the probability distribution of the states.
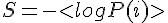
Where S is the entropy and P(i) is the probability of the i'th state. The negative sign is because the probability is always less than or equal to 1. This becomes:
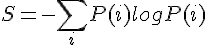
When you add up each state probability value.
Post by: alancalverd on 24/05/2022 12:36:42
This is why temperature is in units of energy.No. The mean energy of a particle within a body is kBT where kB is the Boltzmann constant, whose dimensions are joules per kelvin.
Entropy is dimensionless.No. The dimensions of entropy are joules per kelvin.
Post by: Spring Theory on 24/05/2022 12:56:38
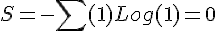
If the are m states equally probably, then the entropy is:
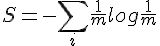
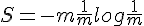
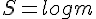
And you now have the generalization of what entropy is. It is the logarithm (you can pick your base) of the variation of different states a system can have.
Post by: Spring Theory on 24/05/2022 12:58:36
This is why temperature is in units of energy.No. The mean energy of a particle within a body is kBT where kB is the Boltzmann constant, whose dimensions are joules per kelvin.Entropy is dimensionless.No. The dimensions of entropy are joules per kelvin.
Kelvin = Energy
Post by: hamdani yusuf on 24/05/2022 13:16:05
There's a discussion elsewhere about dissolving a stressed spring.That's interesting. Can someone give the link?
Post by: hamdani yusuf on 24/05/2022 13:19:43
Kelvin = EnergyIt seems to imply that objects with the same temperature have the same energy, which is demonstrably false.
Post by: Bored chemist on 24/05/2022 13:56:17
The point remains that vibrational + electronic energy is also part of thermal energy.It's any form of energy.Er, no. The potential energy of a strained lattice may be enormous, but as that can't be transferred by thermal conduction to another body, it doesn't affect its temperature.
There's a discussion elsewhere about dissolving a stressed spring.
"The potential energy of a strained lattice may be enormous"
yes, it was the cause of the Windscale fire- it made the graphite very hot...
As I said...
However, when a body has a well defined temperature the energy per degree of freedom will be the same anyway.
Post by: alancalverd on 24/05/2022 21:16:39
"The potential energy of a strained lattice may be enormous"No, it was the release of Wigner potential energy that raised the temperature. Problem is that once you reach the annealing temperature you can initiate a chain reaction that outstrips the cooling capacity of the system - as happened at Windscale.
yes, it was the cause of the Windscale fire- it made the graphite very hot...
Post by: hamdani yusuf on 25/05/2022 07:40:24
When heat capacity is 3R/2, we interpret this as the heat energy is distributed to translational motion equally in 3 spatial axes.(https://upload.wikimedia.org/wikipedia/commons/thumb/0/07/DiatomicSpecHeat1.png/300px-DiatomicSpecHeat1.png)Or it shows that the number of degrees of freedom isn't an integer.
I think that the experimental plot above plays important role in the development of equipartition theory, also the concept of degree of freedom. But the difference in the gradient of the curve shows that at least at some points, the energy distribution among different degrees of freedoms are not equal.
When heat capacity is 5R/2, the gas has 2 additional degrees of freedom, which is thought to come from rotation in 2 axes. Each unit of additional heat energy will be distributed evenly over 5 available degrees of freedom, which are 3 translational and 2 rotational motion.
There's a point in the graphic where heat capacity is 4R/2. How should it be interpreted? Will additional heat energy be distributed evenly over 4 available degrees of freedom?
Post by: Bored chemist on 25/05/2022 09:05:40
So, what happened was the transfer from one particular degree of freedom to all the others."The potential energy of a strained lattice may be enormous"No, it was the release of Wigner potential energy that raised the temperature. Problem is that once you reach the annealing temperature you can initiate a chain reaction that outstrips the cooling capacity of the system - as happened at Windscale.
yes, it was the cause of the Windscale fire- it made the graphite very hot...
As I said...
Quote from: Bored chemist on Yesterday at 08:57:08
However, when a body has a well defined temperature the energy per degree of freedom will be the same anyway.
Post by: Bored chemist on 25/05/2022 09:06:47
It depends.When heat capacity is 3R/2, we interpret this as the heat energy is distributed to translational motion equally in 3 spatial axes.(https://upload.wikimedia.org/wikipedia/commons/thumb/0/07/DiatomicSpecHeat1.png/300px-DiatomicSpecHeat1.png)Or it shows that the number of degrees of freedom isn't an integer.
I think that the experimental plot above plays important role in the development of equipartition theory, also the concept of degree of freedom. But the difference in the gradient of the curve shows that at least at some points, the energy distribution among different degrees of freedoms are not equal.
When heat capacity is 5R/2, the gas has 2 additional degrees of freedom, which is thought to come from rotation in 2 axes. Each unit of additional heat energy will be distributed evenly over 5 available degrees of freedom, which are 3 translational and 2 rotational motion.
There's a point in the graphic where heat capacity is 4R/2. How should it be interpreted? Will additional heat energy be distributed evenly over 4 available degrees of freedom?
Post by: hamdani yusuf on 25/05/2022 13:48:01
It depends.On what, exactly?
Post by: Bored chemist on 25/05/2022 16:39:33
Guess.It depends.On what, exactly?
or, even better, learn science.
Post by: Spring Theory on 25/05/2022 20:09:02
Kelvin = EnergyIt seems to imply that objects with the same temperature have the same energy, which is demonstrably false.
It depends on the experimental set up and what objects you are talking about and the heat bath and equilibrium state, etc. I will have to think about that based on the photon concept.
My point was that Entropy is the log of something. The log of something has no units. To get to temperature, the Boltzmann constant simply converts energy into units of temperature. Kind of like you can convert Joules in units of kg m/s by multiplying it by 1.
The goal was to define what temperature (Kelvin) is. It is in natural units of energy (which is generally accepted in the physics community):
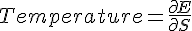
Where E is the energy of the system and S is the Entropy as defined previously.
Post by: alancalverd on 25/05/2022 23:13:57
No, it was the release of Wigner potential energy that raised the temperature. Problem is that once you reach the annealing temperature you can initiate a chain reaction that outstrips the cooling capacity of the system - as happened at Windscale.It's an odd use of "degree of freedom". Wigner energy is potential energy stored as microscopic areas of mechanical stress when a neutron displaces an atom from its lattice position into a metastable trap. Wigner release is the conversion of that potential energy into phonons (i.e. heat) as the atom returns to a stable position. It's the "whole atom" equivalent of thermoluminescent electron trapping resulting from gamma radiation.
So, what happened was the transfer from one particular degree of freedom to all the others.
Post by: alancalverd on 25/05/2022 23:22:50
My point was that Entropy is the log of something. The log of something has no units.
S = -kBlogp where kB, Boltzmann's constant, has dimensions ML2T-2K-1, so S is far from dimensionless.
Post by: Eternal Student on 26/05/2022 01:08:07
There's a point in the graphic where heat capacity is 4R/2. How should it be interpreted? Will additional heat energy be distributed evenly over 4 available degrees of freedom?I don't have any idea where that graphic came from. It looks very simplified. For a real substance you'd never get anything that perfect or perfectly symmetric at each transition zone betwen what is thought to be two states.
Don't interpret it as suggesting that for a brief range of temperatures there was a state which supported 4 degrees - that's not the usual approach and not a good idea or model to have.
A more reasonable way to interpret that location on the graph is that half of the substance is in a state where there are 5 degrees of freedom, while the other half of the substance is in a state where there are 3 degrees of freedom.
Similarly, all the other in-between positions (e.g. where it looks like 3.334 degrees of freedom exist) can be explained by varying the fraction of the substance in the two distinct states.
Overall then , the heat capacity connects or relates to the average number of degrees of freedom that a particle would have. Take a moment to think about this and you'll see that it will work: If there are 2 particles, one with 3 deg. freedom, the other with 5, then you need to deliver 8 units of energy to get the particles to increase the energy per degree of freedom by 1 unit, this corresponds to a temperature increase of 1 unit - that's precisely the same as 8 units of energy distributed to 2 particles with an average of 4 degrees of freedom each.
The flat regions on the graph are where, to within a reasonable approximation, all the particles are in one state and have a set number of degrees of freedom (3, 5 or 7 in your graph).
- - - - - - - - -
I am a little curious about where you are going with this thread. Is there something you think temperature should be?
Best Wishes.
Post by: hamdani yusuf on 26/05/2022 05:15:46
I don't have any idea where that graphic came from. It looks very simplified. For a real substance you'd never get anything that perfect or perfectly symmetric at each transition zone betwen what is thought to be two states.It's from wikipedia commons, which supposed to be a common knowledge and established science.
Post by: hamdani yusuf on 26/05/2022 12:29:25
A more reasonable way to interpret that location on the graph is that half of the substance is in a state where there are 5 degrees of freedom, while the other half of the substance is in a state where there are 3 degrees of freedom.I have considered your hypothesis. One of its implications is the increase of heat capacity would be more granular with fewer gas molecules. The chart would look like a stair.
Similarly, all the other in-between positions (e.g. where it looks like 3.334 degrees of freedom exist) can be explained by varying the fraction of the substance in the two distinct states.
Overall then , the heat capacity connects or relates to the average number of degrees of freedom that a particle would have. Take a moment to think about this and you'll see that it will work: If there are 2 particles, one with 3 deg. freedom, the other with 5, then you need to deliver 8 units of energy to get the particles to increase the energy per degree of freedom by 1 unit, this corresponds to a temperature increase of 1 unit - that's precisely the same as 8 units of energy distributed to 2 particles with an average of 4 degrees of freedom each.
The flat regions on the graph are where, to within a reasonable approximation, all the particles are in one state and have a set number of degrees of freedom (3, 5 or 7 in your graph).
Post by: hamdani yusuf on 26/05/2022 12:32:21
Wigner release is the conversion of that potential energy into phonons (i.e. heat) as the atom returns to a stable position.Are all phonons heat? Are there some forms of phonon which are not heat?
Post by: Bored chemist on 26/05/2022 12:34:16
It is an odd circumstance.No, it was the release of Wigner potential energy that raised the temperature. Problem is that once you reach the annealing temperature you can initiate a chain reaction that outstrips the cooling capacity of the system - as happened at Windscale.It's an odd use of "degree of freedom".
So, what happened was the transfer from one particular degree of freedom to all the others.
The point remains that the vibrations of atoms in a molecule are still heat energy.
Post by: Eternal Student on 26/05/2022 13:41:45
It's from wikipedia commons, which supposed to be a common knowledge and established science.OK. I'm not sure I've heard of it. I'll guess it's a Wikipedia thing. Do they explain it?
I have considered your hypothesis. One of its implications is the increase of heat capacity would be more granular with fewer gas molecules. The chart would look like a stair.I'm not sure it would.
1. No-one tries to consider a temperature for a small number of particles. That graph looks simplified and idealised, presumably they are just communicating an idea.
2. You can divide things up into segments of time if needed. A particle can have 5 deg. freedom for half the time and 3 deg. of freedom for the other half of the time. You'd still observe a heat capacity as if the particle had 4 degrees of freedom on average. Temperature is only ever modelled as if you have an ensemble of particles, a particle having a k.e. of 1 unit half the time and 1.5 units for the other half of the time can not be assigned a temperature. However, the system can be modelled as if there was an ensemble of particles and the average k.e. of a particle was 1.25 units - that is something you can call a temperature.
Best Wishes.
Post by: alancalverd on 26/05/2022 22:28:58
The point remains that the vibrations of atoms in a molecule are still heat energy.Absolutely. But irrelevant to Wigner.
Post by: hamdani yusuf on 27/05/2022 03:10:26
It doesn't sound like a scientific answer.Guess.It depends.On what, exactly?
or, even better, learn science.
Post by: hamdani yusuf on 27/05/2022 03:12:18
OK. I'm not sure I've heard of it. I'll guess it's a Wikipedia thing.You can see the link by quoting the post.
Post by: hamdani yusuf on 27/05/2022 03:52:00
I am a little curious about where you are going with this thread. Is there something you think temperature should be?I just try to understand what temperature is by relating it with other things I already understood. The description by Wikipedia below requires the understanding of other concepts first. I want to understand how those concepts are related to each other consistently and useful to explain and predict observations and experimental results.
Quote
https://en.wikipedia.org/wiki/Temperature
Temperature is a physical quantity that expresses hot and cold or a measure of the average kinetic energy of the atoms or molecules in the system. It is the manifestation of thermal energy, present in all matter, which is the source of the occurrence of heat, a flow of energy, when a body is in contact with another that is colder or hotter. Temperature should not be confused with heat.
International Kelvin scale
Many scientific measurements use the Kelvin temperature scale (unit symbol: K), named in honor of the physicist who first defined it. It is an absolute scale. Its numerical zero point, 0 K, is at the absolute zero of temperature. Since May, 2019, its degrees have been defined through particle kinetic theory, and statistical mechanics. In the International System of Units (SI), the magnitude of the kelvin is defined through various empirical measurements of the average kinetic energies of microscopic particles. It is numerically evaluated in terms of the Boltzmann constant, the value of which is defined as fixed by international convention.[5][6]
Statistical mechanical versus thermodynamic temperature scales
Since May 2019, the magnitude of the kelvin is defined in relation to microscopic phenomena, characterized in terms of statistical mechanics. Previously, since 1954, the International System of Units defined a scale and unit for the kelvin as a thermodynamic temperature, by using the reliably reproducible temperature of the triple point of water as a second reference point, the first reference point being 0 K at absolute zero.[citation needed]
Historically, the triple point temperature of water was defined as exactly 273.16 units of the measurement increment. Today it is an empirically measured quantity. The freezing point of water at sea-level atmospheric pressure occurs at approximately 273.15 K = 0 °C.
The article says that temperature is a measure of the average kinetic energy of the atoms or molecules in the system. But we know that not all kinetic energy of the atoms or molecules are in the form of heat which contribute to the system's temperature. How they move also affects the temperature measurement of the system. Uniform rotation of a solid object can make it have a very high average kinetic energy. But usually it's not called a hot object.
Quote
https://en.wikipedia.org/wiki/Heat
In thermodynamics, heat is energy in transfer to or from a thermodynamic system, by mechanisms other than thermodynamic work or transfer of matter.[1][note 1]
Like thermodynamic work, heat transfer is a process involving more than one system, not a property of any one system. In thermodynamics, energy transferred as heat contributes to change in the system's cardinal energy variable of state, for example its internal energy, or for example its enthalpy. This is to be distinguished from the ordinary language conception of heat as a property of an isolated system.
The quantity of energy transferred as heat in a process is the amount of transferred energy excluding any thermodynamic work that was done and any energy contained in matter transferred. For the precise definition of heat, it is necessary that it occur by a path that does not include transfer of matter.[2]
Though not immediately by the definition, but in special kinds of process, quantity of energy transferred as heat can be measured by its effect on the states of interacting bodies. For example, respectively in special circumstances, heat transfer can be measured by the amount of ice melted, or by change in temperature of a body in the surroundings of the system.[3] Such methods are called calorimetry.
Post by: Bored chemist on 27/05/2022 09:54:32
So why did you introduce Wigner?The point remains that the vibrations of atoms in a molecule are still heat energy.Absolutely. But irrelevant to Wigner.
The point remains that this
is misleading because vibrational and rotational energy also contribute.So, what's your answer to this question : what is temperature?A measure of the internal kinetic energy of a body.
Post by: Bored chemist on 27/05/2022 09:55:36
Suggesting that someone guesses (i.e. forms a hypothesis), then tests it is entirely scientific.It doesn't sound like a scientific answer.Guess.It depends.On what, exactly?
or, even better, learn science.
Post by: Bored chemist on 27/05/2022 09:58:47
For a real substance you'd never get anything that perfect or perfectly symmetric at each transition zone betwen what is thought to be two states.It's a pretty good illustration of the real world.
The transition is smooth and more or less symmetrical because it's the behaviour of a large ensemble of things with 2 (or more) states.
Post by: alancalverd on 27/05/2022 10:41:15
So why did you introduce Wigner?Because you asked about the Windscale fire.
Post by: alancalverd on 27/05/2022 10:42:59
is misleading because vibrational and rotational energy also contribute.And they are kinetic (to do with movement) , i.e. not potential (to do with static stress).
Post by: hamdani yusuf on 27/05/2022 12:26:22
Suggesting that someone guesses (i.e. forms a hypothesis), then tests it is entirely scientific.Saying "I don't know" is a more effective and efficient way to convey the same message as yours.
Post by: Bored chemist on 27/05/2022 13:06:20
My message was that if you thought about it, you might work out the answer.Suggesting that someone guesses (i.e. forms a hypothesis), then tests it is entirely scientific.Saying "I don't know" is a more effective and efficient way to convey the same message as yours.
How is that the same as saying "I don't know"?
Post by: Bored chemist on 27/05/2022 13:09:17
On average, half of the energy of vibrating bodies is potential, not kinetic.is misleading because vibrational and rotational energy also contribute.And they are kinetic (to do with movement) , i.e. not potential (to do with static stress).
And the electronic contribution to temperature s largely due to storage of energy in electrostatic fields.
Why not just accept the fact that it isn't only down to kinetic energy?
Post by: Bored chemist on 27/05/2022 13:14:18
I didn't "ask" I pointed out that it was a counter example to your assertion thatSo why did you introduce Wigner?Because you asked about the Windscale fire.
The potential energy of a strained lattice may be enormous, but as that can't be transferred by thermal conduction to another body,
It's a little beside the point.
Thermal energy still is more than just kinetic energy.
Post by: Spring Theory on 27/05/2022 14:58:59
My point was that Entropy is the log of something. The log of something has no units.
S = -kBlogp where kB, Boltzmann's constant, has dimensions ML2T-2K-1, so S is far from dimensionless.
You are getting bogged down by the constants. One of the most useful tools in analyzing physics is working in natural units. This basically sets your constants equal to 1 (more to that though). Remove all constants from your equation and then make your analysis. Remember rate of change of a constant is zero so it plays no roles in derivatives.
This technique can be used to express all physical properties in terns of energy to some power. An example would be that pressure can be expressed as energy to the fourth power and mass expressed as energy to the first power and length expressed as energy to the minus 1 power.
Or we can beat this horse one more time...
Post by: hamdani yusuf on 27/05/2022 15:24:01
My message was that if you thought about it, you might work out the answer.If you said "I don't know", I might work out to find the answer. What's the difference?
How is that the same as saying "I don't know"?
Post by: hamdani yusuf on 27/05/2022 15:30:04
It's a little beside the point.The main question in this topic is about temperature. Wikipedia article says it's about average kinetic energy.
Thermal energy still is more than just kinetic energy.
Post by: Origin on 27/05/2022 15:47:29
You are getting bogged down by the constants.You are not making sense.
Post by: Bored chemist on 27/05/2022 15:48:48
I offered a suggestion on where to start.My message was that if you thought about it, you might work out the answer.If you said "I don't know", I might work out to find the answer. What's the difference?
How is that the same as saying "I don't know"?
Guess.
or, even better, learn science.
I remain puzzled that, rather than following the recommendation, you pretend that it didn't exist.
Why won't you learn?
Post by: Bored chemist on 27/05/2022 15:49:11
Wiki is not God.It's a little beside the point.The main question in this topic is about temperature. Wikipedia article says it's about average kinetic energy.
Thermal energy still is more than just kinetic energy.
Post by: hamdani yusuf on 27/05/2022 15:52:09
pressure can be expressed as energy to the fourth powerHow do you get this?
Post by: hamdani yusuf on 27/05/2022 15:57:07
I offered a suggestion on where to start.Where should I start?
Post by: alancalverd on 27/05/2022 15:58:56
One of the most useful tools in analyzing physics is working in natural units. This basically sets your constants equal to 1 (more to that though).Numerically, yes, but you have to transfer the dimensions of your constant to some other quantity, and of course it can only work for one constant at a time.
Post by: Bored chemist on 27/05/2022 16:00:04
I offered a suggestion on where to start.Where should I start?
learn science.
Post by: hamdani yusuf on 27/05/2022 16:01:11
I remain puzzled that, rather than following the recommendation, you pretend that it didn't exist.You are puzzled that I disagree with you, but can't point out which point you disagree on.
Why won't you learn?
Post by: Bored chemist on 27/05/2022 17:13:41
You are puzzled that I disagree with youNo
I remain puzzled that, rather than following the recommendation, you pretend that it didn't exist.
And again, you seem to ignore what I actually say.
Post by: Bored chemist on 27/05/2022 17:16:27
If you had spent time learning and thinking, you might know by now.It depends.On what, exactly?
Instead, you spend time saying stupid things like
You are puzzled that I disagree with you, but can't point out which point you disagree on.
Post by: hamdani yusuf on 27/05/2022 22:32:15
Wiki is not God.Of course not. But it represents common knowledge of many contributors, with many of them are expert in their fields. If you disagree with them, at least you need a supporting evidence to show their mistakes.
Post by: hamdani yusuf on 27/05/2022 22:42:00
If you had spent time learning and thinking, you might know by now.In your point of view, everyone who disagree with you is stupid, which is virtually everyone writing in this thread, including Wikipedia authors. Has it ever cross your mind that may be you are the one who made mistakes?
Instead, you spend time saying stupid things like
Post by: Spring Theory on 27/05/2022 23:35:42
One of the most useful tools in analyzing physics is working in natural units. This basically sets your constants equal to 1 (more to that though).Numerically, yes, but you have to transfer the dimensions of your constant to some other quantity, and of course it can only work for one constant at a time.
Set c = 1 = ħ
Post by: Spring Theory on 28/05/2022 00:12:34
pressure can be expressed as energy to the fourth powerHow do you get this?
Natural units per the constants above set to one means the following:
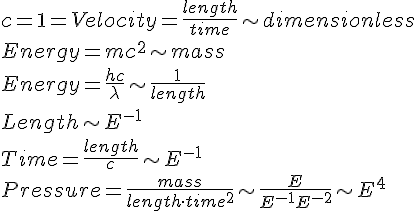
Post by: Bored chemist on 28/05/2022 00:41:39
Or...Wiki is not God.Of course not. But it represents common knowledge of many contributors, with many of them are expert in their fields. If you disagree with them, at least you need a supporting evidence to show their mistakes.
https://en.wikipedia.org/wiki/Lie-to-children
Post by: Bored chemist on 28/05/2022 00:43:40
In your point of view, everyone who disagree with you is stupid, which is virtually everyone writing in this thread,OK
That's misrepresentation but...
I'm happy to take a straw poll on that
Who thinks that HY knows more about science than I do?
Post by: Spring Theory on 28/05/2022 11:35:37
I bend over backwards here trying to help you guys understand concepts and be productive to the topic but the typical response is just an unproductive, smart ass quip.
What a waste of time and effort. You guys deserve neither.
Your welcome.
Post by: hamdani yusuf on 28/05/2022 11:54:54
pressure can be expressed as energy to the fourth powerHow do you get this?
Natural units per the constants above set to one means the following:
What's the dimension of temperature then?
Momentum = Mass * Velocity
Momentum = Energy * 1 = Energy
It doesn't seem right
Post by: alancalverd on 29/05/2022 13:39:59
[h] = ML2T-1
[c] = LT-1
so you can't set both c and h to a dimensionless unity simultaneously.
Post by: Origin on 29/05/2022 14:42:13
I bend over backwards here trying to help you guys understand concepts and be productive to the topic but the typical response is just an unproductive, smart ass quip.Since most of what you bring to the party is pseudoscience, leaving the discussion is no loss.
What a waste of time and effort. You guys deserve neither.
Post by: Bored chemist on 29/05/2022 20:28:19
It just reveals what many of you people are here for, trying to prove how much you know about science. What a farce.Less of a farce than the ones having a contest to show who knows least about it.
Post by: hamdani yusuf on 31/05/2022 23:07:01
What's temperature, if you have to explain to an adult?Or...Wiki is not God.Of course not. But it represents common knowledge of many contributors, with many of them are expert in their fields. If you disagree with them, at least you need a supporting evidence to show their mistakes.
https://en.wikipedia.org/wiki/Lie-to-children
Post by: Origin on 31/05/2022 23:48:20
What's temperature,390 posts and you are still on square one; nice. ::)
Post by: Bored chemist on 01/06/2022 00:28:54
What's temperature, if you have to explain to an adult?We answered that.
You still don't know what temperature is.
It may be possible to deduce something from those to observations.
Post by: hamdani yusuf on 01/06/2022 10:46:30
And you haven't contributed meaningfully.What's temperature,390 posts and you are still on square one; nice. ::)
Post by: hamdani yusuf on 01/06/2022 10:47:31
Can you summarize it?What's temperature, if you have to explain to an adult?We answered that.
You still don't know what temperature is.
It may be possible to deduce something from those to observations.
Post by: hamdani yusuf on 01/06/2022 11:36:36
At least I found an adult in the room with whom I can discuss scientific problems more seriously. Here's what I get so far.I am a little curious about where you are going with this thread. Is there something you think temperature should be?I just try to understand what temperature is by relating it with other things I already understood. The description by Wikipedia below requires the understanding of other concepts first. I want to understand how those concepts are related to each other consistently and useful to explain and predict observations and experimental results.Quotehttps://en.wikipedia.org/wiki/Temperature
Temperature is a physical quantity that expresses hot and cold or a measure of the average kinetic energy of the atoms or molecules in the system. It is the manifestation of thermal energy, present in all matter, which is the source of the occurrence of heat, a flow of energy, when a body is in contact with another that is colder or hotter. Temperature should not be confused with heat.
International Kelvin scale
Many scientific measurements use the Kelvin temperature scale (unit symbol: K), named in honor of the physicist who first defined it. It is an absolute scale. Its numerical zero point, 0 K, is at the absolute zero of temperature. Since May, 2019, its degrees have been defined through particle kinetic theory, and statistical mechanics. In the International System of Units (SI), the magnitude of the kelvin is defined through various empirical measurements of the average kinetic energies of microscopic particles. It is numerically evaluated in terms of the Boltzmann constant, the value of which is defined as fixed by international convention.[5][6]
Statistical mechanical versus thermodynamic temperature scales
Since May 2019, the magnitude of the kelvin is defined in relation to microscopic phenomena, characterized in terms of statistical mechanics. Previously, since 1954, the International System of Units defined a scale and unit for the kelvin as a thermodynamic temperature, by using the reliably reproducible temperature of the triple point of water as a second reference point, the first reference point being 0 K at absolute zero.[citation needed]
Historically, the triple point temperature of water was defined as exactly 273.16 units of the measurement increment. Today it is an empirically measured quantity. The freezing point of water at sea-level atmospheric pressure occurs at approximately 273.15 K = 0 °C.
The article says that temperature is a measure of the average kinetic energy of the atoms or molecules in the system. But we know that not all kinetic energy of the atoms or molecules are in the form of heat which contribute to the system's temperature. How they move also affects the temperature measurement of the system. Uniform rotation of a solid object can make it have a very high average kinetic energy. But usually it's not called a hot object.Quotehttps://en.wikipedia.org/wiki/Heat
In thermodynamics, heat is energy in transfer to or from a thermodynamic system, by mechanisms other than thermodynamic work or transfer of matter.[1][note 1]
Like thermodynamic work, heat transfer is a process involving more than one system, not a property of any one system. In thermodynamics, energy transferred as heat contributes to change in the system's cardinal energy variable of state, for example its internal energy, or for example its enthalpy. This is to be distinguished from the ordinary language conception of heat as a property of an isolated system.
The quantity of energy transferred as heat in a process is the amount of transferred energy excluding any thermodynamic work that was done and any energy contained in matter transferred. For the precise definition of heat, it is necessary that it occur by a path that does not include transfer of matter.[2]
Though not immediately by the definition, but in special kinds of process, quantity of energy transferred as heat can be measured by its effect on the states of interacting bodies. For example, respectively in special circumstances, heat transfer can be measured by the amount of ice melted, or by change in temperature of a body in the surroundings of the system.[3] Such methods are called calorimetry.
In principle, I agree with Wikipedia article which says that temperature is a measure of the average (internal - as Alan mentioned) kinetic energy of the atoms or molecules in the system. Hence we need to exclude external kinetic energy, such as uniform translation and rotation of the particles. Otherwise we will have to say that ISS has high temperature.
An object can absorb energy from other objects, and transform it into some other forms of energy with or without change in temperature. If it's purely transformed into potential energy, then the object's temperature doesn't change. If it's purely transformed into external kinetic energy, then the object's temperature doesn't change either.
Some kind of movements involve both kinetic and potential energy. Let's have a 1 kg ball at 1 m height in 10 m/s² gravitational acceleration. It has 10 Joule potential energy, and 0 kinetic energy. If it then fall freely to the floor, the potential energy starts to convert into kinetic energy, with constant total energy. When it hit the floor, the potential energy is 0 while the kinetic energy is 10 Joule.
Let's make the ball and the floor make perfectly elastic collision. On average, the ball has 5 Joule of kinetic energy and 5 Joule of potential energy.
Mass and spring is another example which is more closely related to temperature.
(https://assets.coursehero.com/study-guides/lumen/images/suny-osuniversityphysics/15-2-energy-in-simple-harmonic-motion/CNX_UPhysics_15_02_EnergyStSp1.jpg)
If 2 Joules of energy is absorbed by a mass-spring system, on average 1 Joule will be in the form of kinetic energy, while 1 Joule is in potential energy.
For comparison, if the mass is put into a perfectly elastic box without attached to spring, 2 Joules of energy will be in the form of kinetic energy and 0 potential energy.
As analogy for temperature, the mass-spring system has twice heat capacity as the spring-less system. We can add the same amount of energy to both systems, but only half is manifested as kinetic energy in the first system, which is comparable to lower increase of temperature.
The widest range of temperature measurement methods I know is by ideal gas law, which can be approached practically using monoatomic noble gases, although they start to deviate at high enough temperature where the gas starts to ionize. The electromagnetic interactions makes the energy no longer strictly in kinetic form.
Post by: Bored chemist on 01/06/2022 11:45:46
What would be the point?Can you summarize it?What's temperature, if you have to explain to an adult?We answered that.
You still don't know what temperature is.
It may be possible to deduce something from those to observations.
Post by: hamdani yusuf on 01/06/2022 11:51:59
So you can contribute meaningfully to the discussion.What would be the point?Can you summarize it?What's temperature, if you have to explain to an adult?We answered that.
You still don't know what temperature is.
It may be possible to deduce something from those to observations.
Post by: Bored chemist on 01/06/2022 11:54:09
In principle, I agree with Wikipedia article which says that temperature is a measure of the average (internal - as Alan mentioned) kinetic energy of the atoms or molecules in the system.How many times must I point out that the vibrational, rotational and electronic energies are also involved?
If they don't all agree (i.e. if the equipartition principle isn't obeyed) then the temperature is not defined.
It's possible to obtain a sample of a gas where most of the atoms are in an excited state.
If you measure the temperature of that gas by looking at the translational kinetic energy you will get some answer.
If you then put the material in a closed insulated container, the atoms will collide with eachother and the extra energy from that excitation will be transferred into kinetic energy.
If you measured the temperature (as determined from the kinetic energy), it would have gone up.
You would have had a closed system where the temperature spontaneously rose.
And that's a breach of the laws of thermodynamics.
So, Alan is wrong, and so is the Wiki article.
It's not usually an important element of temperature.
So leaving it out is one of the lies we tell to children.
Post by: Bored chemist on 01/06/2022 11:55:54
You first...So you can contribute meaningfully to the discussion.What would be the point?Can you summarize it?What's temperature, if you have to explain to an adult?We answered that.
You still don't know what temperature is.
It may be possible to deduce something from those to observations.
Post by: hamdani yusuf on 01/06/2022 13:06:05
It's possible to obtain a sample of a gas where most of the atoms are in an excited state.How would you do that without increasing the temperature?
Post by: hamdani yusuf on 01/06/2022 13:08:21
You first...Here.
If 2 Joules of energy is absorbed by a mass-spring system, on average 1 Joule will be in the form of kinetic energy, while 1 Joule is in potential energy.
For comparison, if the mass is put into a perfectly elastic box without attached to spring, 2 Joules of energy will be in the form of kinetic energy and 0 potential energy.
As analogy for temperature, the mass-spring system has twice heat capacity as the spring-less system. We can add the same amount of energy to both systems, but only half is manifested as kinetic energy in the first system, which is comparable to lower increase of temperature.
The widest range of temperature measurement methods I know is by ideal gas law, which can be approached practically using monoatomic noble gases, although they start to deviate at high enough temperature where the gas starts to ionize. The electromagnetic interactions makes the energy no longer strictly in kinetic form.
Post by: Bored chemist on 01/06/2022 13:12:35
The scientific answer is "Who cares? Trust me; it's possible".It's possible to obtain a sample of a gas where most of the atoms are in an excited state.How would you do that without increasing the temperature?
But here's the basics.
"The next step is "state selection"—in order to get some stimulated emission, it is necessary to create a population inversion of the atoms. This is done in a way that is very similar to the Stern–Gerlach experiment. After passing through an aperture and a magnetic field, many of the atoms in the beam are left in the upper energy level of the lasing transition. From this state, the atoms can decay to the lower state and emit some microwave radiation.
from
https://en.wikipedia.org/wiki/Maser#Some_common_types
Post by: Bored chemist on 01/06/2022 13:15:27
For comparison, if the mass is put into a perfectly elastic box without attached to spring, 2 Joules of energy will be in the form of kinetic energy and 0 potential energy.The fact that you specify a perfectly elastic box means that it spends some of its time stretched.
And at that point you have stored potential energy in it.
Post by: Eternal Student on 01/06/2022 14:30:21
Let's make the ball and the floor make perfectly elastic collision. On average, the ball has 5 Joule of kinetic energy and 5 Joule of potential energy.and also this section....
If 2 Joules of energy is absorbed by a mass-spring system, on average 1 Joule will be in the form of kinetic energy, while 1 Joule is in potential energy.is slightly inaccurate.
You need to be clearer about what you are averaging over. Usually it would be an average over time - but that won't work in these examples. The bouncing ball has the lowest speed at the top of its bounce and so spends much more time there, with high potential energy and low kinetic. Similarly the mass on a spring has the lowest speed when the spring is most extended. Considering an average over time, the total energy in these systems is not equally partitioned between kinetic and potential, much more than half of it will be potential energy.
The equipartition theory is actually quite difficult to derive from fundamental principles. I do not know or concern myself too much with those details. For real life systems we have a reasonable assumption of ergodicity or randomness and this is enough to allow the equi-partition theorem, with just simple time-averages, to hold.
For the systems you have suggested a different average, an average over phase space (the space of generalised momentum and position) should still satisfy the equi-partition result. It doesn't reduce to an average over time because the property of ergodicity is lacking. Ergodicity is explained in Wikipedia here: https://en.wikipedia.org/wiki/Ergodicity. Along with a general discussion of the equipartition theorem here: https://en.wikipedia.org/wiki/Equipartition_theorem#General_formulation_of_the_equipartition_theorem
and finally a guide to its derivation here: https://en.wikipedia.org/wiki/Equipartition_theorem#Derivations
Best Wishes.
Post by: hamdani yusuf on 03/06/2022 12:27:44
The scientific answer is "Who cares? Trust me; it's possible"."Trust me" is never a scientific answer.
Post by: hamdani yusuf on 03/06/2022 12:30:30
The fact that you specify a perfectly elastic box means that it spends some of its time stretched.It merely means that kinetic energy is preserved.
Post by: hamdani yusuf on 03/06/2022 12:33:35
You need to be clearer about what you are averaging over. Usually it would be an average over time - but that won't work in these examples. The bouncing ball has the lowest speed at the top of its bounce and so spends much more time there, with high potential energy and low kinetic. Similarly the mass on a spring has the lowest speed when the spring is most extended.You need to pay attention to the accompanying pictures.
For comparison, if the mass is put into a perfectly elastic box without attached to spring, 2 Joules of energy will be in the form of kinetic energy and 0 potential energy.This means remove the spring and add an elastic wall to the right of the mass.
Post by: hamdani yusuf on 03/06/2022 12:49:45
The bouncing ball has the lowest speed at the top of its bounce and so spends much more time thereTime ratio between top and bottom depends on the height difference, maximum speed, and gravitational acceleration. In ordinary lab desktop experiments with gas at near STP, it's close to 1.
Post by: Bored chemist on 04/06/2022 16:46:04
It merely means that kinetic energy is preserved.How?
Post by: hamdani yusuf on 04/06/2022 17:13:15
The bouncing ball has the lowest speed at the top of its bounce and so spends much more time there, with high potential energy and low kinetic.In this model, the top of the container would have lower temperature than the bottom. Let's say that two identical piezoelectric generators are placed on top and bottom of the container. Bottom generator will produce more power than the top generator.
This can be demonstrated by filling a tall container with heavy gases. The container should be thermally isolated from the outside. The heavier the gas, the shorter the container can be while still showing temperature difference.
Post by: hamdani yusuf on 04/06/2022 17:15:06
That's the definition of elastic collision. It doesn't depend on how long/short the collision happens, as long as the kinetic energy of the system is preserved.It merely means that kinetic energy is preserved.How?
Post by: Bored chemist on 04/06/2022 17:28:45
The thing about "elastic" is that, by definition, it stretches.That's the definition of elastic collision. It doesn't depend on how long/short the collision happens, as long as the kinetic energy of the system is preserved.It merely means that kinetic energy is preserved.How?
And the only reason that kinetic energy is conserved is that it ie loaned to the surface as potential energy and then returned as kinetic energy on the rebound.
So, once you know how the kinetic energy is conserved, you realise that, ironically, it isn't. Briefly, it is converted to potential energy.
Did you not realise that was why I asked "How?" ?
Instead of answering the question, you posted some nonsense about duration.
Post by: hamdani yusuf on 04/06/2022 17:34:38
The thing about "elastic" is that, by definition, it stretches.The time spent by a particle to have potential energy can be much smaller than the time it spent to have kinetic energy. That's why energy of ideal gas is almost exclusively in the form of kinetic energy, which makes its specific heat capacity minimum.
And the only reason that kinetic energy is conserved is that it ie loaned to the surface as potential energy and then returned as kinetic energy on the rebound.
So, once you know how the kinetic energy is conserved, you realise that, ironically, it isn't. Briefly, it is converted to potential energy.
Did you not realise that was why I asked "How?" ?
Instead of answering the question, you posted some nonsense about duration.
Post by: alancalverd on 05/06/2022 10:17:49
Post by: Bored chemist on 06/06/2022 18:21:57
In an ideal gas, all collisions are perfectly elastic, by definition, and therefore the rebound is instantaneous..Only for a monatomic gas.
Molecular gases vibrate.
And, in any event, you are in danger of solving the problem for a spherical horse in a vacuum.
Post by: alancalverd on 06/06/2022 23:57:45
Post by: hamdani yusuf on 10/06/2022 07:41:15
Which is why we distinguish between real gases and ideal gases. In the GCSE fantasy world of weightless strings and frictionless pulleys, all gases are composed of tiny billiard balls and PV = RT for ever.We solve problems starting from the most basic and simple version. When the result is satisfactory, we must expand it to more general and realistic situation by introducing other factors.
In real life, we apply temperature measurement not only to ideal gas. We also have to measure temperature of molecular gases, liquids, solids, and plasmas. We must also measure fluid temperature in various pressure. Plotting the heat capacity in various temperature and pressure can give us insight on the microscopic states shown as temperature of objects.
Post by: alancalverd on 10/06/2022 16:55:25
Post by: Bored chemist on 10/06/2022 17:16:12
from which we infer that temperature is a measure of the mean internalkineticenergy of a body
Post by: alancalverd on 10/06/2022 23:31:21
Post by: hamdani yusuf on 11/06/2022 09:48:37
And when we look at heat transfer between objects, we recognise that there is none between bodies at the same temperature, from which we infer that temperature is a measure of the mean internal kinetic energy of a body.I can accept this. But can you be more specific about the definition of "internal" here?
You need to specify what you mean with internal and kinetic, in contrast to external and potential.So, what's your answer to this question : what is temperature?A measure of the internal kinetic energy of a body.
Post by: alancalverd on 11/06/2022 12:29:46
Kinetic: associated with movement.
Post by: Spring Theory on 11/06/2022 13:25:50
Something missing in the above.
[h] = ML2T-1
[c] = LT-1
so you can't set both c and h to a dimensionless unity simultaneously.
You have never taken a theoretical physics class. They work in natural units.
Here are some good vids you can get educated on natural units:
Sabine Hossenfelder - Curt Jaimungal -
Post by: Spring Theory on 11/06/2022 13:30:16
I bend over backwards here trying to help you guys understand concepts and be productive to the topic but the typical response is just an unproductive, smart ass quip.Since most of what you bring to the party is pseudoscience, leaving the discussion is no loss.
What a waste of time and effort. You guys deserve neither.
I guess that means Leonard Susskind is teaching pseudoscience courses at Stanford University.
Post by: hamdani yusuf on 11/06/2022 13:48:03
Imagine a bullet fired from a high-flying aircraft. Ignoring the residual heat from the propellant and barrel friction, it leaves at ambient temperature, say -20C, which is a measure of its internal kinetic energy. It initially travels at 500 m/s, which defines its external kinetic energy. En route it is slowed by air friction, some of which results in heating, thus depleting the external and increasing the internal k.e.Ok. If the bullet also spins, it should also be an external energy, right?
Kinetic: associated with movement.
Post by: alancalverd on 12/06/2022 00:49:42
Post by: alancalverd on 12/06/2022 00:57:03
Here are some good vids you can get educated on natural units:You might do well to study them yourself and see where your assertion of 29 May is incorrect.
Post by: hamdani yusuf on 14/06/2022 12:07:39
At higher flow, the cooling water has higher kinetic energy. But the temperature is almost the same. Thus the water flow here is considered as external kinetic energy.
The question is, how do the water molecules and reactor wall know the difference between internal and external energy?
Post by: alancalverd on 14/06/2022 12:44:42
Do not confuse "almost" with "equals".
And if the cooling water does not leave at a higher temperature than it arrives, it is not cooling anything - check both gauges!
Anyway, the answer is "temperature".
Post by: hamdani yusuf on 14/06/2022 14:39:10
Quote
Cooling curves - stearic acid cooling from a liquid to a solid
Changes in state cause bonds to be broken or made. It takes energy to break bonds, and making bonds releases energy.
Post by: hamdani yusuf on 14/06/2022 14:49:38
Quote
Universal technique for condensing common gases and studying their liquid phases. Have you seen blue oxygen?
This video is part of the Flinn Scientific Best Practices for Teaching Chemistry Video Series, a collection of over 125 hours of free professional development training for chemistry teachers - http://elearning.flinnsci.com
ATTENTION: This demonstration is intended for and should only be performed by certified science instructors in a safe laboratory/classroom setting.
Post by: hamdani yusuf on 14/06/2022 14:51:50
"know"? Chemical engineers are is clever and know a lot, chemicals are dumb and know nothing.The reactor wall seems to be able to "distinguish" between internal and external kinetic energy in the cooling water. What's our best model to distinguish them?
Do not confuse "almost" with "equals".
And if the cooling water does not leave at a higher temperature than it arrives, it is not cooling anything - check both gauges!
Anyway, the answer is "temperature".
As far as possible, I try to avoid circular reasoning. Like saying that temperature is internal kinetic energy, and continues explaining that internal kinetic energy is temperature.
Post by: Bored chemist on 14/06/2022 21:10:11
Here's an interesting experiment related to this thread.Did you imagine that we are not aware of that sort of behaviour?QuoteCooling curves - stearic acid cooling from a liquid to a solid
Changes in state cause bonds to be broken or made. It takes energy to break bonds, and making bonds releases energy.
Post by: Bored chemist on 14/06/2022 21:13:26
ATTENTION: This demonstration is intended for and should only be performed by certified science instructors in a safe laboratory/classroom setting.
Do you understand that I have done those sorts of experiments?
Post by: alancalverd on 14/06/2022 23:03:42
The reactor wall seems to be able to "distinguish" between internal and external kinetic energy in the cooling water. What's our best model to distinguish them?Temperature is a measure of internal kinetic energy.
As far as possible, I try to avoid circular reasoning. Like saying that temperature is internal kinetic energy, and continues explaining that internal kinetic energy is temperature.
Beware of using anthropic terms like "distinguish" - you will confuse yourself.
If a body A has more of anything than body B, which way will "anything" flow? (NB there are a couple of significant contradictions to the obvious answer).
Post by: hamdani yusuf on 15/06/2022 06:04:33
Did you imagine that we are not aware of that sort of behaviour?If you don't think that it is interesting, you can just ignore it.
One thing that could be interesting to discus is why heating curve has different shape than cooling curve, among others.
Post by: hamdani yusuf on 15/06/2022 06:06:32
So what?ATTENTION: This demonstration is intended for and should only be performed by certified science instructors in a safe laboratory/classroom setting.
Do you understand that I have done those sorts of experiments?
Post by: hamdani yusuf on 15/06/2022 06:20:56
Let me simplify the case.The reactor wall seems to be able to "distinguish" between internal and external kinetic energy in the cooling water. What's our best model to distinguish them?Temperature is a measure of internal kinetic energy.
As far as possible, I try to avoid circular reasoning. Like saying that temperature is internal kinetic energy, and continues explaining that internal kinetic energy is temperature.
Beware of using anthropic terms like "distinguish" - you will confuse yourself.
If a body A has more of anything than body B, which way will "anything" flow? (NB there are a couple of significant contradictions to the obvious answer).
At the beginning, reactor wall and cooling water has the same temperature, say 30°C. The water doesn't flow, all of its kinetic energy is internal type. No heat transfer occurs.
Then with some method, the water is made to flow while keeping its total kinetic energy. Some of its internal kinetic energy is converted to external kinetic energy. This makes the water temperature to drop. Let's say we can manage to reduce the temperature to 25°C.
The last action produces temperature difference between reactor wall and cooling water, which causes heat transfer from reactor to water. Why it reacts differently even though the water has the same total kinetic energy? What's the distinguishing characteristics of internal kinetic energy that makes it different than external kinetic energy?
Post by: hamdani yusuf on 15/06/2022 06:30:44
If a body A has more of anything than body B, which way will "anything" flow? (NB there are a couple of significant contradictions to the obvious answer).(https://cdn.britannica.com/64/91964-050-4FDB21E8/vicinity-mass-distance-distortion-continuum-amount-relativity.jpg?w=690&h=388&c=crop)
Which way will they go?
Post by: Bored chemist on 15/06/2022 08:24:08
So there's not much point posting them.So what?ATTENTION: This demonstration is intended for and should only be performed by certified science instructors in a safe laboratory/classroom setting.
Do you understand that I have done those sorts of experiments?
Post by: hamdani yusuf on 15/06/2022 09:45:34
So there's not much point posting them.Do you understand that you are not the only one who use this forum?
Post by: alancalverd on 15/06/2022 11:16:22
Which way will they go?They all move towards the center of mass. But nothing flows from one to another.
Post by: alancalverd on 15/06/2022 11:18:44
What's the distinguishing characteristics of internal kinetic energy heat that makes it different than external kinetic energy gross motion?[/quote]
Post by: hamdani yusuf on 15/06/2022 18:05:57
Except if they have atmosphere.Which way will they go?They all move towards the center of mass. But nothing flows from one to another.
Post by: hamdani yusuf on 15/06/2022 18:07:03
What's the distinguishing characteristics of internal kinetic energy heat that makes it different than external kinetic energy gross motion?[/quote]As you said, the reactor wall doesn't understand the difference between heat and gross motion.
Post by: alancalverd on 16/06/2022 14:33:34
Except if they have atmosphere.Are you suggesting that air is some kind of gravitational shield? Wow!
Post by: alancalverd on 16/06/2022 14:35:00
Er...but it clearly does, since it transmits heat but not gross motion.What's the distinguishing characteristics of internal kinetic energy heat that makes it different than external kinetic energy gross motion?[/quote]As you said, the reactor wall doesn't understand the difference between heat and gross motion.
Post by: hamdani yusuf on 17/06/2022 03:31:15
No. If their atmospheres are large enough to overlap with each other, then some flow of material is to be expected.Except if they have atmosphere.Are you suggesting that air is some kind of gravitational shield? Wow!
Post by: hamdani yusuf on 17/06/2022 06:02:21
Er...but it clearly does, since it transmits heat but not gross motion.What is the mechanism?
Post by: Bored chemist on 17/06/2022 08:37:22
What is the mechanism?
Because heat and gross motion are not the same thing.
Post by: alancalverd on 17/06/2022 10:31:50
Now imagine you are at a drive-in movie next to a stationary car full of monkeys. What determines your enjoyment of the film: the speed of the car or the amount of noise the monkeys are making?
Post by: hamdani yusuf on 17/06/2022 11:02:56
How does the wall know the difference, thus react accordingly?What is the mechanism?
Because heat and gross motion are not the same thing.
Post by: hamdani yusuf on 17/06/2022 11:03:13
How does the wall know the difference, thus react accordingly?How does the wall know the difference, thus react accordingly?
Post by: alancalverd on 17/06/2022 11:27:38
Post by: hamdani yusuf on 18/06/2022 06:41:59
Same way as your skin "knows" the difference between the external kinetic energy of a moving car and the internal kinetic energy of a stationary car. Different causes, different effects.What's the difference?
That's the question.
Post by: alancalverd on 18/06/2022 10:37:55
If you want to make things complicated for yourself, solve the equations for the velocity profile of laminar flow and thermal diffusivity through a boundary and a moving fluid. In fact there's no need to solve them: write then down, or just draw a diagram, and you will see why the boundary material doesn't need to "know" anything.
Post by: hamdani yusuf on 18/06/2022 11:58:15
If the kinetic energy causes heat transfer, it's called internal kinetic energy, represented as temperature.
On the other hand, if the kinetic energy doesn't cause heat transfer, it's called external kinetic energy, represented as flow.
Post by: Deecart on 18/06/2022 13:31:06
It could also "exists " because it is the not measured part of some physic formula (like if you add some black energy to confirm some formula), but by chance we can measure it.
Therefore (because it be can measured) because there are multiple possibilies to measure it, for every temperature we are talking about, we need to explain how we are able to give its value (or temperature become senseless).
Temperature is some kind of concept like we have with time.
"Temp" if you refer to latin is somewhat refering to time..
Post by: alancalverd on 18/06/2022 14:29:34
If the kinetic energy causes heat transfer,It doesn't. Internal kinetic energy is heat. Temperature difference causes heat transfer.
Post by: Bored chemist on 18/06/2022 15:02:59
Temperature is some kind of concept like we have with time.No
"Temp" if you refer to latin is somewhat refering to time..
"late Middle English: from French température or Latin temperatura, from temperare ‘restrain’. The word originally denoted the state of being tempered or mixed, later becoming synonymous with temperament. The modern sense dates from the late 17th century."
Post by: hamdani yusuf on 18/06/2022 15:20:18
If you want to be pedantic, it would read,If the kinetic energy causes heat transfer,It doesn't. Internal kinetic energy is heat. Temperature difference causes heat transfer.
If the difference in the kinetic energy causes heat transfer, it's called internal kinetic energy. But in practice, the chance to have two objects with exactly the same amount of kinetic energy is almost zero. Especially if quantum fluctuation and cosmic rays are taken into account.
Post by: alancalverd on 18/06/2022 17:07:49
And the fact that A has more internal kinetic energy per particle than B does not guarantee heat transfer. There must be some means of exchanging that kinetic energy.
But as you say, it is extremely difficult to establish thermal equilibrium in a simple experiment.
Post by: Spring Theory on 18/06/2022 21:47:36
Here are some good vids you can get educated on natural units:You might do well to study them yourself and see where your assertion of 29 May is incorrect.
You can lead a horse to water, but you can't make him drink...
Post by: hamdani yusuf on 05/07/2022 14:28:31
Quote
Boltzmann's Entropy Equation: A History from Clausius to Planck
Boltzmann's entropy formula was created by Max Planck in 1900! So, why did Planck create this equation and how did it end up on Boltzmann's grave? I used primary sources to explain the history of this famous and confusing equation.
Post by: hamdani yusuf on 15/07/2022 14:03:28
The experiments shown in this video can give us more complete picture about temperature. Although the actual temperature still needs confirmation from contacting sensors as pointed out by some viewers.
Post by: Bored chemist on 15/07/2022 14:12:21
:-)
Post by: hamdani yusuf on 15/07/2022 15:20:46
At about 08:28 he shows that he doesn't understand why the sunset is red.He said he could not get rid of (separate) the brown substance from the finish product.
:-)
Post by: Bored chemist on 15/07/2022 16:34:48
And there is no brown substance (any more than there is a "red" substance in the air which causes red sunsets.At about 08:28 he shows that he doesn't understand why the sunset is red.He said he could not get rid of (separate) the brown substance from the finish product.
:-)
Post by: hamdani yusuf on 16/07/2022 00:16:27
And there is no brown substance (any more than there is a "red" substance in the air which causes red sunsets.But there is substance that makes the sunset looks red, even if it only works for some angle.
Post by: Bored chemist on 16/07/2022 11:40:38
And that substance is air.And there is no brown substance (any more than there is a "red" substance in the air which causes red sunsets.But there is substance that makes the sunset looks red, even if it only works for some angle.
What colour is it?
The point is that he is looking at the effect of scattering by a white material, and saying there is something brown or yellow present.
That strongly signals that he doesn't really know what he is talking about.
Post by: puppypower on 16/07/2022 11:48:45
If we solve for T, then T=(H-G)/S. Temperature is a measure of system free energy, divided by system entropy. The same amount of energy, inputted into different materials, can give different temperatures. This will be based on the entropy within each material. Temperature is not energy, alone.
The lower the system entropy, the higher the temperature for any energy input. The higher the system entropy the lower the temperature, with the same energy input.
Entropy was originally defined as lost energy. The less loss energy within the complexity of a dynamic system, the higher the temperature that will be measured for any energy input. Some systems are better at losing energy, so they will appear cooling for the same energy input.
I like the Gibb's free energy equation since it is connected to applied science. Applied science is where you need to apply science, to make things in reality, that have to work in reality, or you will go out of business. Theoretical science is useful, but it does not have the same amount of social pressure. It can look pretty and sit over there and everyone is happy with just that. Applied science has to deal with angry customers. It may not pretty enough to make up for any type of flaw. You need to make her perfect, instead of just pretty.
Post by: Bored chemist on 16/07/2022 12:15:35
I like the Gibb's free energy equation since it is connected to applied science.Josiah Willard Gibbs was a theoretical physicist, not an applied one.
All applied science is based on theoretical science (it is the theoretical science which gets "applied" to things".
They are essentially the same thing.
Why do you keep posting nonsense?
Post by: alancalverd on 16/07/2022 12:33:54
All applied science is based on theoretical scienceThere is an admonitory adage in physics: "Thermodynamics owes more to the steam engine than the steam engine owes to thermodynamics", and the history of science bears that out.
If you subscribe to the "observe - hypothesise - test" model of scientific method, observation and experiment always precede theory and take precedence over it..
Post by: hamdani yusuf on 17/07/2022 08:46:38
Post by: Bored chemist on 17/07/2022 09:17:58
Humans have been using fire long before they have a scientific model of fire. Boats have been widely used before Archimedes came up with theory of buoyancy. Arrows have been widely used before humans understand mechanics, gravity, and aerodynamic.Do you have any 21st century examples?
Post by: alancalverd on 17/07/2022 13:32:59
I'm not sure that can ever be rigorously demonstrated in real time, but as far as I am concerned I type stuff into this machine and by magic, somebody, somewhere, disagrees.
On a more mundane level, an apparently simple machine like a Boeing 747 contains so many functional elements that the manufacturers happily state that no single human knows how it works! Sadly, it seems that nobody bothered to tell the pilots how to fly a 737MAX because everyone assumed it would work by itself.
Post by: Bored chemist on 17/07/2022 13:42:04
, but as far as I am concerned I type stuff into this machine and by magic, somebody, somewhere, disagrees.It doesn't require magic for you to be wrong.
Post by: puppypower on 17/07/2022 17:19:02
I like the Gibb's free energy equation since it is connected to applied science.Josiah Willard Gibbs was a theoretical physicist, not an applied one.
All applied science is based on theoretical science (it is the theoretical science which gets "applied" to things".
They are essentially the same thing.
Why do you keep posting nonsense?
Not exactly. Theoretical science will more often than not, get published, for prestige and for all to see and appreciate. Applied science is more likely to have a restricted audience, through copyrights, patents, intellectual property rights, as well as classified information. Such science may even be stored behind security walls.
Much of applied science will not be easy to access, since these special applications of science, create practical advantages in reality, that equate to money and power. If you get a job with a company, you need to promise not to share their trade secrets; for your eyes only. You will not find it in the stacks.
If you had universal security Q-clearance for full access to all applied science, all over the world, you would see the limitations of the theoretical. Practical has more unique data and angles, that may not be shared since they give unique advantages. I am willing to share, not the secrets of others, but my own secret recipes. You may need some new background, to look at the same things.
You can go to university and get a graduate degree in food science based on the known science literature. But this alone will not allow you to infer the secret recipe for making Coke. This will need to be shared from the inside. But you will need to sign secrecy agreements, since this knowledge is unique and others cannot easily infer it with the freely published science.
Theoretical science is like a pretty girt that you can see from a distance or up close. Although you are free to look, she does not allow everyone the same intimacy. There is a deeper side to her, that only some get to see. Many are very protective of these fond memories.
Post by: puppypower on 17/07/2022 18:40:31
All applied science is based on theoretical scienceThere is an admonitory adage in physics: "Thermodynamics owes more to the steam engine than the steam engine owes to thermodynamics", and the history of science bears that out.
If you subscribe to the "observe - hypothesise - test" model of scientific method, observation and experiment always precede theory and take precedence over it..
Applied science often begins by testing existing theory and new hypothesis. It is very rare that things work out as expected, with the first test. As problems appear and are overcome, the theory is modified at each iteration of experiments, until a new steady state is reached. Theoretical science may have to be modified to fit the data anomalies that keep coming. The final result is often protected, since the final application may have value in the free market.
I remember a development project I was given that involved developing a biological process, that could work under extreme circumstances. The experts said it was not possible, based on the then current technology and theory. The final goal was to run an anaerobic experiment in a 2.5 million gallon open and leaky basin, with an initial composition that exceeded all known safe closed bio-reactors variable, by order of magnitude. My boss had faith in my ingenuity.
My first problem was I never took any biology courses in high school or college. II like life but biology was to memory intensive and empirical for my tastes. I was good at organic chemistry, polymers, and engineering all of which are based on basic theory and ingenuity. So I had to teach myself a cram course in biology and bioreactors, and then follow my hunches in the lab, based on the POV of a biology outsider.
It turned out, I was a natural bacteria whisperer and the little bugs would help me out. I could get them go where they were not expected to go. To make a long story short, without any formal biology training, I was able to push the biology technology of the day, into the future.
My advantage was, I was not biased by the educational traditions that used a black box. My coursework as an engineer assumed something simple and more rational. When I looked at the project with my naive eyes, my bacteria were more robust than expected.
The final test of concept was my largest experiment of my career; 2.5 million gallons. It took 150 ton of powered limestone to neutralize the acid pond; caravan of dump trucks that I leased. I also gave my bacteria steak to eat, with a 5000 gallon tanker truck of 100% acetic acid. I used about 30 gallons of concentrated phosphoric acid for the phosphate needs of my bacteria's DNA and RNA. The little bugs were happy I am could see bioactivity the next day. It took about two weeks to really kick in. The little bugs even reduced heavy metal concentrations to discharge limits. This was part of a secondary need, I used some concentrated sulfuric acid that the bacteria would anaerobically reduce to sulfide to form Heavy metal sulfides. This cause the pond to stick like crap, so I used used a larger aerator/fountain, to beat in oxygen into the pond; shift to aerobic, until all the bacteria steak was also gone. Good observation, logic and ingenuity in the field can challenge theory based on consensus in a black box.
The tragic thing was, what should have been a good thing, made many people angry. It altered the priority of a parallel engineering project; monument, that now became obsolete. I felt political pressure afterwards and would eventually make me feel unwelcome and need to quit. I am less sensitive today. Now I fight and do not quit. Although I now try to be more diplomatic.
Temperature as a function of energy divided by entropy tells us how the energy will be distributed based on the entropic information connected to the state of the system. This allows for more complex modeling.
Picture a 1 mile cube in the atmosphere. This cube is gas that has clouds that phase separated from the oxygen and nitrogen and other trace gases like CO2. We are looking for the final temperature in the cube, based on adding X Kilojoules of solar energy to the cube.
Since entropy is not the same for the water cloud; gas predicate, as the rest of the gas solution, the movement to steady temperature will not be straight forward. We will get some cooler spots, that will then need a type of secondary equilibration. This is not an ideal gas, but a mixture of gases and gas phase that can self segregate. My little equation can address this. The entropy data can be found in the CRC.
Post by: Bored chemist on 17/07/2022 18:47:11
Did you realise that your story didn't actually tell us anything apart from the fact that, you needed to learn the theoretical aspect of biology in order to plan your project (which, incidentally sounds like a big heap of ... manure)?All applied science is based on theoretical scienceThere is an admonitory adage in physics: "Thermodynamics owes more to the steam engine than the steam engine owes to thermodynamics", and the history of science bears that out.
If you subscribe to the "observe - hypothesise - test" model of scientific method, observation and experiment always precede theory and take precedence over it..
Applied science often begins by testing existing theory and new hypothesis. It is very rare that things work out as expected, with the first test. As problems appear and are overcome, the theory is modified at each iteration of experiments, until a new steady state is reached. Theoretical science may have to be modified to fit the data anomalies that keep coming. The final result is often protected, since the final application may have value in the free market.
I remember a development project I was given that involved developing a biological process, that could work under extreme circumstances. The experts said it was not possible, based on the then current technology and theory. The final goal was to run an anaerobic experiment in a 2.5 million gallon open and leaky basin, with an initial composition that exceeded all known safe closed bio-reactors variable, by order of magnitude. My boss had faith in my ingenuity.
My first problem was I never took any biology courses in high school or college. II like life but biology was to memory intensive and empirical for my tastes. I was good at organic chemistry, polymers, and engineering all of which are based on basic theory and ingenuity. So I had to teach myself a cram course in biology and bioreactors, and then follow my hunches in the lab, based on the POV of a biology outsider.
It turned out, I was a natural bacteria whisperer and the little bugs would help me out. I could get them go where they were not expected to go. To make a long story short, without any formal biology training, I was able to push the biology technology of the day, into the future.
My advantage was, I was not biased by the educational traditions that used a black box. My coursework as an engineer assumed something simple and more rational. When I looked at the project with my naive eyes, my bacteria were more robust than expected.
The final test of concept was my largest experiment of my career; 2.5 million gallons. It took 150 ton of powered limestone to neutralize the acid pond, I also gave bacteria steak to eat with a 5000 gallon tanker truck 100% acetic acid. I used about 30 gallons of concentrated phosphoric acid for the phosphate needs of DNA and RNA. It took about two weeks to kick in and ran like a charm and was done a few weeks later It even reduced heavy metal concentrations to discharge limits. This was part of a secondary trick using sulfuric acid that the bacteria would reduce to sulfide to form Heavy metal sulfides. This cause the pond to stick so I used an larger aerator to beat in oxygen until all the food was also gone. Good observation, logic and ingenuity can challenge theory based on consensus in a black box.
The tragic thing was, wha was a good thing, made many people angry. It altered the priority of a parallel engineering project; monument, that became obsolete. I felt political pressure afterwards and would eventually need to quit. I am less sensitive today. Now I fight and do not quit. Although I now try to be more diplomatic.
Temperature as a function of energy divided by entropy tells us how the energy is distributed based on the entropic information in the system. This allows for more complex modeling.
Picture a 1 mile cube in the atmosphere.l This is gas that has clouds that phase separated from the oxygen and nitrogen and other trace gases like CO2. We are looking for the final temperature in the cube, based on adding X Kilojoules of energy to the cube.
Since entropy is not the same for the water cloud gas predicate, as the rest of the gas solution, the movement to steady temperature will not be straight forward. We will get some cooler spots, that will then need a secondary equilibration. This is not an ideal gas, but a mixture of gases and gas phase that can self segregate. My little equation can address this. The entropy data can be found in the CRC.
Post by: puppypower on 17/07/2022 19:52:31
Did you realise that your story didn't actually tell us anything apart from the fact that, you needed to learn the theoretical aspect of biology in order to plan your project (which, incidentally sounds like a big heap of ... manure)?All applied science is based on theoretical scienceThere is an admonitory adage in physics: "Thermodynamics owes more to the steam engine than the steam engine owes to thermodynamics", and the history of science bears that out.
If you subscribe to the "observe - hypothesise - test" model of scientific method, observation and experiment always precede theory and take precedence over it..
Applied science often begins by testing existing theory and new hypothesis. It is very rare that things work out as expected, with the first test. As problems appear and are overcome, the theory is modified at each iteration of experiments, until a new steady state is reached. Theoretical science may have to be modified to fit the data anomalies that keep coming. The final result is often protected, since the final application may have value in the free market.
I remember a development project I was given that involved developing a biological process, that could work under extreme circumstances. The experts said it was not possible, based on the then current technology and theory. The final goal was to run an anaerobic experiment in a 2.5 million gallon open and leaky basin, with an initial composition that exceeded all known safe closed bio-reactors variable, by order of magnitude. My boss had faith in my ingenuity.
My first problem was I never took any biology courses in high school or college. II like life but biology was to memory intensive and empirical for my tastes. I was good at organic chemistry, polymers, and engineering all of which are based on basic theory and ingenuity. So I had to teach myself a cram course in biology and bioreactors, and then follow my hunches in the lab, based on the POV of a biology outsider.
It turned out, I was a natural bacteria whisperer and the little bugs would help me out. I could get them go where they were not expected to go. To make a long story short, without any formal biology training, I was able to push the biology technology of the day, into the future.
My advantage was, I was not biased by the educational traditions that used a black box. My coursework as an engineer assumed something simple and more rational. When I looked at the project with my naive eyes, my bacteria were more robust than expected.
The final test of concept was my largest experiment of my career; 2.5 million gallons. It took 150 ton of powered limestone to neutralize the acid pond, I also gave bacteria steak to eat with a 5000 gallon tanker truck 100% acetic acid. I used about 30 gallons of concentrated phosphoric acid for the phosphate needs of DNA and RNA. It took about two weeks to kick in and ran like a charm and was done a few weeks later It even reduced heavy metal concentrations to discharge limits. This was part of a secondary trick using sulfuric acid that the bacteria would reduce to sulfide to form Heavy metal sulfides. This cause the pond to stick so I used an larger aerator to beat in oxygen until all the food was also gone. Good observation, logic and ingenuity can challenge theory based on consensus in a black box.
The tragic thing was, wha was a good thing, made many people angry. It altered the priority of a parallel engineering project; monument, that became obsolete. I felt political pressure afterwards and would eventually need to quit. I am less sensitive today. Now I fight and do not quit. Although I now try to be more diplomatic.
Temperature as a function of energy divided by entropy tells us how the energy is distributed based on the entropic information in the system. This allows for more complex modeling.
Picture a 1 mile cube in the atmosphere.l This is gas that has clouds that phase separated from the oxygen and nitrogen and other trace gases like CO2. We are looking for the final temperature in the cube, based on adding X Kilojoules of energy to the cube.
Since entropy is not the same for the water cloud gas predicate, as the rest of the gas solution, the movement to steady temperature will not be straight forward. We will get some cooler spots, that will then need a secondary equilibration. This is not an ideal gas, but a mixture of gases and gas phase that can self segregate. My little equation can address this. The entropy data can be found in the CRC.
I only studied for about a couple of weeks and decided to wing it. The goal was not to repeat the limitations of my peers, but make the project work, even if the current theory thought it could not work. The needed direction was not yet part of their literature, so I had to wing it. I started more empirical and observational until my 55-gallon drum evolved to my needs. I learned from that.
The only reason I was able to run the big test, was an emergency. The final test basin was reported in the local papers as a pollution hazard, and this news went up the chain, to state and then national EPA. I was the only one who could be ready to go in days, instead of months and years. I could improvise instead of buy off the shelf.
My project had actually been killed several months earlier. It made a come back because of these unique needs. I was invited to the big table meeting since the Plant Manager wanted all his options on the table. Nobody was willing to commit to anything, for the short term, until I spoke up and gave 5 to 1 odds it would work. I was disliked for this, since, after being killed, I dared stepped on the toes.
The success of start up, helped the plant manager, since he was under a lot of stress. After all was done, he accepted the awards we received from the DOE, since it was a team effort, once it got going, from top to bottom. I was then reassigned to another big project. They slowed me down with a long term project.
In retrospect, it was a career builder project, that could have lasted me to retirement. I was to develop all the needed science and technology to decommission the Lithium Isolate Separation Facility that was used in the 1950-60's to collect Lithium 6. I must have been overdose by mercury, while exploring that historic facility. I lost my way with paranoia, so I had to quit, since I could not function properly and became off the wall.
Post by: Bored chemist on 17/07/2022 20:26:37
even if the current theory thought it could not work.Is there actual evidence for the assertion that current theories said it couldn't work?
What are these theories?
Or are you just reporting the views of some people who were basing their assessment on "practical experience"?
Post by: hamdani yusuf on 22/07/2022 09:27:36
People usually don't realize that they don't understand or misunderstand how things work, especially if they can make them work. They will only realize when someone comes up with a better explanation.Humans have been using fire long before they have a scientific model of fire. Boats have been widely used before Archimedes came up with theory of buoyancy. Arrows have been widely used before humans understand mechanics, gravity, and aerodynamic.Do you have any 21st century examples?
Post by: Bored chemist on 22/07/2022 13:07:10
I presume that was a stupid way of saying "no".People usually don't realize that they don't understand or misunderstand how things work, especially if they can make them work. They will only realize when someone comes up with a better explanation.Humans have been using fire long before they have a scientific model of fire. Boats have been widely used before Archimedes came up with theory of buoyancy. Arrows have been widely used before humans understand mechanics, gravity, and aerodynamic.Do you have any 21st century examples?
Post by: Eternal Student on 22/07/2022 22:52:03
Apologies: There's no way I've read all the posts since I last wrote something here. It's just a quiet day and I'd like to join a discussion.
Are you asking for an example of something people currently use but clearly don't understand?At a cursory glance, someone was asking for that (Bored Chemist ?) and someone was trying to provide examples (Hamdani Yusuf ?). I think we can let Hamdani off the hook on that one and help out a bit.
I don't have a crystal ball for seeing into the future but this seems like a safe enough bet:
Gravity ---> This is a reasonable 21st century example of something we use but don't understand yet. In particular we don't have a quantum theory gravity but it seems like a reasonable guess that there will be one. If one is developed, it then seems undeniable that gravity wasn't exactly what we thought but we did have some models and approximations and were able to use it and even provide scientific and mathematical models for it that weren't too bad.
About the rest of the thread:
I'm still not sure where @hamdani yusuf was going with this thread or what is left to discuss. If you feel so inclined, it might be worth writing a short summary of what has been done so far and/or what you feel is missing or still needs to be done. The thread is now 25 pages deep and if there were any new members joining, I'm sure they couldn't read all of that.
Best Wishes.
Post by: hamdani yusuf on 23/07/2022 04:15:26
Lord Kelvin was as confident as you are about the scientific knowledge of his contemporaries.I presume that was a stupid way of saying "no".People usually don't realize that they don't understand or misunderstand how things work, especially if they can make them work. They will only realize when someone comes up with a better explanation.Humans have been using fire long before they have a scientific model of fire. Boats have been widely used before Archimedes came up with theory of buoyancy. Arrows have been widely used before humans understand mechanics, gravity, and aerodynamic.Do you have any 21st century examples?
Post by: hamdani yusuf on 23/07/2022 04:54:12
I'm still not sure where @hamdani yusuf was going with this thread or what is left to discuss. If you feel so inclined, it might be worth writing a short summary of what has been done so far and/or what you feel is missing or still needs to be done. The thread is now 25 pages deep and if there were any new members joining, I'm sure they couldn't read all of that.I want to draw the line between what's called temperature and what's not temperature. So far, most of us agree that temperature is a kind of kinetic energy, which is related to motion. Thus a kind of energy not related to motion can not be called temperature.
On the other hand, not all kind of kinetic energy is called temperature. Uniform translational and rotational motions are not called temperature. Electrons on a radio transmission antenna or a power distribution transformer may have high kinetic energy, but we don't usually say that they have high temperature. Measured from surface of the earth, ISS has high kinetic energy, but we don't usually say that it has high temperature. Audio speakers and ceiling fans are some other examples.
Alan offered an idea calling that kinetic energy causes change in temperature is called internal kinetic energy, and consequently, external kinetic energy would not cause change in temperature. Unfortunately, we have not found a microscopic model which can be used to distinguish between internal and external kinetic energy to predict how a kind of movement would affect temperature measurement of an object. It means that for the time being, the distinction is not much better than semantics.
Post by: alancalverd on 23/07/2022 10:22:29
I want to draw the line between what's called temperature and what's not temperature.Go back to page 1. Temperature is a measure of the internal kinetic energy of the constituents of a body.
Thew "microscopic model" you are looking for is simple. Any body is defined by its boundaries, and stuff is either inside or outside the boundary.
Your deliberate self-confusion should disappear once you remember that speed is not absolute.The internal ke is a measure of the velocities of the constituent particles relative to each other, and the external ke is a measure of the mean group velocity of the whole body relative to the rest of the universe or whatever external frame of reference you choose.
Consider a 0.2 gram fly buzzing around at 1 m/s in an otherwise-empty car. It has 10-4 joules of kinetic energy relative to the shell of the car. If the car is travelling at 10 m/s over the ground, the fly has an additional 10-2 joules of kinetic energy relative to the planet.
For simplicity (because this is physics, not engineering) let the car have zero mass. If we lean the car gently against a wall, the fly could in principle transfer up to 10-4 joules of ke to the wall by crashing inelastically into the shell of the car. If we splatter the zero-mass car into the wall at 10 m/s, it will transfer 100 times as much energy, though the temperature of the car (the ke of the fly relative to the car) hasn't changed.
Now let's get practical. What has a zero-mass boundary? Any solid or liquid body. You can distinguish between inside and outside, and the boundary is the infinitesimal imaginary membrane between them.
What about gases, I ask, anticipating the next question of a troublemaker? Quite simply, you can't define the temperature of an unconfined gas because it will expand to fill the entire universe and the concept of average relative velocity will be meaningless. But you can obviously define the temperature of a bounded sample of gas or plasma.
Post by: Bored chemist on 23/07/2022 12:16:26
Lord Kelvin was as confident as you are about the scientific knowledge of his contemporaries.And you confidently assert things that are clearly wrong.
Thus a kind of energy not related to motion can not be called temperature.It quite often is.
The electronically excited neon atoms in a HeNe laser which emit light have a negative electronic temperature. Nobody cares much about their movement.
https://en.wikipedia.org/wiki/Negative_temperature#Lasers
You really need to stop ignoring reality.
Post by: Bored chemist on 23/07/2022 12:23:51
Electrons on a radio transmission antenna or a power distribution transformer may have high kinetic energy,They might.
But only if the metal is hot.
And turning the transmitter on or off hardly affects their motion.
Post by: hamdani yusuf on 23/07/2022 15:31:30
What about gases, I ask, anticipating the next question of a troublemaker? Quite simply, you can't define the temperature of an unconfined gas because it will expand to fill the entire universe and the concept of average relative velocity will be meaningless. But you can obviously define the temperature of a bounded sample of gas or plasma.Does earth atmosphere have a boundary? What about Jupiter's or Venus'?
Post by: hamdani yusuf on 23/07/2022 15:37:37
And you confidently assert things that are clearly wrong.Name one.
Post by: hamdani yusuf on 23/07/2022 15:47:14
The electronically excited neon atoms in a HeNe laser which emit light have a negative electronic temperature. Nobody cares much about their movement.The article says something about vortices momenta, which depend on their motion.
https://en.wikipedia.org/wiki/Negative_temperature#Lasers
You really need to stop ignoring reality.
Quote
The possibility of negative temperatures was first predicted by Lars Onsager in 1949.[1] Onsager was investigating 2D vortices confined within a finite area, and realized that since their positions are not independent degrees of freedom from their momenta, the resulting phase space must also be bounded by the finite area.How do you get a frequency without motion?
Quote
The Hamiltonian for a single mode of a luminescent radiation field at frequency ν is...You really need to stop pretending to understand something that you don't.
Post by: hamdani yusuf on 23/07/2022 15:59:18
They might.Have you ever worked with high powered radio transmission or power distribution transformers?
But only if the metal is hot.
And turning the transmitter on or off hardly affects their motion.
FYI, most of the power delivered to the antenna is not converted to heat.
Post by: alancalverd on 23/07/2022 19:19:00
Post by: Bored chemist on 23/07/2022 19:26:54
Name one.I already named one of the things you confidently got wrong.
Quote from: hamdani yusuf on Today at 04:54:12
Thus a kind of energy not related to motion can not be called temperature.
It quite often is.
The electronically excited neon atoms in a HeNe laser which emit light have a negative electronic temperature. Nobody cares much about their movement.
https://en.wikipedia.org/wiki/Negative_temperature#Lasers
Did you not understand it?
Post by: Bored chemist on 23/07/2022 19:29:26
Have you ever worked with high powered radio transmission or power distribution transformers?Yes, I have.
Now, here are a couple of questions for you.
What is the typical thermal velocity of an electron in a conductor near room temperature and
What is the drift velocity of an electron in a typical conductor in a transformer or antenna?
I don't need any great precision- just order of magnitude is fine.
Post by: Bored chemist on 23/07/2022 19:35:28
The article says something about vortices momenta, which depend on their motion.The relevant bit talks about an electronic population inversion in a laser.
I'm sorry the article is long and has other things that distracted you.
Here is the bit you need to focus on.
"This phenomenon can also be observed in many lasing systems, wherein a large fraction of the system's atoms (for chemical and gas lasers) or electrons (in semiconductor lasers) are in excited states. This is referred to as a population inversion."
"β must itself be negative, implying a negative temperature."
Once you recognise that the laser as a whole does not have a negative temperature, and that a negative temperature can't be due to a negative kinetic energy (because, even if the particles all suddenly moved backwards, their energies would still be positive), you will see that it's the electronic temperature which is negative, and that isn't due to the kinetic energies of the particles.
Post by: hamdani yusuf on 24/07/2022 07:47:33
If the electrons stop moving, will they still have negative temperature?Name one.I already named one of the things you confidently got wrong.Quote from: hamdani yusuf on Today at 04:54:12
Thus a kind of energy not related to motion can not be called temperature.
It quite often is.
The electronically excited neon atoms in a HeNe laser which emit light have a negative electronic temperature. Nobody cares much about their movement.
https://en.wikipedia.org/wiki/Negative_temperature#Lasers
Did you not understand it?
Post by: hamdani yusuf on 24/07/2022 09:00:21
What's the proportion of transmitter power heating up the antenna compared to the power transmitting radio wave into space around it?Have you ever worked with high powered radio transmission or power distribution transformers?Yes, I have.
Quote
Now, here are a couple of questions for you.I never measured them. Have you?
What is the typical thermal velocity of an electron in a conductor near room temperature and
What is the drift velocity of an electron in a typical conductor in a transformer or antenna?
I don't need any great precision- just order of magnitude is fine.
Post by: Bored chemist on 24/07/2022 10:18:42
If the electrons stop moving, will they still have negative temperature?It isn't the electrons that have a negative temperature, it's the ensemble of excited and unexcited neon atoms.
If you understood the science, you wouldn't be asking questions that make no sense.
Try learning.
Post by: Bored chemist on 24/07/2022 10:43:51
What's the proportion of transmitter power heating up the antenna compared to the power transmitting radio wave into space around it?Who cares?
Why do they care?
In an ideal world, no energy is dissipated as heat in the antenna, it's all transmitted.
In practice the reflected power may be larger than that lost to heat via resistance.
I never measured them. Have you?I'm sensible enough to usually let other people do the actual measurements for me.
On the other hand, I did once do one of them.
Measuring the speed of sound in air is a high-school experiment.
https://www.bbc.co.uk/bitesize/guides/z8d2mp3/revision/3#:~:text=Clap%2Decho%20method,to%20the%20wall%20and%20back).
I'm pretty sure we got an answer near 330m/s.
And I know how sound works, so I know that the speed of the molecules in air is about the speed of sound.
And I understand the equipartition principle.
So I know that the thermal KE of the electrons in a wire is the same as that of the air molecules (at the same temperature as the wire).
And I know that an air molecule weighs about 60,000 times as much as an electron, so , since they have the same KE, the electrons must be travelling square root (60,000) times as fast.
That's about 80 km/s.
It's only an approximation, but it's good enough to make the point.
It's also fairly easy to find the drift speed of an electron.
https://en.wikipedia.org/wiki/Drift_velocity#Numerical_example
Tells you " the electrons are flowing at the rate of 23 μm/s".
So, now that you know that the current through a wire only changes the speeds of the electrons by a tiny fraction- about a part in a billion- do you see why it doesn't really matter if the transmitter is turned on or off?
More importantly, do you understand that if you actually knew science, you would already have known that?
Do you realise you wouldn't have said
Electrons on a radio transmission antenna or a power distribution transformer may have high kinetic energy, but we don't usually say that they have high temperature.
because you would have known that the use of electrons in an antenna hardly changes their KE at all.
Do you understand how, if you had spent time learning, you would ave avoided looking stupid?
Post by: alancalverd on 24/07/2022 12:10:52
And I know how sound works, so I know that the speed of the molecules in air is about the speed of sound.Except that they don't actually go anywhere! The speed of sound is the phase velocity, not the group velocity of the air mass. Beware of adding to HY's deliberate confusion!
This misunderstanding caused lots of problems in the music business during the major COVID panic. Brass instruments were first banned, then only permitted if fitted with a particle filter, and all the brass section had to face the same way "so as not to project bugs at high velocity towards one another". Clearly nonsense. I play the tuba, and exhale at pretty much the same rate with or without the instrument in place. Indeed since the exit port is about 50 cm in diameter, the exit group velocity is a factor of 10,000 lower than from my 0.5 cm nostrils! Even more stupid was the requirement to cover the bell of a saxophone, where the music comes out of the umpteen holes in the side!
If you think of a loudspeaker (I also play bass guitar) it makes the same sound with no net air flow. The only reason we blow into wind instruments is to make the initial excitation (brass lip buzz, woodwind reed vibration, or flute/whistle pure edge tone) from which the resonant horn then selects (more or less, depending on your skill) the desired frequency.
Sound in a gas is a series of longitudinal pressure waves: each molecule moves forwards and backwards, ending up in pretty much the same place. Now the same applies to electrons in a naive model of an antenna. They may all be jiggling about at random, but what is propagating along the wire is a density wave, not a gross displacement.
Post by: Bored chemist on 24/07/2022 13:59:20
That may be the record for "largest number of words used to explain that I don't understand the meaning of the word "about".And I know how sound works, so I know that the speed of the molecules in air is about the speed of sound.Except that they don't actually go anywhere! The speed of sound is the phase velocity, not the group velocity of the air mass. Beware of adding to HY's deliberate confusion!
This misunderstanding caused lots of problems in the music business during the major COVID panic. Brass instruments were first banned, then only permitted if fitted with a particle filter, and all the brass section had to face the same way "so as not to project bugs at high velocity towards one another". Clearly nonsense. I play the tuba, and exhale at pretty much the same rate with or without the instrument in place. Indeed since the exit port is about 50 cm in diameter, the exit group velocity is a factor of 10,000 lower than from my 0.5 cm nostrils! Even more stupid was the requirement to cover the bell of a saxophone, where the music comes out of the umpteen holes in the side!
If you think of a loudspeaker (I also play bass guitar) it makes the same sound with no net air flow. The only reason we blow into wind instruments is to make the initial excitation (brass lip buzz, woodwind reed vibration, or flute/whistle pure edge tone) from which the resonant horn then selects (more or less, depending on your skill) the desired frequency.
Sound in a gas is a series of longitudinal pressure waves: each molecule moves forwards and backwards, ending up in pretty much the same place. Now the same applies to electrons in a naive model of an antenna. They may all be jiggling about at random, but what is propagating along the wire is a density wave, not a gross displacement.
Post by: alancalverd on 24/07/2022 17:15:15
Perhaps you should enter politics: there's a job on offer this week with good pay and no liabilities. Anyone who can state that 80,000 is "about" 300 with a straight face is destined for at least Home Secretary (yes, you can get all the illegal immigrants on one plane) or Health Secretary (we had to kill about 300 people to save the NHS), but as Prime Minister you can say anything you like and walk on water (the slime will support you).
Post by: Bored chemist on 24/07/2022 17:20:18
Not sure whether that is an admission or a denial, but it was really for HY's education and the amusement of others.Snap.
Post by: hamdani yusuf on 25/07/2022 11:27:46
Let me try.If the electrons stop moving, will they still have negative temperature?It isn't the electrons that have a negative temperature, it's the ensemble of excited and unexcited neon atoms.
If you understood the science, you wouldn't be asking questions that make no sense.
Try learning.
If the atoms stop moving, will they still have negative temperature?
Post by: hamdani yusuf on 25/07/2022 11:31:51
Who cares?The business owners.
Why do they care?
In an ideal world, no energy is dissipated as heat in the antenna, it's all transmitted.
In practice the reflected power may be larger than that lost to heat via resistance.
If their antenna is too thin, or the material has too high specific resistance, then heat dissipation won't be negligible anymore. In extreme cases, the antenna can melt down and stop working.
Post by: hamdani yusuf on 25/07/2022 12:38:50
Quote
If you can't explain something to a first year student, then you haven't really understood it.Although I realize that some concepts can have high complexity they require a lot of connections that they need a lot of memory space, more than an average human can have. So, I'd like to replace the first year student with an AI which has adequate resources to cover the whole concept. It would be better if the AI also has natural language processing capability, so the communication with the human explainer can be free from unnecessary language barrier.
Richard Feynman
Post by: Bored chemist on 25/07/2022 12:40:45
If the atoms stop movingThey can't, because they are a gas.
Try harder.
Post by: Bored chemist on 25/07/2022 12:46:31
The business owners.But nobody would be so stupid as to design an antenna that way.
If their antenna is too thin, or the material has too high specific resistance, then heat dissipation won't be negligible anymore. In extreme cases, the antenna can melt down and stop working.
The resistive losses are usually small compared to others.
Oh, hang on I already told you that.
In an ideal world, no energy is dissipated as heat in the antenna, it's all transmitted.
In practice the reflected power may be larger than that lost to heat via resistance.
The point remains, the speed of the electrons is almost entirely governed by the temperature of the wire.
You seem to sort of understand that the people who use transmitters try to reduce the resistive losses.
So the change in temperature when they turn the transmitter on or off will be small.
So this
Electrons on a radio transmission antenna or a power distribution transformer may have high kinetic energy, but we don't usually say that they have high temperature.
is still nonsense.
Post by: Bored chemist on 25/07/2022 12:48:53
Although I realize that some concepts can have high complexity they require a lot of connections that they need a lot of memory space, more than an average human can have.... the human explainer ...You seem to think the the human doing the explaining has more memory space than the student.
Post by: hamdani yusuf on 25/07/2022 12:53:23
Use solid state laser.If the atoms stop movingThey can't, because they are a gas.
Try harder.
Post by: hamdani yusuf on 25/07/2022 12:56:56
But nobody would be so stupid as to design an antenna that way.
The resistive losses are usually small compared to others.
Oh, hang on I already told you that.
That's because they cared, unlike you here.
Who cares?
Why do they care?
Post by: hamdani yusuf on 25/07/2022 12:58:18
You seem to think that the words "some" and "average" are meaningless.Although I realize that some concepts can have high complexity they require a lot of connections that they need a lot of memory space, more than an average human can have.... the human explainer ...You seem to think the the human doing the explaining has more memory space than the student.
Post by: Bored chemist on 25/07/2022 13:22:29
The problem was solved ages ago when someone did the design.But nobody would be so stupid as to design an antenna that way.
The resistive losses are usually small compared to others.
Oh, hang on I already told you that.
That's because they cared, unlike you here.Who cares?
Why do they care?
Why would you still care now?
In any event...
this
Quote from: hamdani yusuf on 23/07/2022 04:54:12
Electrons on a radio transmission antenna or a power distribution transformer may have high kinetic energy, but we don't usually say that they have high temperature.
is still nonsense.
The kinetic energy of the electrons is dependent on temperature, not on whether or not it is an antenna.
Do you accept that?
Post by: Bored chemist on 25/07/2022 13:25:54
NoYou seem to think that the words "some" and "average" are meaningless.Although I realize that some concepts can have high complexity they require a lot of connections that they need a lot of memory space, more than an average human can have.... the human explainer ...You seem to think the the human doing the explaining has more memory space than the student.
Try again.
Use solid state laser.But I'm clearly talking about a laser that is a gas laser.
I only need to show one example of a case where your idea is impossible to show that your idea is wrong.
It doesn't matter if your idea works in other circumstances.
Do you act like this in real life, or only on line?
Post by: alancalverd on 25/07/2022 15:19:48
Most schoolkids understand the concept of temperature. At least we used to in the 1950s. No point in teaching it to any machine (apart from a thermostat - and they seem to grasp it immediately time).QuoteIf you can't explain something to a first year student, then you haven't really understood it.Although I realize that some concepts can have high complexity they require a lot of connections that they need a lot of memory space, more than an average human can have. So, I'd like to replace the first year student with an AI which has adequate resources to cover the whole concept. It would be better if the AI also has natural language processing capability, so the communication with the human explainer can be free from unnecessary language barrier.
Richard Feynman
Post by: hamdani yusuf on 26/07/2022 00:33:46
Not sure whether that is an admission or a denial, but it was really for HY's education and the amusement of others.It seems like you got unexpected results. It means that you have made at least one false assumption.
Post by: hamdani yusuf on 26/07/2022 00:42:37
The problem was solved ages ago when someone did the design.The problem still exists, the designs put countermeasures to render it insignificant.
Why would you still care now?
If you believe that there is no problem in the first place, you are likely to forget about the countermeasures, and the system will fail.
Post by: hamdani yusuf on 26/07/2022 00:53:16
But I'm clearly talking about a laser that is a gas laser.The gas condenses when cooled down to near abolute zero, and no longer be a gas. It introduces unnecessary complexity.
I only need to show one example of a case where your idea is impossible to show that your idea is wrong.
It doesn't matter if your idea works in other circumstances.
You need to learn a concept from the simplest cases. Mendel chose a specific kind of pea. Keppler was lucky that planets are far apart that their gravitational influence among them are insignificant compared to the sun's.
Post by: hamdani yusuf on 26/07/2022 00:57:02
The kinetic energy of the electrons is dependent on temperature, not on whether or not it is an antenna.Yes.
Do you accept that?
Electric current in the wire adds electrons kinetic energy.
Do you accept that?
Post by: hamdani yusuf on 26/07/2022 01:00:55
Most schoolkids understand the concept of temperature.Or misunderstand it, depends on what was taught to them.
Post by: hamdani yusuf on 26/07/2022 01:04:06
No point in teaching it to any machine (apart from a thermostat - and they seem to grasp it immediately time).It seems like you are not familiar with AI.
Have you ever taught a thermostat?
What did you teach it?
Post by: Bored chemist on 26/07/2022 08:51:16
Electric current in the wire adds electrons kinetic energy.Do you understand how little kinetic energy the current adds?
Do you accept that?
Only about a part in a billion.
Do you see how that makes your statement nonsense?
It introduces unnecessary complexity.Then don't.
You need to learn a concept from the simplest cases.Yes.
A monatomic gas like neon is much simpler than the heterojunction between two layers of semiconductor in a laser diode.
But that's beside the point.
The neon case shows that you are wrong.
It does no matter what you say.
You were still wrong.
So it's still an example of this
Name one.I already named one of the things you confidently got wrong.Quote from: hamdani yusuf on Today at 04:54:12
Thus a kind of energy not related to motion can not be called temperature.
It quite often is.
The electronically excited neon atoms in a HeNe laser which emit light have a negative electronic temperature. Nobody cares much about their movement.
https://en.wikipedia.org/wiki/Negative_temperature#Lasers
Did you not understand it?
Post by: hamdani yusuf on 26/07/2022 14:29:11
Do you understand how little kinetic energy the current adds?Little or big are relative.
Only about a part in a billion.
How do you get that number?
Post by: hamdani yusuf on 26/07/2022 14:32:17
A monatomic gas like neon is much simpler than the heterojunction between two layers of semiconductor in a laser diode.If the gases are cooled down to near abolute zero, will they still have negative temperature?
Is there a magnitude of negative temperature? Can an object have more negative temperature than another object? What would happen if they interact?
Post by: alancalverd on 26/07/2022 18:28:08
Have you ever taught a thermostat?The point at which I wanted it to switch the heater on or off. We summarised all the stuff about kinetic energy by using a bimetallic strip that bends according to the mean k.e. of the atoms inside two different bits of metal.
What did you teach it?
Post by: alancalverd on 26/07/2022 18:30:33
Or misunderstand it, depends on what was taught to them."Temperature is a measure of the average kinetic energy of the constituent atoms and molecules in a body". There being no other definition in my lifetime, that was always what was taught.
Post by: Bored chemist on 26/07/2022 19:52:51
How do you get that number?Like this
What's the proportion of transmitter power heating up the antenna compared to the power transmitting radio wave into space around it?Who cares?
Why do they care?
In an ideal world, no energy is dissipated as heat in the antenna, it's all transmitted.
In practice the reflected power may be larger than that lost to heat via resistance.I never measured them. Have you?I'm sensible enough to usually let other people do the actual measurements for me.
On the other hand, I did once do one of them.
Measuring the speed of sound in air is a high-school experiment.
https://www.bbc.co.uk/bitesize/guides/z8d2mp3/revision/3#:~:text=Clap%2Decho%20method,to%20the%20wall%20and%20back).
I'm pretty sure we got an answer near 330m/s.
And I know how sound works, so I know that the speed of the molecules in air is about the speed of sound.
And I understand the equipartition principle.
So I know that the thermal KE of the electrons in a wire is the same as that of the air molecules (at the same temperature as the wire).
And I know that an air molecule weighs about 60,000 times as much as an electron, so , since they have the same KE, the electrons must be travelling square root (60,000) times as fast.
That's about 80 km/s.
It's only an approximation, but it's good enough to make the point.
It's also fairly easy to find the drift speed of an electron.
https://en.wikipedia.org/wiki/Drift_velocity#Numerical_example
Tells you " the electrons are flowing at the rate of 23 μm/s".
So, now that you know that the current through a wire only changes the speeds of the electrons by a tiny fraction- about a part in a billion- do you see why it doesn't really matter if the transmitter is turned on or off?
More importantly, do you understand that if you actually knew science, you would already have known that?
Do you realise you wouldn't have saidElectrons on a radio transmission antenna or a power distribution transformer may have high kinetic energy, but we don't usually say that they have high temperature.
because you would have known that the use of electrons in an antenna hardly changes their KE at all.
Do you understand how, if you had spent time learning, you would ave avoided looking stupid?
Pay attention; you might learn something.
Post by: Bored chemist on 26/07/2022 19:55:32
If the gases are cooled down to near abolute zero, will they still have negative temperature?Could you at least try to think about your questions a bit before you ask them?
If you have something at a negative temperature, and you want to get to absolute zero you don't cool it down, you warm it up.
Post by: hamdani yusuf on 28/07/2022 02:50:33
Your definition of teaching is unconventional.Have you ever taught a thermostat?The point at which I wanted it to switch the heater on or off. We summarised all the stuff about kinetic energy by using a bimetallic strip that bends according to the mean k.e. of the atoms inside two different bits of metal.
What did you teach it?
Post by: hamdani yusuf on 28/07/2022 02:54:22
As you can see, not everyone here agreed with that definition.Or misunderstand it, depends on what was taught to them."Temperature is a measure of the average kinetic energy of the constituent atoms and molecules in a body". There being no other definition in my lifetime, that was always what was taught.
A resonating tuning fork has higher average kinetic energy than a silent tuning fork.
Post by: hamdani yusuf on 28/07/2022 04:17:06
So I know that the thermal KE of the electrons in a wire is the same as that of the air molecules (at the same temperature as the wire).You look careful with terminology. But you called for further questions. What does thermal KE means? If you say it's KE which affects temperature, then it creates a circular logic, thus meaningless.
Is there such a thing as thermal potential energy?
Post by: hamdani yusuf on 28/07/2022 04:21:00
So, now that you know that the current through a wire only changes the speeds of the electrons by a tiny fraction- about a part in a billion- do you see why it doesn't really matter if the transmitter is turned on or off?What makes you think that ideal gas model can accurately describes behavior of electrons in a conductor?
Where does most of power fed by radio transmitter to the antenna go?
Post by: Bored chemist on 28/07/2022 09:09:50
Any definition is unorthodox once you start applying it to teaching machines.Your definition of teaching is unconventional.Have you ever taught a thermostat?The point at which I wanted it to switch the heater on or off. We summarised all the stuff about kinetic energy by using a bimetallic strip that bends according to the mean k.e. of the atoms inside two different bits of metal.
What did you teach it?
And you did that.
You can't complain about it now.
Post by: Bored chemist on 28/07/2022 09:12:43
What makes you think that ideal gas model can accurately describes behavior of electrons in a conductor?The fact that the electron gas model is widely accepted and in broad agreement with experimental results.
https://en.wikipedia.org/wiki/Free_electron_model#Properties_of_an_electron_gas
Why do you ask?
Have you a better model?
Where does most of power fed by radio transmitter to the antenna go?If it is properly set up, most of the power is radiated away into space.
Ideally, all of it would be radiated.
The important bit is that practically none goes into increasing the KE of the electrons.
Post by: alancalverd on 28/07/2022 11:01:35
A resonating tuning fork has higher average kinetic energy than a silent tuning fork.And a tuning fork inside a rocket has more kinetic energy than one on the ground. Or maybe not, if you are in the rocket. According to Einstein, it's the one on the ground that is receding at umpteen mph and thus has more kinetic energy.
Hence the rather pedantic distinction between internal (thermal) and total (gross motion) kinetic energy. Physics is pedantic.
Post by: hamdani yusuf on 28/07/2022 15:09:00
Any definition is unorthodox once you start applying it to teaching machines.A more widely used word for machine learning is training. The machine is trained by feeding it data which are used to adjust its internal parameters until it reach desired behaviors. The process is similar to teaching kids, and less similar to designing thermostats.
And you did that.
You can't complain about it now.
Post by: hamdani yusuf on 28/07/2022 15:12:01
So, what kind of motion is needed to increase temperature of a tuning fork?A resonating tuning fork has higher average kinetic energy than a silent tuning fork.And a tuning fork inside a rocket has more kinetic energy than one on the ground. Or maybe not, if you are in the rocket. According to Einstein, it's the one on the ground that is receding at umpteen mph and thus has more kinetic energy.
Hence the rather pedantic distinction between internal (thermal) and total (gross motion) kinetic energy. Physics is pedantic.
Post by: hamdani yusuf on 28/07/2022 15:26:57
The fact that the electron gas model is widely accepted and in broad agreement with experimental results.
https://en.wikipedia.org/wiki/Free_electron_model#Properties_of_an_electron_gas
Why do you ask?
Have you a better model?
Read the article to the end. You'll find this part.
Quote
The free electron model presents several inadequacies that are contradicted by experimental observation.
No, I have no better model, yet. At least until I finish some of experiments.
Post by: hamdani yusuf on 28/07/2022 15:36:59
If it is properly set up, most of the power is radiated away into space.Which part of the antenna change when electric power is fed into it? Is it the electrons, or metal atoms, or both, or none?
Ideally, all of it would be radiated.
The important bit is that practically none goes into increasing the KE of the electrons.
If the electrons are prevented from moving along with the electric signal, will the antenna still radiate the power?
Post by: alancalverd on 28/07/2022 15:54:49
Wrong way round. If you heat the tuning fork, you will increase the mean kinetic energy of the atoms within it. In most cases this will increase the length of the arms and decrease its gross vibration frequency.So, what kind of motion is needed to increase temperature of a tuning fork?A resonating tuning fork has higher average kinetic energy than a silent tuning fork.And a tuning fork inside a rocket has more kinetic energy than one on the ground. Or maybe not, if you are in the rocket. According to Einstein, it's the one on the ground that is receding at umpteen mph and thus has more kinetic energy.
Hence the rather pedantic distinction between internal (thermal) and total (gross motion) kinetic energy. Physics is pedantic.
Post by: alancalverd on 28/07/2022 15:56:07
If the electrons are prevented from moving along with the electric signal, will the antenna still radiate the power?Obviously not, which is why we use conductors for antennas.
Post by: hamdani yusuf on 28/07/2022 16:33:23
Just stop the gas molecules from moving. What happens to the temperature?If the gases are cooled down to near abolute zero, will they still have negative temperature?Could you at least try to think about your questions a bit before you ask them?
If you have something at a negative temperature, and you want to get to absolute zero you don't cool it down, you warm it up.
Post by: hamdani yusuf on 28/07/2022 16:36:47
What kind of motion does the heated tuning fork have more than unheated one?Wrong way round. If you heat the tuning fork, you will increase the mean kinetic energy of the atoms within it. In most cases this will increase the length of the arms and decrease its gross vibration frequency.So, what kind of motion is needed to increase temperature of a tuning fork?A resonating tuning fork has higher average kinetic energy than a silent tuning fork.And a tuning fork inside a rocket has more kinetic energy than one on the ground. Or maybe not, if you are in the rocket. According to Einstein, it's the one on the ground that is receding at umpteen mph and thus has more kinetic energy.
Hence the rather pedantic distinction between internal (thermal) and total (gross motion) kinetic energy. Physics is pedantic.
Post by: alancalverd on 28/07/2022 16:45:19
Post by: hamdani yusuf on 28/07/2022 16:50:26
OK, let's wait and see if other members agree with us.If the electrons are prevented from moving along with the electric signal, will the antenna still radiate the power?Obviously not, which is why we use conductors for antennas.
Post by: hamdani yusuf on 28/07/2022 16:57:30
None, until you ping it. Then it vibrates, but slower.Let's be consistent with our definitions. Higher temperature tuning fork has higher internal kinetic energy. Hence it is related to motion of metal lattice atoms in the tuning fork.
"Temperature is a measure of the average kinetic energy of the constituent atoms and molecules in a body". There being no other definition in my lifetime, that was always what was taught.
Post by: Deecart on 28/07/2022 16:58:50
Quote
And a tuning fork inside a rocket has more kinetic energy than one on the ground. Or maybe not, if you are in the rocket. According to Einstein, it's the one on the ground that is receding at umpteen mph and thus has more kinetic energy.
Hence the rather pedantic distinction between internal (thermal) and total (gross motion) kinetic energy. Physics is pedantic.
I dont think so.
I am not a specialist but it is how i understand it :
External kinetic energy is some energy related to "something" and not related to "manything" (not billions particles)
Per example, a moving rocket, have or do not have, some additionnal kinetic energy, depending on the comparison point you choose (two rockets flying parallel with the same speed do not have kinetic energy regarding each other).
Because of the need of an external relativ state, we can call it "external".
Internal thermal kinetic is related to a statistic quantity (many particles. so we use the kinetic relativ of the particles relativ to the mean speed of the particles volume.)
Because of this "mean" reference we can call it "internal".
The reference frame is the volume itself (itself imply internal), and you can also do it with the rocket (because it is composed of billions of particles moving slightly using covalent bounds).
Post by: hamdani yusuf on 28/07/2022 17:04:08
Is there a magnitude of negative temperature? Can an object have more negative temperature than another object? What would happen if they interact?
Post by: alancalverd on 28/07/2022 18:26:35
Post by: hamdani yusuf on 29/07/2022 05:41:58
You still have not grasped the difference between internal kinesis and gross motion. This is worrying. By the age of about 6 months, a dog has a very clear concept of "inside" and takes care always to pee "outside".What's the difference again? In simple English please.
Post by: Bored chemist on 29/07/2022 10:32:04
What's the difference again? In simple English please.It seems that no English is simple enough for you.
You need to face the fact that you will never understand this.
Post by: alancalverd on 29/07/2022 14:06:05
Ask an English-speaking dog. Or a mathematician who can tell you all about bounded sets in language that is as complicated as you like.You still have not grasped the difference between internal kinesis and gross motion. This is worrying. By the age of about 6 months, a dog has a very clear concept of "inside" and takes care always to pee "outside".What's the difference again? In simple English please.
Here's an old mathematical joke.
How do you get an elephant into a jam jar?
Consider an elephant outside a jam jar, then perform a trivial inversion of vector space.
In physics, it's known as a Fourier transform and hugely useful in crystallography, medical imaging and communications engineering.
Post by: hamdani yusuf on 30/07/2022 07:23:37
You can say that X is Y, and then continue saying that Y is X, forming a circular logic. It doesn't solve your delusion problem either.
Post by: alancalverd on 30/07/2022 11:16:31
Post by: hamdani yusuf on 30/07/2022 15:50:22
I don't think that distinguishing between inside and outside is a circular argument. Though there is an ancient Greek story about a philosopher walking round a huge pillar whilst facing it, and wailing "I'm trapped".A spinning ball can have high kinetic energy, while maintaining its position in an inertial frame of reference. What makes you think that rotational energy as outside/external?
Post by: Bored chemist on 30/07/2022 18:04:13
We answered that 11 pages ago/I don't think that distinguishing between inside and outside is a circular argument. Though there is an ancient Greek story about a philosopher walking round a huge pillar whilst facing it, and wailing "I'm trapped".A spinning ball can have high kinetic energy, while maintaining its position in an inertial frame of reference. What makes you think that rotational energy as outside/external?
Quote from: hamdani yusuf on 19/03/2022 03:56:58It is sad, but it seems that you are really not able to keep enough facts in your head at the same time tounderstand temperature.
The question is, what distinguishes thermal energy from kinetic energy?
The motion having some sort of structure, rather than being random. Essentially, it's an effect of entropy.
As I said, you should forget about trying.
If you haven't got it sorted out after 12 pages of coaching, you probably never will.
Post by: hamdani yusuf on 30/07/2022 23:27:23
We answered that 11 pages ago/How would entropy relate total kinetic energy and temperature of an object? Is it simply a multiplication?
Quote from: Bored chemist on 19/03/2022 11:45:18
Quote from: hamdani yusuf on 19/03/2022 03:56:58
The question is, what distinguishes thermal energy from kinetic energy?
The motion having some sort of structure, rather than being random. Essentially, it's an effect of entropy.
How can we get negative temperature?
Is there a magnitude of negative temperature? Can an object have more negative temperature than another object? What would happen if they interact?
Post by: hamdani yusuf on 30/07/2022 23:34:49
What are the criteria to make it possible?QuoteHow is the temperature measured before reaching equilibrium?Sometimes you can do this spectroscopically.
Sometimes it's impossible.
Post by: hamdani yusuf on 30/07/2022 23:40:21
If you haven't got it sorted out after 12 pages of coaching, you probably never will.Here's what I've got in second page. How much further do I need to go?
Afaik, thermal energy has highest entropy among other kinds of energy, which makes it the most random form.
Black body radiation is the simplest spectral distribution of thermal electromagnetic radiation. The object's temperature can be calculated from the peak frequency. But most objects are not black body. Spectrum of low pressure gas has much different shape than black body radiation.
Post by: Bored chemist on 31/07/2022 10:42:48
How can we get negative temperature?I answered this earlier.
https://en.wikipedia.org/wiki/Negative_temperature#Lasers
As I said, it seems you can't keep an idea in your head long enough to learn about this sort of thing.
Give up; you are just wasting everyone's time including your own.
Post by: hamdani yusuf on 31/07/2022 18:02:55
I answered this earlier.If you think that you already understand something by simply pointing to an online article, which mentions it, you are likely being delusional.
If you really understand it, you should be able to answer these questions easily.
What are the criteria to make it possible?QuoteHow is the temperature measured before reaching equilibrium?Sometimes you can do this spectroscopically.
Sometimes it's impossible.
Post by: hamdani yusuf on 31/07/2022 18:03:31
Is there a magnitude of negative temperature? Can an object have more negative temperature than another object? What would happen if they interact?
Post by: Bored chemist on 31/07/2022 18:28:12
You have repeatedly demonstrated that you don't even understand the easy stuff.I answered this earlier.If you think that you already understand something by simply pointing to an online article, which mentions it, you are likely being delusional.
If you really understand it, you should be able to answer these questions easily.What are the criteria to make it possible?QuoteHow is the temperature measured before reaching equilibrium?Sometimes you can do this spectroscopically.
Sometimes it's impossible.
Why waste everyone's time with the more sophisticated bits?
Post by: hamdani yusuf on 01/08/2022 10:56:18
You have repeatedly demonstrated that you don't even understand the easy stuff.It's okay to say that you don't know. Pretending to know something that you don't know is not a scientific attitude.
Why waste everyone's time with the more sophisticated bits?
Post by: Bored chemist on 01/08/2022 12:04:31
What you said is true.You have repeatedly demonstrated that you don't even understand the easy stuff.It's okay to say that you don't know. Pretending to know something that you don't know is not a scientific attitude.
Why waste everyone's time with the more sophisticated bits?
However, it's unconnected to what you quoted me as saying.
Why did you quote me?
Post by: hamdani yusuf on 01/08/2022 13:25:59
What you said is true.Your comments seem to imply that you already understand something that I don't. But so far, you failed to answer some simple questions, like these.
However, it's unconnected to what you quoted me as saying.
Why did you quote me?
Is there a magnitude of negative temperature? Can an object have more negative temperature than another object? What would happen if they interact?
Post by: hamdani yusuf on 01/08/2022 13:27:01
Post by: hamdani yusuf on 01/08/2022 13:42:21
Post by: Bored chemist on 01/08/2022 13:47:00
But so far, you failed to answer some simple questions, like these.The questions are simple. the answers are not.
And, since you don't understand the simple stuff, you will not understand the complicated stuff.
Why would I bother posting a reply that you will never comprehend?
Post by: Bored chemist on 01/08/2022 13:48:34
How do we know the temperature of the cube?It obviously does not have one.
Bits are clearly much hotter than others.
Something like a thermal camera could give us an indication of the temperature distribution.
This is the sort of thing I mean when I say
you don't understand the simple stuff
Post by: hamdani yusuf on 01/08/2022 14:20:58
Post by: Bored chemist on 01/08/2022 14:26:01
Talking about heat shield, you might find this video quite informative.Yes. I discovered that they still talk about "friction" causing heating even though that's a minor part of the cause. Most is due to near adiabatic compression.
Also, they are wrong about another aspect.
He complains that a guy in a film falls from a spacecraft + burns up.
He says that's nonsense because the vehicle is moving along with the planet.
But he misses the point.
If you fall, you accelerate.
If you start from a spaceship, you aren't slowed down by the air (there isn't any).
So you end up going very fast indeed- well over the speed of sound.
And then you hit the air.
That air slows you down to terminal velocity and as it does so, the air ahead of you is compressed forming a shockwave. That heats it.
And that very hot air, just in front of you heats you up
Essentially, it's the same thing that he does talk about. As you hit the air, you have lots of energy (gravitational rather than kinetic). That energy has to be dissipated somehow (or you will crash).
And the only realistic fate for that energy is to be converted to heat.
That's what burns you up.
The effect was noticeable when Felix Baumgartner jumped from a balloon.
There's another point he glosses over.
Retrorockets have to point forwards.
That means you end up flying into the output from your rocket.
It's not a big deal in space because the gas is going much faster than you are so you never "hit" it.
But if you fire a retrorocket into air the hot exhaust gasses are slowed down a lot.
And that means you end up flying into the rocket exhaust.
You need roughly the same amount of heat shielding to address that problem as you do to shield you from the heat created by aerobraking.
He's sort of right. the very front surface of the blunt end of a re-entry vehicle is an aerobrake.
But you could make that from a sheet of metal. It doesn't need to be very strong (in the context of the vehicle weight, for example). The forces on it are , in principle, in simple compression. And, since the acceleration caused by the brakes is only of the order of a few G (1.7 G for the space shuttle, for example), the forces are only of the order of a few times the weight.
If it was't a heat shield, you could make it from 6mm aluminum sheet (properly braced) and it would probably be "over engineered".
But it does have to avoid being destroyed by the thermal radiation from the superheated air in front of it; and it also needs to protect the rest of the vehicle from that heat too.
So... it's a heat shield.
So, yes, I can learn a lot from that video but...
Post by: hamdani yusuf on 01/08/2022 14:27:53
The first two questions are just closed yes/no questions. You can answer them with yes or no. Or just say that you don't know.But so far, you failed to answer some simple questions, like these.The questions are simple. the answers are not.
And, since you don't understand the simple stuff, you will not understand the complicated stuff.
Why would I bother posting a reply that you will never comprehend?
The last question can be answered by saying if there will be energy transfer. If it is, the next required answer would be which way is the direction of the energy transfer.
If they are too complicated for you, I can't help but think that you don't really understand it.
Post by: Bored chemist on 01/08/2022 14:39:13
Post by: Bored chemist on 01/08/2022 14:43:32
Yes
That's clear from the article I cited; unfortunately, you don't understand it.
Can an object have more negative temperature than another object?
Yes; that's clear from the answer above.
What would happen if they interact?
What do you mean by "they"?
That's the sort of thing I mean when I say
Could you at least try to think about your questions a bit before you ask them?
Post by: alancalverd on 01/08/2022 14:53:18
Post by: hamdani yusuf on 02/08/2022 00:00:00
What would happen if they interact?Those two objects with negative temperature but different magnitude.
What do you mean by "they"?
Could you at least try to think about your questions a bit before you ask them?
Post by: Bored chemist on 02/08/2022 10:29:21
It depends on the objects and what sort of negative temperatures they have.What would happen if they interact?Those two objects with negative temperature but different magnitude.
What do you mean by "they"?Could you at least try to think about your questions a bit before you ask them?
Post by: hamdani yusuf on 03/08/2022 13:54:43
It depends on the objects and what sort of negative temperatures they have.How many kinds of negative temperature are there? What makes them different?
Post by: Bored chemist on 03/08/2022 14:27:05
In the ammonia maser the rotational (I think- might be something odd like a spin flip) temperature is negative.
In the hydrogen maser the electron spin temperature is negative.
There are other sorts too.
Post by: hamdani yusuf on 08/08/2022 03:35:34
Demonstrating the effect of emissivity on infrared thermal camera measurement.
Post by: hamdani yusuf on 08/08/2022 11:11:55
In a HeNe laser the electronic temperature is negative.Negative value can be assigned to a physical parameter if it negates or cancels out the positive value of the same physical parameter. Otherwise, it shouldn't be assigned a negative value.
In the ammonia maser the rotational (I think- might be something odd like a spin flip) temperature is negative.
In the hydrogen maser the electron spin temperature is negative.
There are other sorts too.
Post by: alancalverd on 08/08/2022 11:58:52
Post by: hamdani yusuf on 08/08/2022 12:13:09
Quote
NASA’s Cold Atom Lab aboard the International Space Station cools atoms down to a billionth of a degree above absolute zero, or the temperature at which atoms should stop moving entirely. Nowhere in the universe are there atoms that reach this temperature naturally. But how do scientists accomplish this feat? It’s a three-step process that starts with scientists hitting the atoms with precisely-tuned lasers to slow them down.
The colder atoms are, the slower they move, and the easier they are to study. Ultracold atoms can also form a fifth state of matter, called a Bose-Einstein condensate (BEC). Learning about the fundamental properties of atoms has laid the foundation for technologies that most of us use every day, such as computers. As the first ultracold atom facility in Earth orbit, Cold Atom Lab is opening up new avenues for investigation.
Here are some other videos explaining about laser cooling.
From 2:22 of the last video, we can infer that to make it work, the frequency of the laser is set to be slightly lower than transition frequency of the atoms to be cooled. Thus, a certain frequency of laser can cool some type of atoms, but heat other types of atoms instead. Which means that it doesn't always have negative temperature.
Post by: Bored chemist on 08/08/2022 12:49:24
Negative value can be assigned to a physical parameter if it negates or cancels out the positive value of the same physical parameter.So what?
Post by: Bored chemist on 08/08/2022 12:51:13
Which means that it doesn't always have negative temperature.No
You have missed the point.
It's sad, but no surprise.
You just don't seem to have what it takes to understand this.
You should probably stop wasting time trying.
The process called "laser cooling" but that is a misnomer.
You don't need a laser to do it.
Using a laser is by far the most sensible way but, in principle you could use a different light source.
So laser cooling has nothing to do with the negative temperature in a laser.
Post by: alancalverd on 08/08/2022 13:24:10
Post by: hamdani yusuf on 09/08/2022 03:01:31
Using a laser is by far the most sensible way but, in principle you could use a different light source.What other kinds of light can be used for cooling? Do you have any source?
Post by: hamdani yusuf on 09/08/2022 03:03:44
The concept of negative temperature derives from a definition of temperature in terms of entropy. All it means is a condition where adding energy to a system decreases its entropy.Do laser cutters decrease entropy?
Post by: hamdani yusuf on 09/08/2022 04:52:45
Gravitational potential is very real and always negative.
Quote
In classical mechanics, the gravitational potential at a location is equal to the work (energy transferred) per unit mass that would be needed to move an object to that location from a fixed reference location. It is analogous to the electric potential with mass playing the role of charge. The reference location, where the potential is zero, is by convention infinitely far away from any mass, resulting in a negative potential at any finite distance.When the reference location has negative gravitational potential, gravitational potential at some location can have positive value.
https://en.wikipedia.org/wiki/Gravitational_potential
Post by: hamdani yusuf on 09/08/2022 04:58:21
If it cannot negate positive value of the same parameter, it should not be assigned a negative value.Negative value can be assigned to a physical parameter if it negates or cancels out the positive value of the same physical parameter.So what?
Post by: hamdani yusuf on 09/08/2022 05:09:38
In a HeNe laser the electronic temperature is negative.What's the magnitude, in Kelvin?
Post by: alancalverd on 09/08/2022 10:42:20
Do laser cutters decrease entropy?no.
Post by: hamdani yusuf on 09/08/2022 11:02:56
So, they don't have negative temperature.Do laser cutters decrease entropy?no.
Post by: Bored chemist on 09/08/2022 16:05:44
But it can so... what was your point?If it cannot negate positive value of the same parameter, it should not be assigned a negative value.Negative value can be assigned to a physical parameter if it negates or cancels out the positive value of the same physical parameter.So what?
Post by: Bored chemist on 09/08/2022 16:07:54
I'd have to do lots of complicated maths but, for the sake of discussion, let's pretend that I have done it and the answer is minus 5000K.In a HeNe laser the electronic temperature is negative.What's the magnitude, in Kelvin?
What use are you going to make of that information?
Because, if you don't have a good answer, I'm going to take it as further proof that you are a troll.
It will prove that you were just trying to trick me into doing lots of pointless work.
Post by: Bored chemist on 09/08/2022 16:09:52
I already explained that there's nothing magical about lasers.So, they don't have negative temperature.Do laser cutters decrease entropy?no.
You can use a focused beam of sunlight to cut stuff with.
No laser required; no negative temperature required.
There's also nothing special about lasers for cooling.
Post by: hamdani yusuf on 09/08/2022 23:20:14
There's also nothing special about lasers for cooling.
How to cool things using light other than laser?Using a laser is by far the most sensible way but, in principle you could use a different light source.What other kinds of light can be used for cooling? Do you have any source?
Post by: hamdani yusuf on 09/08/2022 23:46:38
I'd have to do lots of complicated maths but, for the sake of discussion, let's pretend that I have done it and the answer is minus 5000K.It seems like you are trying so hard to look like you know more things than you actually do.
What use are you going to make of that information?
Because, if you don't have a good answer, I'm going to take it as further proof that you are a troll.
It will prove that you were just trying to trick me into doing lots of pointless work.
I wanted to verify the justification for assigning a negative value for temperature of laser. If in an isolated container an object with the same heat capacity but have positive 5000K temperature is let to exchange heat with the object having negative 5000K temperature, the result is expected to be objects at 0K. If that's not the case, then saying that the laser has negative 5000K is erroneous in the first place.
Post by: Bored chemist on 10/08/2022 09:17:53
with the same heat capacityHeat capacity isn't well defined here.
The heat capacity depends on the available degrees of freedom.
Since, in the case of the negative electron temperature in lasers we are talking about the heat capacity of a specific electronic transition it's not gong to be possible to get equilibrium within that transition without getting a more general shift towards equipartition.
It seems like you are trying so hard to look like you know more things than you actually do.I do know the stuff; I just know that there's not much point trying to explain it to you because you don't understand and or don't listen.
If that's not the case, then saying that the laser has negative 5000K is erroneous in the first place.or it means that your understanding is faulty.
In principle, what you actually get is an infinitely negative temperature.
Please try to realise that it isn't my fault that you do not understand this.
I am not your teacher.
Post by: Bored chemist on 10/08/2022 09:18:45
How to cool things using light other than laser?The same way that you would do it using a laser, but using a different light source.
The fact that you ask this proves that you don't understand how laser cooling works.
Post by: alancalverd on 11/08/2022 22:35:15
Now introduce an energetic dog who rounds them up into a pen, where they all stand still and face the same way. He has done work and reduced their entropy.
Therefore if you define temperature in terms of entropy, T = (dS/dE)-1, he has reduced the temperature of the sheep by doing work on them, so Tsheep is negative.
Post by: hamdani yusuf on 12/08/2022 09:56:03
Is there any report of the experiment? Or is it just your hypothesis?How to cool things using light other than laser?The same way that you would do it using a laser, but using a different light source.
The fact that you ask this proves that you don't understand how laser cooling works.
Post by: Bored chemist on 12/08/2022 12:52:17
Or is it just your hypothesis?No, it's a deduction.
There is no way for the atoms being cooled to know if the photons hitting them came from a laser or from some other source, is there?
So the idea that they might behave differently is a bit silly, isn't it?
So, once again, we are into the territory of you asking questions that would not be asked by anyone who understood the science.
so, once again, you are only proving your own lack of understanding.
Why do you keep doing this?
Post by: hamdani yusuf on 12/08/2022 16:59:28
No, it's a deduction.Until it's confirmed experimentally, your deduction will remain as a hypothesis.
There is no way for the atoms being cooled to know if the photons hitting them came from a laser or from some other source, is there?What are the differences between laser and other light sources?
So the idea that they might behave differently is a bit silly, isn't it?
Quote
A laser differs from other sources of light in that it emits light which is coherent. Spatial coherence allows a laser to be focused to a tight spot, enabling applications such as laser cutting and lithography. Spatial coherence also allows a laser beam to stay narrow over great distances (collimation), enabling applications such as laser pointers and lidar (light detection and ranging). Lasers can also have high temporal coherence, which allows them to emit light with a very narrow spectrum. Alternatively, temporal coherence can be used to produce ultrashort pulses of light with a broad spectrum but durations as short as a femtosecond.
https://en.m.wikipedia.org/wiki/Laser
Post by: Bored chemist on 12/08/2022 17:14:41
What are the differences between laser and other light sources?The difference is (1) irrelevant, and that's the point (2) the way in which the light is produced- by stimulated, rather than spontaneous emission.
Until it's confirmed experimentally, your deduction will remain as a hypothesis.Not really, no.
We know from other experiments that a photon is a photon is a photon.
If it has the same wavelength and polarisation it will act the same as any other photon with that wavelength and polarisation.
Did you not realise this?
I tried to explain it to you.
"There is no way for the atoms being cooled to know if the photons hitting them came from a laser or from some other source"
But it seems the point went over your head.
Post by: Bored chemist on 12/08/2022 17:30:03
A laser differs from other sources of light in that it emits light which is coherent.Again, this is in the "lies we tell to children" category.
Unlike "laser" cooling, in the field of holography you actually need to concern yourself with coherence.
You can't really make holograms without coherent radiation. (Ok, technically, you can but it's doing things the hard way)
And yet, Gabor invented and demonstrated holography without using a laser.
https://en.wikipedia.org/wiki/Dennis_Gabor
So we know that the wiki article isn't entirely correct.
However, it is nearly correct.
By far the easiest way to get coherent light is to use a laser.
It would be very unusual to use any other source of coherent radiation in an experiment today.
But, here's the point you missed.
You do not need coherence to do "laser" cooling.
So why did you quote the (not entirely accurate) wiki article about something irrelevant?
Are you trolling, or did you just not understand?
Post by: hamdani yusuf on 12/08/2022 17:39:06
https://www.esa.int/gsp/ACT/projects/electroluminescent_cooling_using_LEDs/
Quote
Electroluminescent cooling using light-emitting diodesThe main difference from laser cooling, is the light cools the LED body, instead of the cooled gas molecules. Have you ever thought why can't we just simply replace the lasers in laser cooling with LED or other incoherent light sources?
There is a multitude of areas where sub-zero temperatures are required in space, from the storage of lunar material [1] to the cooling of infrared (IR) cameras and other sensors [2]. Because the transportation of equipment and materials from Earth results in huge costs, with an estimated price of around 500 000 €/kg to the Moon in 2008 [3], lightweight cooling systems are highly sought [1].
For almost 60 years [4], we have known that when an electron-hole pair recombines in certain light-emitting diodes (LEDs), the energy of the emitted photon (light, energy Eout = hν) can exceed the energy of the injected charge carriers (applied current, energy Ein = qV). For this phenomenon, hν > qV, to make physical sense, a portion of the energy of the photon must be taken from somewhere - in this case phonons (heat) in the crystal lattice. The result is that the LED can function as a cooling element, drawing heat from the crystal. In this situation, the wall-plug efficiency (WPE), defined as the ratio of the total optical output power to the input electrical power and therefore also called the electrical-to-optical power-conversion efficiency, has a value above unity.
Electroluminescent cooling (ELC) is only feasible, however, if the combined cooling effect from all radiative recombination events exceeds the heating caused by all parasitic, non-radiative recombination events. For every time an injected electron fails to recombine with a hole or a photon is absorbed in the structure, heat is produced.
Only if the external quantum efficiency (EQE, the ratio of successfully extracted photons to injected charge carriers) of the LED is close to unity or the photon energy hν is considerably higher than the applied voltage qV to the LED, the WPE = EQE × ( hν / qV ) can exceed unity [5].
Although the theoretical potential of ELC has been known for a long time [6], it was only recently that ELC was demonstrated in the laboratory - although for extremely low cooling powers [5]. With the increase in the efficiencies of LEDs in general, several groups have started looking into the use of ELC for practical purposes [7-10].
Post by: Bored chemist on 12/08/2022 18:53:31
Have you ever thought why can't we just simply replace the lasers in laser cooling with LED or other incoherent light sources?Yes, I have.
I didn't need to think about it for very long before I realised the answer.
Now it's your turn.
Can you think of some property of laser light that might explain why lasers are typically used for cooling and other light sources are not?
Here's a hint
Nd YAG 1064 nm
NeNe 632.8nm
HeCd 422 nm
Post by: hamdani yusuf on 12/08/2022 23:41:53
Not really, no.Photon model can explain some experimental results, but has difficulties to explain some others. We should not take it as a complete representation of physical reality, and assume that it will produce correct predictions for experiments yet to be done.
We know from other experiments that a photon is a photon is a photon.
If it has the same wavelength and polarisation it will act the same as any other photon with that wavelength and polarisation.
Post by: hamdani yusuf on 12/08/2022 23:45:18
Yes, I have.What's the answer?
I didn't need to think about it for very long before I realised the answer.
Post by: hamdani yusuf on 12/08/2022 23:58:54
Can you think of some property of laser light that might explain why lasers are typically used for cooling and other light sources are not?Laser frequency and wavelength can be tuned at will, by changing the length of light-emitting cavity or its temperature.
Here's a hint
Nd YAG 1064 nm
NeNe 632.8nm
HeCd 422 nm
Temperature can also change frequency of LED.
Incandescent light produces continuous spectrum. Prism or diffraction grating can be used to select desired frequency.
Post by: Bored chemist on 13/08/2022 01:14:01
Good, you are apparently learning.Can you think of some property of laser light that might explain why lasers are typically used for cooling and other light sources are not?Laser frequency and wavelength can be tuned at will, by changing the length of light-emitting cavity or its temperature.
Here's a hint
Nd YAG 1064 nm
NeNe 632.8nm
HeCd 422 nm
Temperature can also change frequency of LED.
Incandescent light produces continuous spectrum. Prism or diffraction grating can be used to select desired frequency.
I wonder how long it will last.
Post by: Bored chemist on 13/08/2022 01:14:19
but has difficulties to explain some others.Which?
Post by: Bored chemist on 13/08/2022 01:17:32
In order to get "laser" cooling to work, you need to produce a beam of light with a very carefully defined wavelength.Yes, I have.What's the answer?
I didn't need to think about it for very long before I realised the answer.
That's relatively easy with a tuneable laser.
You can do it with a conventional light source and a monochromator and collimator , but it's horribly inefficient.
But, in principle, you can do it.
So, do you now (a few days later than everyone else) understand that you do not need a laser to produce that sort of cooling, so that sort of cooling can't be anything to do with a negative electronic temperature in a laser?
Post by: hamdani yusuf on 13/08/2022 04:33:00
Diffraction, interference, polarization, and refraction are better explained using wave model.but has difficulties to explain some others.Which?
Post by: hamdani yusuf on 13/08/2022 04:55:27
You can do it with a conventional light source and a monochromator and collimator , but it's horribly inefficient.Yet no one has come up with experimental results to determine if your principle is correct.
But, in principle, you can do it.
Post by: alancalverd on 13/08/2022 11:33:08
Like the man said, if you are trying to efficiently stimulate a quantum phenomenon, you need to use tightly specified photons. LED spectra are fairly narrow but difficult to modify, unlike a tuneable laser.
Post by: Bored chemist on 13/08/2022 12:18:02
No.You can do it with a conventional light source and a monochromator and collimator , but it's horribly inefficient.Yet no one has come up with experimental results to determine if your principle is correct.
But, in principle, you can do it.
Because every single experiment with light- all of them- every single one- has shown that you can change the light source and not affect the outcome as long as you maintain the polarisation, intensity and spectrum.
Every experiment where someone first did it with candle light or sunlight and which has subsequently been repeated using artificial light is a demonstration that your bizarre idea is wrong.
Post by: hamdani yusuf on 15/08/2022 07:26:59
Every experiment where someone first did it with candle light or sunlight and which has subsequently been repeated using artificial light is a demonstration that your bizarre idea is wrong.Why can't I find any link to the report?
Post by: Bored chemist on 15/08/2022 08:54:35
Because you are the only person who thinks it is plausible to write a report on every single instance of an observation of Youngs slits (for example) done using a tungsten lamp or an LED )or anything other than the daylight which Young used).Every experiment where someone first did it with candle light or sunlight and which has subsequently been repeated using artificial light is a demonstration that your bizarre idea is wrong.Why can't I find any link to the report?
How would you go about writing a report on an experiment done in every high-school physics class?
Did you not understand that?
Every time someone uses some other sort of light source, and gets the same result, they prove that a photon doesn't "remember" what source it came from.
How did you imagine that they might?
Did you think photons carried notebooks?
This is what I mean when I say you need to learn some science; it avoids you saying silly things like that.
Post by: hamdani yusuf on 16/08/2022 05:04:18
Because you are the only person who thinks it is plausible to write a report on every single instance of an observation of Youngs slits (for example) done using a tungsten lamp or an LED )or anything other than the daylight which Young used).If something is important enough or interesting enough, someone somewhere will write something about it. I think it's an interesting phenomenon, if it's true though.
How would you go about writing a report on an experiment done in every high-school physics class?
Post by: hamdani yusuf on 16/08/2022 05:06:21
Every time someone uses some other sort of light source, and gets the same result, they prove that a photon doesn't "remember" what source it came from.Most of the time, shining light on an object would increase its temperature instead of decreasing it.
Post by: hamdani yusuf on 16/08/2022 05:13:48
How did you imagine that they might?How do you think that photon can go backward in time like in quantum eraser experiment? Or be in two places at once?
Did you think photons carried notebooks?
You can accept that weird things actually happened in an observed phenomenon, or modify the model you use to describe it by changing one or more assumptions to make it less weird.
Post by: hamdani yusuf on 16/08/2022 05:19:15
This is what I mean when I say you need to learn some science; it avoids you saying silly things like that.I learn science because it's necessary for achieving the universal terminal goal. Any other things are secondary bonus.
Quote
Philosophy of Science - Replication
This video examines some of the challenges involved in determining whether or not an experimental result has been replicated.
-- Collins, Harry. (1985). Changing Order: Replication and Induction in Scientific Practice. London: Sage.
-- Norton, John. (2015). "Replicability of experiment." Theoria 30(2): 229-248. https://sites.pitt.edu/~jdnorton/pape...
-- Popper, Karl. (1959). The Logic of Scientific Discovery. New York: Harper & Row.
-- Ramscar, Michael. "The unspeakable in pursuit of the unrepeatable." https://ramscar.wordpress.com/2015/08...
0:00 - Introduction
2:49 - Two types of replication
8:19 - Seven steps to replication
10:13 - 1 What is the subject matter?
13:37 - 2 What is science?
14:59 - 3 Identity of the experimenter
18:03 - 4 What is an experiment?
24:54 - 5 Is the experiment a competent copy?
32:29 - 6 Is the result positive?
40:29 - 7 Replication
Post by: Bored chemist on 16/08/2022 08:56:31
Most of the time, shining light on an object would increase its temperature instead of decreasing it.Nobody has ever suggested anything else, have they.
So that was s silly thing to say, wasn't it?
Post by: Bored chemist on 16/08/2022 08:59:35
If something is important enough or interesting enough, someone somewhere will write something about it.So, you think " I'm a physics teacher; today in class I demonstrated that the double slit experiment worked- just the same as it has for the last hundred years" is important enough, or interesting enough to get written up?
Really?
You think that thousands of school repetitions of an experiment will get published?
Why would anyone bother?
What's "important enough or interesting enough" about it?
Post by: Bored chemist on 16/08/2022 09:00:39
I learn scienceNo you do not.
That's why we have to keep repeating stuff, and explaining it to you repeatedly.
Post by: alancalverd on 17/08/2022 23:50:54
Obviously you can't precisely replicate an experiment.The stars have moved in the firmament and some of the original atoms have disintegrated. But that isn't the point. The questions scientists ask are "does the experiment support the hypothesis?" and "are the results sufficiently robust to invest money or entrust lives to their application?" So for the most part we are interested either in partial replication with known perturbations (test of robustness) , or reasonable approximations to the original experiment (trying to minimise random errors and systematic uncertainties). And just occasionally we ask "has he made a serious mistake, is he lying, or have we really been getting it wrong for the last 20 years?" To which the answer, in my experience, is "sometimes", "rarely", and "surprisingly often". But you don't usually need precise replication to come to those conclusions.
Post by: hamdani yusuf on 18/08/2022 05:15:57
If facts sound silly to you, maybe there's something wrong with you.Most of the time, shining light on an object would increase its temperature instead of decreasing it.Nobody has ever suggested anything else, have they.
So that was s silly thing to say, wasn't it?
Post by: hamdani yusuf on 18/08/2022 05:17:55
Repeating false things doesn't make them any truer.I learn scienceNo you do not.
That's why we have to keep repeating stuff, and explaining it to you repeatedly.
Post by: Bored chemist on 18/08/2022 07:04:28
If facts sound silly to you, maybe there's something wrong with you.There's such a thing as context.
If I ask for directions to the railway station, and you tell me that the capital of Peru is Lima, you sound silly, don't you?
Nobody had suggested anything other than the idea that shining light on something normally makes it hotter, had they?
Repeating false things doesn't make them any truer.Again, nobody had said otherwise, had they?
Post by: hamdani yusuf on 18/08/2022 12:11:55
In principle, what you actually get is an infinitely negative temperature.Doesn't this sound silly to you?
Post by: alancalverd on 18/08/2022 15:04:11
It happens, for perfectly good reasons, that the dimensions of torque and energy are the same, but we treat them as different quantities, and only measure torque.
Post by: Bored chemist on 18/08/2022 18:12:43
Well, that depends.In principle, what you actually get is an infinitely negative temperature.Doesn't this sound silly to you?
Are you answering a pointless question ,which the questioner has already said they can't think of a use for, or are you trying to achieve something?
If all I'm doing is answering a question from some guy on the internet who can't really give a good reason for his questions then, it's not that silly; there's some hope the guy might learn from it.
If it's matter of trying to make practical use of an infinitely negative electronic temperature then, yes, it's a bit daft.
Have you now realised why I was reluctant to waste time on the calculation?
In particular, have you now realise that this was wrong?
It seems like you are trying so hard to look like you know more things than you actually do.
It's not that I don't know the answer; it's that i know that the answer isn't going to be much use.
Post by: Bored chemist on 18/08/2022 18:14:24
It happens, for perfectly good reasons, that the dimensions of torque and energy are the same, but we treat them as different quantities, and only measure torque.I'm sure I heard some wacky story somewhere of some group trying to measure energy.
I think their problem was they failed to ask a chemist.
Post by: alancalverd on 18/08/2022 18:24:21
I know how to calculate all sorts of things that involve energy and energy transfer, but I can only sort-of-directly measure the energy of a photon.
Post by: Bored chemist on 18/08/2022 18:35:26
How to measure energy?
Ask the experts
https://energysavingtrust.org.uk/advice/guide-to-smart-meters
Well maybe not quite "experts"...
:-)
Post by: alancalverd on 18/08/2022 20:08:16
I did indeed have a chemical power integrator some years ago but it wasn't particularly accurate. Quite handy and a lot of fun as a low-power run-time integrator with a moving color band.
Post by: Bored chemist on 18/08/2022 22:37:09
to give you an inflated ideaIn what way?
I accept that the supply company rips you off, but that's different.
Post by: hamdani yusuf on 19/08/2022 09:00:12
Here are some videos about negative temperature.This video shows what makes laser different than other sources of light.
Post by: Bored chemist on 19/08/2022 10:26:50
This video shows what makes laser different than other sources of light.We already know.
Why post the vid?
Post by: hamdani yusuf on 19/08/2022 23:45:06
It contradicts your statement.This video shows what makes laser different than other sources of light.We already know.
Why post the vid?
Post by: alancalverd on 19/08/2022 23:57:31
I've just watched that last video. If that is the quality of modern physics teaching, the next generation is doomed to a life of ignorance and superstition.
Post by: hamdani yusuf on 21/08/2022 03:27:36
It can not be done if the source is incoherent. That's why focusing sunlight can't get higher temperature than surface of the sun.
Post by: hamdani yusuf on 21/08/2022 03:31:06
The source may well be different, but a photon is a photon.And it's just a mathematical model. No one has isolated it, AFAIK.
Post by: alancalverd on 21/08/2022 10:30:14
Post by: hamdani yusuf on 21/08/2022 12:30:26
I have equipment that counts them individually.How do you know that it does indeed count a single photon, not less nor more, and not counting something else?
Post by: Bored chemist on 21/08/2022 18:57:01
AFAIK.That is the essence of the problem with many of your posts.
You just don't K very F because you fail to learn.
Not as far as I can tell.It contradicts your statement.This video shows what makes laser different than other sources of light.We already know.
Why post the vid?
Which bit is troubling you?
Post by: Bored chemist on 21/08/2022 19:02:37
The most distinctive feature of laser over the other light sources is coherence.I take it you didn't understand this.
You can't really make holograms without coherent radiation. (Ok, technically, you can but it's doing things the hard way)
And yet, Gabor invented and demonstrated holography without using a laser.
https://en.wikipedia.org/wiki/Dennis_Gabor
Post by: alancalverd on 21/08/2022 19:35:19
Something to do with quantum mechanics, I guess. And the fact that the counting stops when I switch off the source.I have equipment that counts them individually.How do you know that it does indeed count a single photon, not less nor more, and not counting something else?
Post by: hamdani yusuf on 22/08/2022 06:06:13
AFAIK.That is the essence of the problem with many of your posts.
You just don't K very F because you fail to learn.Not as far as I can tell.It contradicts your statement.This video shows what makes laser different than other sources of light.We already know.
Why post the vid?
Which bit is troubling you?
why focusing sunlight can't get higher temperature than surface of the sun.Why focusing laser can?
Post by: hamdani yusuf on 22/08/2022 06:10:06
Do you understand the difference between hard and impossible?The most distinctive feature of laser over the other light sources is coherence.I take it you didn't understand this.You can't really make holograms without coherent radiation. (Ok, technically, you can but it's doing things the hard way)
And yet, Gabor invented and demonstrated holography without using a laser.
https://en.wikipedia.org/wiki/Dennis_Gabor
Post by: hamdani yusuf on 22/08/2022 06:11:25
Does temperature of the detector affect the counter?Something to do with quantum mechanics, I guess. And the fact that the counting stops when I switch off the source.I have equipment that counts them individually.How do you know that it does indeed count a single photon, not less nor more, and not counting something else?
Post by: Bored chemist on 22/08/2022 09:55:44
I didn't say anything about the temperature you can get by focussing a laser or the sun.AFAIK.That is the essence of the problem with many of your posts.
You just don't K very F because you fail to learn.Not as far as I can tell.It contradicts your statement.This video shows what makes laser different than other sources of light.We already know.
Why post the vid?
Which bit is troubling you?why focusing sunlight can't get higher temperature than surface of the sun.Why focusing laser can?
How did you come to the conclusion that the video contradicts what I said?
Post by: Bored chemist on 22/08/2022 10:00:20
Do you understand the difference between hard and impossible?es, in this case "difficult" means constructing a hologram by computing the array of light and dark patches you need to form a hologram and then somehow "panting" them onto something.
on the other hand, "impossible" is making a hologram in the "normal" way - i.e. using an interference pattern to make that pattern.
Why is it that, whenever you don't understand something, you assume that it's me who doesn't understand?
It's impossible to make "conventional" holograms without coherent light.
Gabor did it without a laser.
Therefore it is possible to get coherent light without a laser.
Therefore the wiki article isn't quite accurate.
Post by: hamdani yusuf on 22/08/2022 11:07:33
I didn't say anything about the temperature you can get by focussing a laser or the sun.
How did you come to the conclusion that the video contradicts what I said?
There's also nothing special about lasers for cooling.
How to cool things using light other than laser?The same way that you would do it using a laser, but using a different light source.
The fact that you ask this proves that you don't understand how laser cooling works.
Post by: alancalverd on 22/08/2022 12:25:00
Does temperature of the detector affect the counter?
Not much. Thermal noise is all at quite low energy compared with an x-ray photon.
Post by: Bored chemist on 22/08/2022 12:36:09
Do you realise that heating is not the same as cooling?I didn't say anything about the temperature you can get by focussing a laser or the sun.
How did you come to the conclusion that the video contradicts what I said?There's also nothing special about lasers for cooling.How to cool things using light other than laser?The same way that you would do it using a laser, but using a different light source.
The fact that you ask this proves that you don't understand how laser cooling works.
Post by: hamdani yusuf on 23/08/2022 08:42:09
But the effect does exist.Does temperature of the detector affect the counter?
Not much. Thermal noise is all at quite low energy compared with an x-ray photon.
Post by: hamdani yusuf on 23/08/2022 09:04:21
Do you realise that heating is not the same as cooling?Heating increases temperature, cooling decreases temperature.
Post by: alancalverd on 23/08/2022 11:30:03
Yes. It's all in the electronics textbooks, which is why some photon counters, particularly those looking for low-energy photons, are cooled. Less of a problem with medical x and γ radiations.But the effect does exist.Does temperature of the detector affect the counter?
Not much. Thermal noise is all at quite low energy compared with an x-ray photon.
Post by: Sheilataylor on 23/08/2022 12:18:20
Exactly, how simple but at the same time how hard.Do you realise that heating is not the same as cooling?Heating increases temperature, cooling decreases temperature.
Post by: Bored chemist on 23/08/2022 12:53:54
So... you do understand that the heating effect you from from focussing sunlight is nothing to do with the "laser" cooling effect, don't you?Do you realise that heating is not the same as cooling?Heating increases temperature, cooling decreases temperature.
Post by: Bored chemist on 23/08/2022 12:56:39
Several effects- all perfectly well explained and catalogued - exist.But the effect does exist.Does temperature of the detector affect the counter?
Not much. Thermal noise is all at quite low energy compared with an x-ray photon.
https://en.wikipedia.org/wiki/Shot_noise
https://en.wikipedia.org/wiki/Johnson%E2%80%93Nyquist_noise
https://en.wikipedia.org/wiki/Flicker_noise
So what?
Post by: hamdani yusuf on 24/08/2022 04:38:35
So... you do understand that the heating effect you from from focussing sunlight is nothing to do with the "laser" cooling effect, don't you?You said that photon from laser is indistinguishable from other sources, wich make them equally good for cooling. The video shows that they are different.
Post by: hamdani yusuf on 24/08/2022 04:40:27
Several effects- all perfectly well explained and catalogued - exist.So you can't claim that when the detector registers a spike, it means that a photon is just hitting it.
https://en.wikipedia.org/wiki/Shot_noise
https://en.wikipedia.org/wiki/Johnson%E2%80%93Nyquist_noise
https://en.wikipedia.org/wiki/Flicker_noise
So what?
Post by: Bored chemist on 24/08/2022 08:49:52
It doesn't show that they are different in a relevant way, does it?So... you do understand that the heating effect you from from focussing sunlight is nothing to do with the "laser" cooling effect, don't you?You said that photon from laser is indistinguishable from other sources, wich make them equally good for cooling. The video shows that they are different.
You could still use a non-laser light source for cooling. (I didn't say they were "good" for it, did I?. I said they were not good.You made that up. Why did you do that?)
The point I was making was that laser cooling has nothing to do with the negative electronic temperature in a laser.
Do you understand that?
Incidentally, the video is wrong. The "explanation" about lenses is irrelevant if you use non-imaging optics.
Post by: hamdani yusuf on 25/08/2022 07:48:46
It doesn't show that they are different in a relevant way, does it?What do you mean by relevant?
You could still use a non-laser light source for cooling. (I didn't say they were "good" for it, did I?. I said they were not good.You made that up. Why did you do that?)What makes them less good for cooling?
Post by: hamdani yusuf on 25/08/2022 08:52:42
The point I was making was that laser cooling has nothing to do with the negative electronic temperature in a laser.Why is electronic temperature in a laser said to be negative?
Post by: Bored chemist on 25/08/2022 10:49:57
Because it is.The point I was making was that laser cooling has nothing to do with the negative electronic temperature in a laser.Why is electronic temperature in a laser said to be negative?
There are more electrons in an upper state than in a lower one.
This still has nothing to do with "laser cooling".
Post by: Bored chemist on 25/08/2022 10:55:13
What do you mean by relevant?I mean relevant.
The differences between laser- and conventional- light are not relevant to the use of light to produce cooling.
For example:
You do not need a temperature inversion to produce cooling.
You do not need coherence to produce cooling (and you do not need a laser to produce coherence anyway.)
Why don't you try thinking about what things mean rather than asking about obvious stuff?
Post by: Bored chemist on 25/08/2022 10:56:01
What makes them less good for cooling?Why not try to work this out for yourself?
Or are you not going to do that, because it would require you to actually learn a bit of science?
Post by: hamdani yusuf on 27/08/2022 04:23:54
It sounds more like undefined or indeterminate.Because it is.The point I was making was that laser cooling has nothing to do with the negative electronic temperature in a laser.Why is electronic temperature in a laser said to be negative?
There are more electrons in an upper state than in a lower one.
This still has nothing to do with "laser cooling".
How does this negative electronic temperature affects the temperature of the container of laser medium? Is it cooled down, or heated up instead?
Post by: hamdani yusuf on 27/08/2022 04:28:32
What are the necessary conditions for a light beam to produce cooling effect on an object it's shone on?What do you mean by relevant?I mean relevant.
The differences between laser- and conventional- light are not relevant to the use of light to produce cooling.
For example:
You do not need a temperature inversion to produce cooling.
You do not need coherence to produce cooling (and you do not need a laser to produce coherence anyway.)
Post by: hamdani yusuf on 27/08/2022 04:33:38
I'll try to figure it out through experiments when I have the time. There are still many others in my list.What makes them less good for cooling?Why not try to work this out for yourself?
Or are you not going to do that, because it would require you to actually learn a bit of science?
Demonstration videos would also help to reduce efforts. If someone here has the links, I'll be thankful if you can share them here.
Post by: Bored chemist on 27/08/2022 12:23:39
It sounds more like undefined or indeterminate.To whom?
What are the necessary conditions for a light beam to produce cooling effect on an object it's shone on?They are the conditions which people arrange when they wish to perform laser cooling experiments.
If you see how they do those experiments, you should be able to work out what the conditions are.
I'll try to figure it out through experiments when I have the time.I'm not expecting you to do experiments; I'm hoping you will think.
Post by: hamdani yusuf on 28/08/2022 12:34:43
To anyone who wants to understand how temperature emerges from microscopic interactions.It sounds more like undefined or indeterminate.To whom?
Post by: hamdani yusuf on 28/08/2022 12:35:52
They are the conditions which people arrange when they wish to perform laser cooling experiments.Do you know? What are they?
If you see how they do those experiments, you should be able to work out what the conditions are.
Post by: Bored chemist on 28/08/2022 13:34:18
Do you know?Yes.
That's why I can tell you that you don't need a laser.
What are they?
I'm hoping you will think.
Post by: hamdani yusuf on 29/08/2022 08:57:48
It makes your "knowledge" unfalsifiable, hence unscientific.Do you know?Yes.
That's why I can tell you that you don't need a laser.What are they?I'm hoping you will think.
Post by: Bored chemist on 29/08/2022 10:21:39
It makes your "knowledge" unfalsifiable, hence unscientific.How did you come to that odd conclusion?
Me telling you to look something up rather than spoon-feeding you the information doesn't make it unscientific; it makes it educational.
I'm hoping that you will become accustomed to thinking for yourself.
Post by: hamdani yusuf on 29/08/2022 14:43:26
By not specifying your knowledge, you protect yourself from being contradicted by experimental results.It makes your "knowledge" unfalsifiable, hence unscientific.How did you come to that odd conclusion?
Me telling you to look something up rather than spoon-feeding you the information doesn't make it unscientific; it makes it educational.
I'm hoping that you will become accustomed to thinking for yourself.
Post by: Bored chemist on 29/08/2022 15:45:14
By not specifying your knowledge, you protect yourself from being contradicted by experimental results.My knowledge is generally based on experimental results so your idea makes no sense, does it?
How could the experiments contradict themselves?
Post by: hamdani yusuf on 31/08/2022 06:09:43
You can't contradict anything which is not specified.By not specifying your knowledge, you protect yourself from being contradicted by experimental results.My knowledge is generally based on experimental results so your idea makes no sense, does it?
How could the experiments contradict themselves?
Post by: Bored chemist on 31/08/2022 08:51:16
You can't contradict anything which is not specified.Nobody said that you could.
Post by: hamdani yusuf on 01/09/2022 13:13:06
One experimental results might be interpreted by several different theories. You didn't specify which theory you accept.By not specifying your knowledge, you protect yourself from being contradicted by experimental results.My knowledge is generally based on experimental results so your idea makes no sense, does it?
How could the experiments contradict themselves?
You don't always agree with Google nor Wikipedia. How do we know which one is the correct answer? Do you think you're always right?It makes your "knowledge" unfalsifiable, hence unscientific.How did you come to that odd conclusion?
Me telling you to look something up rather than spoon-feeding you the information doesn't make it unscientific; it makes it educational.
I'm hoping that you will become accustomed to thinking for yourself.
Post by: Bored chemist on 01/09/2022 13:55:22
Do you think you're always right?No
How do we know which one is the correct answer?It is difficult to be sure you have the right answer.
But it is often quite easy to identify wrong answers.
For example, an answer that contradicts itself is wrong.
An answer that contradicts an observation is wrong.
That's true, even if the observation was made a long time ago.
Post by: hamdani yusuf on 02/09/2022 14:17:27
How could the experiments contradict themselves?If an experiment is not repeatable, then there must be some uncontrolled factors involved.
Post by: hamdani yusuf on 02/09/2022 14:22:34
Post by: Bored chemist on 02/09/2022 16:25:39
This video gives important information to learn abouttemperaturesuperconductors.
Post by: hamdani yusuf on 04/09/2022 03:52:59
Can't you see its relationship with temperature?This video gives important information to learn abouttemperaturesuperconductors.
Post by: Bored chemist on 04/09/2022 09:52:09
Can't you see its relationship with temperature?Practically the whole of thermodynamics was sorted out before thee were any high temperature superconductors to play with.
Post by: hamdani yusuf on 08/09/2022 04:37:26
If two thermometers show different measurements from the same object at the same time, how to determine which one is more correct?Can't you see its relationship with temperature?Practically the whole of thermodynamics was sorted out before thee were any high temperature superconductors to play with.
Post by: Bored chemist on 08/09/2022 08:38:45
Post by: alancalverd on 09/09/2022 01:14:14
If two thermometers show different measurements from the same object at the same time, how to determine which one is more correct?The one traceable to a national standards laboratory.
True story. I attended the induction of recruits to the UK National Physical Laboratory. The Director said: "How long is a piece of string? In law, it is as long as I say it is. Your job is to tell me what to say."
Post by: hamdani yusuf on 18/09/2022 14:08:01
Post by: Deecart on 18/09/2022 19:13:19
He is doing the comparison of the "temperature" of two systems composed of the same objects.
One has more kinetic energy as the other, so it is hotter (this is what the guy say).
This is wrong.
I think he is doing confusion between temperaure and heat.
Temperature is not a concequence of heat, even if there is a possible link between the two concepts using mass !
He use a specific case (same molecules (same mass) in two systems) to demonstrate a false general conclusion.
Heat and Temperature are totally different things !
First, temperatures cant be added with temperature, but heat can be added with heat.
Here the guy is talking about heat, not about temperature.
To be convinced, just think about "tin air" (few molecules) : It has very low heat, but it can have very high temperature if the speed of the molecules is high.
You could grip it with you hand and dont be burned (no heat...) altought it has very hight temperature.
The best temperature measurement tool is the one who change the less the temperature measured (so when mass of the measurement tool tend to 0).
So here there is no heat involved at all (no "equilibrum" needed unlike the guy say incorrectly).
Now, yes the heat is also a mechanical energy, but he say it himself... it is a statistical mechanical energy.
Post by: Bored chemist on 18/09/2022 20:19:44
In this video, thermal energy is distinguished from other forms of kinetic energy by introducing a term: non-mechanical.If heat was only about kinetic energy, then hot things would not glow.
Post by: alancalverd on 19/09/2022 00:30:22
In this video, thermal energy is distinguished from other forms of kinetic energy by introducing a term: non-mechanical.Pretty much what we discussed on page 2 of this nonsense, talking about gross motion and internal motion.
Post by: hamdani yusuf on 21/09/2022 11:43:50
Here the guy is talking about heat, not about temperature.Here's another example.
A mixture of ice and water may have the same temperature, but they have different heat content.
Post by: hamdani yusuf on 21/09/2022 11:50:47
Now, yes the heat is also a mechanical energy, but he say it himself... it is a statistical mechanical energy.What's the difference between statistical mechanical energy and non-statistical mechanical energy?
How would it compare to temperature?
Is there a statistical mechanical energy which is not kinetic? What would it be called?
Post by: Origin on 21/09/2022 13:14:49
Post by: hamdani yusuf on 21/09/2022 14:47:31
If heat was only about kinetic energy, then hot things would not glow.What kind of energy makes hot things glow?
Post by: hamdani yusuf on 21/09/2022 14:50:04
How do you define gross motion and internal motion?In this video, thermal energy is distinguished from other forms of kinetic energy by introducing a term: non-mechanical.Pretty much what we discussed on page 2 of this nonsense, talking about gross motion and internal motion.
What are the main differences?
Post by: hamdani yusuf on 21/09/2022 14:56:36
The first stage is where you find something of which the temperature is known, and see which thermometer measures that correctly.It would be problematic if the thermometers have narrow range and we have nothing with known temperature within the range.
Post by: hamdani yusuf on 21/09/2022 14:57:58
It would make physical science more like a social construct.If two thermometers show different measurements from the same object at the same time, how to determine which one is more correct?The one traceable to a national standards laboratory.
True story. I attended the induction of recruits to the UK National Physical Laboratory. The Director said: "How long is a piece of string? In law, it is as long as I say it is. Your job is to tell me what to say."
Post by: hamdani yusuf on 21/09/2022 15:14:37
After 35 pages are you any closer to understanding what temperature is?I think so.
But the conversation here shows that there are still some disagreements among participants of this thread. Some videos shown here also indicates that disagreements even exist among science educators.
I hope to find some convincing argumentations to back up those positions in later posts. BTW, I haven't found your meaningful contribution here. I hope it would change next time.
Post by: hamdani yusuf on 22/09/2022 15:25:52
For example, which temperature measurement is correct?The first stage is where you find something of which the temperature is known, and see which thermometer measures that correctly.It would be problematic if the thermometers have narrow range and we have nothing with known temperature within the range.
Here's my first video on investigation of thermodynamics.
Demonstrating the effect of emissivity on infrared thermal camera measurement.
Post by: Origin on 22/09/2022 17:52:59
BTW, I haven't found your meaningful contribution here. I hope it would change next time.The question was answered in the first page so there has not really been all that much meaningful content in these past 35 pages...
Post by: paul cotter on 22/09/2022 18:49:06
Post by: alancalverd on 22/09/2022 21:01:51
It would make physical science more like a social construct.If you are a philosopher, you probably think so. Argumentative, but pointless.
Post by: alancalverd on 22/09/2022 21:02:55
For example, which temperature measurement is correct?The one traceable to a national standards laboratory, always. That is what defines "correct".
Post by: hamdani yusuf on 23/09/2022 15:31:51
Which nation?For example, which temperature measurement is correct?The one traceable to a national standards laboratory, always. That is what defines "correct".
What if you want to measure temperature higher than the maximum limit of your national standards laboratory?
Post by: Bored chemist on 23/09/2022 18:44:34
Which nation?The one you are in, but it hardly matters.
Standards like these are international.
https://tsapps.nist.gov/publication/get_pdf.cfm?pub_id=830622
The triple point of gallium is not dependent on nationality
.
What if you want to measure temperature higher than the maximum limit of your national standards laboratory?Technically, as Alan pointed out, that's their problem.
The answer is likely to be spectroscopy
Post by: alancalverd on 23/09/2022 20:05:01
The trick is to make a device that is modular and inherently linear and extendable, then calibrate the modules and add them together.
It is left as an exercise to the reader to work out how to do this with temperature. Hint: Johnson noise is good to the boiling point of carbon, and a buddy of mine uses spectroscopy to measure the temperature of plasmas with a diffraction grating traceable to common mechanical standards.
Post by: hamdani yusuf on 23/09/2022 22:37:14
This could proceed ad infinitum without a hint of progress.It's a possibility, if we keep posting the same things over and over again without coming up with something new.
Another possibility is that everyone stop posting and just ignore this, which means that we agree to disagree.
Yet another possible outcome is someone will come up with a convincing argumentations which lead to some agreement.
Post by: hamdani yusuf on 23/09/2022 22:54:21
I've measured voltages higher than the national standard, whilst working at a national standards lab!Have you measured temperature of sun's corona?
The trick is to make a device that is modular and inherently linear and extendable, then calibrate the modules and add them together.
It is left as an exercise to the reader to work out how to do this with temperature. Hint: Johnson noise is good to the boiling point of carbon, and a buddy of mine uses spectroscopy to measure the temperature of plasmas with a diffraction grating traceable to common mechanical standards.
https://www.nasa.gov/feature/goddard/2018/nasa-s-parker-solar-probe-and-the-curious-case-of-the-hot-corona
Quote
Something mysterious is going on at the Sun. In defiance of all logic, its atmosphere gets much, much hotter the farther it stretches from the Sun’s blazing surface.
Temperatures in the corona — the tenuous, outermost layer of the solar atmosphere — spike upwards of 2 million degrees Fahrenheit, while just 1,000 miles below, the underlying surface simmers at a balmy 10,000 F. How the Sun manages this feat remains one of the greatest unanswered questions in astrophysics; scientists call it the coronal heating problem. A new, landmark mission, NASA’s Parker Solar Probe — scheduled to launch no earlier than Aug. 11, 2018 — will fly through the corona itself, seeking clues to its behavior and offering the chance for scientists to solve this mystery.
Quote
Not until 70 years later did a Swedish physicist discover the element responsible for the emission is iron, superheated to the point that it’s ionized 13 times, leaving it with just half the electrons of a normal atom of iron. And therein lies the problem: Scientists calculated that such high levels of ionization would require coronal temperatures around 2 million degrees Fahrenheit — nearly 200 times hotter than the surface.
Post by: hamdani yusuf on 23/09/2022 22:56:11
Quote
Nasa
Search NASA.gov
NASA TV
MORE STORIES
Parker Solar ProbeIllustration of Parker Solar Probe circling the Sun.
Jul 20, 2018
Traveling to the Sun: Why Won’t Parker Solar Probe Melt?
This summer, NASA’s Parker Solar Probe will launch to travel closer to the Sun, deeper into the solar atmosphere, than any mission before it. If Earth was at one end of a yard-stick and the Sun on the other, Parker Solar Probe will make it to within four inches of the solar surface.
Inside that part of the solar atmosphere, a region known as the corona, Parker Solar Probe will provide unprecedented observations of what drives the wide range of particles, energy and heat that course through the region — flinging particles outward into the solar system and far past Neptune.
Inside the corona, it’s also, of course, unimaginably hot. The spacecraft will travel through material with temperatures greater than a million degrees Fahrenheit while being bombarded with intense sun light.
So, why won’t it melt?
Parker Solar Probe has been designed to withstand the extreme conditions and temperature fluctuations for the mission. The key lies in its custom heat shield and an autonomous system that helps protect the mission from the Sun’s intense light emission, but does allow the coronal material to “touch” the spacecraft.
NASA's Parker Solar Probe is heading to the Sun. Why won't the spacecraft melt? Thermal Protection System Engineer Betsy Congdon (Johns Hopkins APL) outlines why Parker can take the heat.
Credits: NASA's Goddard Space Flight Center
Download this video in HD formats from NASA Goddard's Scientific Visualization Studio
The Science Behind Why It Won’t Melt
One key to understanding what keeps the spacecraft and its instruments safe, is understanding the concept of heat versus temperature. Counterintuitively, high temperatures do not always translate to actually heating another object.
In space, the temperature can be thousands of degrees without providing significant heat to a given object or feeling hot. Why? Temperature measures how fast particles are moving, whereas heat measures the total amount of energy that they transfer. Particles may be moving fast (high temperature), but if there are very few of them, they won’t transfer much energy (low heat). Since space is mostly empty, there are very few particles that can transfer energy to the spacecraft.
Post by: hamdani yusuf on 23/09/2022 23:00:36
If you are already satisfied by the answers in the first page, then you're welcome. You can continue your life ignoring disagreements posted after that.BTW, I haven't found your meaningful contribution here. I hope it would change next time.The question was answered in the first page so there has not really been all that much meaningful content in these past 35 pages...
Post by: hamdani yusuf on 23/09/2022 23:01:39
You just haven't seen the point, yet.It would make physical science more like a social construct.If you are a philosopher, you probably think so. Argumentative, but pointless.
Post by: hamdani yusuf on 23/09/2022 23:12:31
The one you are in, but it hardly matters.What's the method selected as international standard?
Standards like these are international.
https://tsapps.nist.gov/publication/get_pdf.cfm?pub_id=830622
The triple point of gallium is not dependent on nationality
Why was it chosen over the alternatives?
Has it ever been changed or replaced?
Post by: hamdani yusuf on 24/09/2022 00:01:05
These can be seen as rhetorical questions. But since no one is interested to answer them, I'll give it a try, starting with this video.Now, yes the heat is also a mechanical energy, but he say it himself... it is a statistical mechanical energy.What's the difference between statistical mechanical energy and non-statistical mechanical energy?
How would it compare to temperature?
Is there a statistical mechanical energy which is not kinetic? What would it be called?
Quote
Entropy is a fundamental concept in Data Science because it shows up all over the place - from Decision Trees, to similarity metrics, to state of the art dimension reduction algorithms. It's also surprisingly simple, but often poorly explained. Traditionally the equation is presented with the expectation that you memorize it without thoroughly understanding what it means and where it came from. This video takes a very different approach by showing you, step-by-step, where this simple equation comes from, making it easy to remember (and derive), understand and explain to your friends at parties.
Post by: Bored chemist on 24/09/2022 00:55:02
if we keep posting the same thingsRoyal "We"?
Post by: Bored chemist on 24/09/2022 00:56:42
There's a point where you have to let the experts be experts.The one you are in, but it hardly matters.What's the method selected as international standard?
Standards like these are international.
https://tsapps.nist.gov/publication/get_pdf.cfm?pub_id=830622
The triple point of gallium is not dependent on nationality
Why was it chosen over the alternatives?
Has it ever been changed or replaced?
This is true even (some might say "especially") when you don't understand the question.
Post by: Bored chemist on 24/09/2022 00:58:22
But since no one is interested to answer themOthers have spent 15 pages answering them
Try not to tell obvious lies in your nest post.
Post by: JLindgaard on 24/09/2022 02:07:57
Maybe you could consider an event horizon? Matter is created while some matter is absorbed. And that increases what created the event horizon. That has nothing to do with heat. Heat is nothing more than conserved electromagnetic radiation being released as e = hv. And it's collisions between molecules that release heat/electromagnetic radiation.
Post by: hamdani yusuf on 24/09/2022 03:50:18
In this video, thermal energy is distinguished from other forms of kinetic energy by introducing a term: non-mechanical.What's called non-mechanical kinetic energy in this video can be interpreted as kinetic energy with high entropy. Alan called this internal kinetic energy.
On the other hand, non-thermal kinetic energy or mechanical kinetic energy in this video can be interpreted as kinetic energy with low entropy. Alan called this gross kinetic energy.
Post by: hamdani yusuf on 24/09/2022 04:14:37
The Misunderstood Nature of Entropy
Reversing Entropy with Maxwell's Demon
Post by: hamdani yusuf on 24/09/2022 04:21:38
Any we.if we keep posting the same thingsRoyal "We"?
Post by: hamdani yusuf on 24/09/2022 04:22:10
Aren't you interested to be an expert yourself?There's a point where you have to let the experts be experts.The one you are in, but it hardly matters.What's the method selected as international standard?
Standards like these are international.
https://tsapps.nist.gov/publication/get_pdf.cfm?pub_id=830622
The triple point of gallium is not dependent on nationality
Why was it chosen over the alternatives?
Has it ever been changed or replaced?
This is true even (some might say "especially") when you don't understand the question.
Post by: hamdani yusuf on 24/09/2022 04:23:14
Point one.But since no one is interested to answer themOthers have spent 15 pages answering them
Try not to tell obvious lies in your nest post.
Try not to tell obvious lies in your next post.
Post by: hamdani yusuf on 24/09/2022 04:33:57
hamdani, gravity is the opposite of entropy. While stars grow to great size, they will not increase in mass. Yet one day the universe will be 1 star that will collapse into itself causing another big bang. The big bang theory says the universe will become 1 small piece of matter that is extremely dense.I don't think that your prediction above is in the mainstream view of astrophysics community.
Maybe you could consider an event horizon? Matter is created while some matter is absorbed. And that increases what created the event horizon. That has nothing to do with heat. Heat is nothing more than conserved electromagnetic radiation being released as e = hv. And it's collisions between molecules that release heat/electromagnetic radiation.
Post by: alancalverd on 24/09/2022 11:55:50
Post by: Bored chemist on 24/09/2022 12:03:37
so it will surelyYou may be sure, but the rest of us aren't.
Eventually, it all ends up moving fast enough to overcome the gravitational attraction- it's above escape velocity- so it becomes more and more diffuse.
Post by: Bored chemist on 24/09/2022 12:04:40
I don't think that your prediction above is in the mainstream view of astrophysics community.Hamdani is correct in his belief here.
JL's post is nonsense
Post by: alancalverd on 24/09/2022 14:39:51
so it will surelyYou may be sure, but the rest of us aren't.
Eventually, it all ends up moving fast enough to overcome the gravitational attraction- it's above escape velocity- so it becomes more and more diffuse.
Stephen Hawking thought pretty much the same as JL and myself, in "Black holes and baby universes". Not sure whether he ever recanted.
Post by: Bored chemist on 24/09/2022 17:44:44
so it will surely coalesce back to a new primordial lump.On the way to doing that it would form black holes- which would evaporate into photons (at least, that's what Hawking said) and those photons will diffuse away.
Post by: alancalverd on 24/09/2022 23:36:54
Post by: Bored chemist on 25/09/2022 08:40:49
Isn't the essence of a black hole, that photons cannot leave it? Hence black, and hole.It depends on whether Hawking was right or not.
https://en.wikipedia.org/wiki/Hawking_radiation
Post by: paul cotter on 25/09/2022 10:03:19
Post by: alancalverd on 25/09/2022 15:34:21
Has it ever been changed or replaced?Yes. The usual reason is that someone finds a process that is more precisely or more easily reproducible. Problem with temperature is that you need two accessible points to define a temperature scale. Whilst 0K is a calculable point it isn't actually accessible, thermometers that work well at low temperatures don't always work at high ones, and the linearity of any practical thermometer is limited so you really need several reference points.
We tend to use the platinum resistance thermometer to provide a standard scale up to a few hundred K, accepting that it is not inherently linear but sufficiently consistent. The triple point of water is a simple reference for most industrial purposes but as we have discussed, it is a very difficult substance to use with precision, whereas gallium is rather "better behaved" around its triple point. Most people would agree that temperature measurement above about 700K is very difficult to establish to better than ±0.1K but it is a matter of little practical consequence.
Post by: alancalverd on 25/09/2022 15:35:35
Hawking radiation is a very slow form of black hole ablation.And you have to coalesce a lot of stuff to make the black hole in the first place.
Post by: Bored chemist on 25/09/2022 15:54:02
Hawking radiation is a very slow form of black hole ablation.
Post by: Bored chemist on 25/09/2022 17:44:10
Everything does.
So, let's try to calculate how long it would take the earth to evaporate in a universe at 1K.
Data on volatility of planets is a bit hard to come by.
So I will choose tungsten as a model substance. Not, as you might imagine, because I have taken leave of my senses.
(1) To a very good approximation, everything is more volatile than tungsten, so it's a "worst case"
(2) The data on evaporation rates is available for solid tungsten, because it's important to the people who make light bulbs and such.
I found it in my copy of this.
https://www.wob.com/en-gb/books/fred-rosebury/handbook-of-electron-tube-and-vacuum-techniques
(I like to keep up with cutting edge technology).
[ Invalid Attachment ]
The first column is the temperature (Kelvin) and the second is the rate of evaporation in g/ cm2 / sec
Those of you who ever studied physical chemistry will recognise the significance of the other two columns. It's a a magic trick called a Van't Hoff plot which allows you to linearise rates of things as a function of temperature.
https://en.wikipedia.org/wiki/Van_%27t_Hoff_equation
And that allows me to interpolate the data down to a lower temperature.
OK over 3 orders of magnitude looks like I'm pushing it but...
Here's the graph
[ Invalid Attachment ]
and it looks linear enough to me.
That extrapolation tells me the the rate of evaporation is of the order of 10^-50,000 g/cm^2 per second.
And this puts us in an interesting world of extrapolation where I can ignore the fact that the mass of the world is more than 1 gram.
Because that would only add something like 27 orders of magnitude to the answer.
And I can ignore the fact that the earth has an area bigger than 1 square centimetre because that would only contribute about a couple of dozen orders of magnitude, and I can ignore the fact that I want an answer in years , rather than seconds because that only makes about 7 or 8 orders of magnitude difference.
My estimate for the time taken for an earth sized lump of tungsten the size of the earth to evaporate at 1K is about 10^50000 years.
Quite a while...
But infinitely shorter than "forever".
Post by: alancalverd on 25/09/2022 19:47:28
Because that would only add something like 27 orders of magnitude to the answer.Possibly apocryphal but I heard it from one of his research associates:
Fred Hoyle: "Sorry, I've made an error somewhere. There's a missing factor of 1045."
Student: "Plus or minus 45?"
Hoyle: "It doesn't make much difference".
Post by: hamdani yusuf on 29/09/2022 12:42:50
What I'm looking for is the fundamental definition of temperature, and how it relates to other physical concepts.Has it ever been changed or replaced?Yes. The usual reason is that someone finds a process that is more precisely or more easily reproducible. Problem with temperature is that you need two accessible points to define a temperature scale. Whilst 0K is a calculable point it isn't actually accessible, thermometers that work well at low temperatures don't always work at high ones, and the linearity of any practical thermometer is limited so you really need several reference points.
We tend to use the platinum resistance thermometer to provide a standard scale up to a few hundred K, accepting that it is not inherently linear but sufficiently consistent. The triple point of water is a simple reference for most industrial purposes but as we have discussed, it is a very difficult substance to use with precision, whereas gallium is rather "better behaved" around its triple point. Most people would agree that temperature measurement above about 700K is very difficult to establish to better than ±0.1K but it is a matter of little practical consequence.
So far, the closest thing I get is by comparison to the kinetic energy of ideal gas in thermal equilibrium with the object we want to measure.
Post by: Georginajackson on 30/09/2022 12:00:14
Isn't the essence of a black hole, that photons cannot leave it? Hence black, and hole.Yes, but besides this there are other reasons why this would not be so.
Post by: hamdani yusuf on 01/10/2022 03:34:33
Quickly rotating magnets or electrets in a box have large kinetic energy,You ignored the word "internal" in my quote. It isn't there for padding!
Rotating magnets or electrets in a box have low entropy. The rotation can be described by small amount of information.
Vibrating tuning forks also have low entropy, because it can be described by small amount of information..
These can be seen as rhetorical questions. But since no one is interested to answer them, I'll give it a try, starting with this video.Now, yes the heat is also a mechanical energy, but he say it himself... it is a statistical mechanical energy.What's the difference between statistical mechanical energy and non-statistical mechanical energy?
How would it compare to temperature?
Is there a statistical mechanical energy which is not kinetic? What would it be called?QuoteEntropy is a fundamental concept in Data Science because it shows up all over the place - from Decision Trees, to similarity metrics, to state of the art dimension reduction algorithms. It's also surprisingly simple, but often poorly explained. Traditionally the equation is presented with the expectation that you memorize it without thoroughly understanding what it means and where it came from. This video takes a very different approach by showing you, step-by-step, where this simple equation comes from, making it easy to remember (and derive), understand and explain to your friends at parties.
My conclusion so far, is that temperature of an object is proportional to its kinetic energy, as well as its entropy. I'm still open to suggestion that there might be some other factors involved.
Post by: alancalverd on 01/10/2022 11:05:56
There is little point in arguing about the meaning of words in physics - the important ones are all defined, mostly English (or Latin/Greek/German incorporated into English), and fully understood by those who use them daily.
Post by: Bored chemist on 01/10/2022 17:08:24
My conclusion so far, is that temperature of an object is proportional to its kinetic energy, as well as its entropy.Then your conclusion is wrong.
Temperature is sometimes proportional to the energy in some simple cases.
But the heat capacity is not actually a constant, so your idea is not actually correct.
It's not proportional to the entropy.
Post by: hamdani yusuf on 05/10/2022 10:47:31
The temperature of an object is indeed proportional to its internal kinetic energy, as several people stated on Page 1 and all the textbooks. The constant of proportionality is called the temperature scale, and most scientists use the Kelvin scale starting at absolute zero and incrementing by Celsius degrees.The proportionality between temperature and kinetic energy is well established for ideal gases. But for other things, the kinetic energy is also manifested in other things which are not measurable by thermometers. The point of this thread is to identify and quantify these other things. You've identified one of those as "gross" motion. The next questions are how to quantify it, and how to separate it from the types of motion which contribute to temperature measurement.
There is little point in arguing about the meaning of words in physics - the important ones are all defined, mostly English (or Latin/Greek/German incorporated into English), and fully understood by those who use them daily.
Here is a more complete table of molar heat capacity I compiled from NIST website.
Temp (K) Hydrogen Deuterium Helium Argon Radon
300 28.85 29.19 20.79 20.79 20.79
1000 30.20 31.64 20.79 20.79 20.79
3000 37.09 38.16 20.79 20.79 20.79
6000 41.97 42.25 20.79 20.79 20.79
From the table we can conclude that increase of temperature also increases the portion of rotational and vibrational movements in kinetic energy of diatomic gases. In noble gases, those types of motion are virtually non-existent.
Post by: hamdani yusuf on 05/10/2022 10:50:19
What's your conclusion?My conclusion so far, is that temperature of an object is proportional to its kinetic energy, as well as its entropy.Then your conclusion is wrong.
Temperature is sometimes proportional to the energy in some simple cases.
But the heat capacity is not actually a constant, so your idea is not actually correct.
It's not proportional to the entropy.
Post by: Bored chemist on 05/10/2022 12:47:30
What's your conclusion?
Temperature is sometimes proportional to the energy in some simple cases.
But the heat capacity is not actually a constant, so your idea is not actually correct.
It's not proportional to the entropy.
The proportionality between temperature and kinetic energy is well established for ideal gases.There are no ideal gases.
The next questions are how to quantify it, and how to separate it from the types of motion which contribute to temperature measurement.If all the modes in which energy can be present have (on average) the same amount of energy then you have a temperature and you can calculate it based on any of those modes.
If they aren't the same, you don't have a defined temperature.
Post by: paul cotter on 05/10/2022 15:59:24
Post by: hamdani yusuf on 06/10/2022 08:23:19
But the heat capacity is not actually a constantWhat makes it change?
Post by: hamdani yusuf on 06/10/2022 08:37:19
There are no ideal gases.Noble gases are pretty close approximation at low enough temperature and pressure.
At high enough temperature, ionizations break the approximation down.
At high enough pressure, electromagnetic interactions among many gas atoms also break the approximation down.
Post by: Bored chemist on 06/10/2022 08:40:07
Go and read the thread again.But the heat capacity is not actually a constantWhat makes it change?
I already answered that and I'm not wasting even more time repeating myself just because you are lazy.
Post by: hamdani yusuf on 06/10/2022 09:32:59
I was going to query how such a simple question could expand to 38 pages and still appear to be alive.Other seemingly simple question such as morality has been going and alive among philosophers for thousands of years.
Depending on the level of understanding details we want to achieve, and the robustness of definitions, we can discuss about other simple and fundamental terms such as mass and time for as long as we like.
Post by: hamdani yusuf on 06/10/2022 14:09:25
Go and read the thread again.Do you mean this?
Sort of, because the energy levels are quantised.
For a cold diatomic gas like nitrogen there's not enough to excite vibrations and so the only contributions to the heat capacity are translation and rotation.
That's 3 translational degrees and 2 rotational ones (rotation about the axis between the centres of the two atoms doesn't count) making 5 in total
When the gas is hot there is enough energy to get the molecules vibrating and that adds some more degrees of freedom into which energy can be placed.
That adds another degree of freedom , making 6 in total.
So the calculated heat capacities under those conditions are 2.5kT and 3 kT
But, at an intermediate temperature some, but not all, of the molecules will have enough energy to induce vibrations.
And under those conditions the average number of degrees of freedom will be somewhere between 5 and 6.
What determines the required energy to excite vibrations?
How does these degrees of freedom affect temperature of liquids and solids?
Post by: hamdani yusuf on 19/10/2022 09:10:58
I was going to query how such a simple question could expand to 38 pages and still appear to be alive. However having scanned a number of pages I do see education at work, albeit at a snail's pace. I have one small addition from the early pages: the op referenced Jean Piere Robitaille, this guy is a notorious crank and not a scientist. He is a medic of some description, an anaesthetist as far as I remember.
I searched for online sources about him.
Quote
https://medicine.osu.edu/find-faculty/clinical/radiology/pierre-marie-robitaille-phd
Pierre Marie Robitaille, PhD
Professor, Department of Radiology
Background
I joined the Department of Radiology in 1989. At the time, my research centered on spectroscopic methods, with a focus on the experimental and theoretical aspects of nuclear magnetic resonance and magnetic resonance imaging (MRI). I devoted considerable attention to NIH-funded spectroscopic analysis of in-vivo cardiac metabolism in the normal and failing heart, using both 13C- and 31P- NMR methods. I also focused on the development of new instrumentation for MRI. This included the design and assembly of the first torque compensated asymmetric gradient coil.
From 1995-2000, I was responsible for conceiving and assembling, at Ohio State, the world's first ultra high field clinical MRI system. This 8 Tesla/80cm MRI system was utilized to acquire many of the highest resolution images in existence. At the same time, early results with this instrument prompted a reconsideration of RF power requirements in MRI and of signal to noise. In turning my attention to these problems, I initially sought to consider NMR is a "thermal" process. In the early days of this modality, the T1 relaxation time was also known as the "thermal" relaxation time. This would lead to a detailed study of Kirchhoff's Law of Thermal Emission, a topic on which I have subsequently published extensively.
Kirchhoff's Law stands at the very heart of spectroscopic analysis, not only in medicine, but also in fields as seemingly remote as astronomy. For me, revisiting Kirchhoff's Law of Thermal Emission has resulted in questioning many established ideas in astronomy, including the origin of the microwave background and, most importantly, the nature of the sun itself. That is because the standard model of the sun, relies on the validity of Kirchhoff's Law, in order to justify a gaseous state. Conversely, if Kirchhoff's Law is not valid, then the sun cannot be a gaseous in nature. Along these lines, I have recently advanced forty lines of evidence that the sun is comprised of condensed matter.
He recently published a new video here, called "Responding to Loose Ends!"
If you found any flaws in his methodology, or his results, you can share them so more people can scrutinize more thoroughly.
Post by: Bored chemist on 19/10/2022 13:03:48
But we know it is a gas, so we know the law is valid.
This is a surprise to nobody except cranks because Kirchhoff's radiation law is just a special case of the conservation of energy.
I'm not sure there's any point to watching a video by a guy who either doubts, or fails to understand the conservation of
energy.
This isn't making me think better of him.
https://rationalwiki.org/wiki/Pierre-Marie_Robitaille
Post by: Bored chemist on 19/10/2022 13:05:22
What determines the required energy to excite vibrations?E= h ν
Post by: Origin on 19/10/2022 13:12:28
He recently published a new video here, called "Responding to Loose Ends!"
The guy is a major crank, this is from Rationalwiki:
In 2000, he was asked to step down from his position as director (though he remains a professor) when he began to promote theories that were outside his actual realm of expertise, specifically related to non-mainstream beliefs in the areas of astronomy and physics: he maintains that satellite measurements of the cosmic microwave background radiation, believed by most astronomers to be an afterglow of the Big Bang, are actually observations of a glow from Earth's oceans.[note 1]
He also maintains that the sun is not a ball of plasma but is, in fact, made of liquid metallic hydrogen. None of his ideas have been accepted by any reputable physics publication.
edit: I see Bored Chemist already brought up Rationalwiki.
Post by: paul cotter on 19/10/2022 13:31:04
Post by: hamdani yusuf on 31/10/2022 12:10:29
Graham's Hierarchy of Disagreement
Quote
When you discuss a topic and everyone agrees, the conversation often dies out quickly. But when you disagree, you're putting yourself in opposition to what was said, and the discussion continues. Paul Graham, a computer engineer, therefore proposed a “Hierarchy of Disagreement” in 2008. Learn at which level you are able to articulate your disagreement. Hopefully it’s not just name-calling or responding to tone.When we disagree, it would be helpful to identify the level of our disagreement.
CHAPTER
00:00 Opening quotes and statement
00:52 Introduction
01:19 Graham's hierarchy of disagreement
01:32 Level 1: Name-calling
01:48 Level 2: Ad hominem
02:14 Level 3: Responding to tone
02:41 Level 4: Contradiction
03:08 Level 5: Counterargument
03:41 Level 6: Refutation
04:13 Level 7: Refuting the central point
05:05 Benefit of knowing the form of argument
06:06 What do you think?
06:58 Patrons credits
07:07 Ending
Post by: Bored chemist on 31/10/2022 18:07:25
it would be helpful to identify the level of our disagreement.So... do you need me to point out that I posted a refutation of his point, or can you work that out for yourself?
Post by: hamdani yusuf on 25/11/2022 11:35:46
Feel free to write down your reasoning here.it would be helpful to identify the level of our disagreement.So... do you need me to point out that I posted a refutation of his point, or can you work that out for yourself?
Post by: hamdani yusuf on 25/11/2022 11:39:49
It's an interesting video related to this topic.
Post by: Bored chemist on 25/11/2022 11:48:22
Feel free to answer my question.Feel free to write down your reasoning here.it would be helpful to identify the level of our disagreement.So... do you need me to point out that I posted a refutation of his point, or can you work that out for yourself?
do you need me to point out that I posted a refutation of his point,
This may help you; it's the bit where I posted a refutation of the point made.
"Conversely, if Kirchhoff's Law is not valid, then the sun cannot be a gaseous in nature"
But we know it is a gas, so we know the law is valid.
This is a surprise to nobody except cranks because Kirchhoff's radiation law is just a special case of the conservation of energy.
Post by: hamdani yusuf on 26/11/2022 04:44:16
Feel free to answer my question.That was my answer.
Feel free to write down your reasoning here.
"Conversely, if Kirchhoff's Law is not valid, then the sun cannot be a gaseous in nature"How do you know that it's a gas?
But we know it is a gas, so we know the law is valid.
This is a surprise to nobody except cranks because Kirchhoff's radiation law is just a special case of the conservation of energy.
Post by: Bored chemist on 26/11/2022 11:17:54
How do you know that it's a gas?At over 5000C what else could it be?
But my point is that anyone who doubts Kirchhoff's law is doubting the conservation of energy and thus not trustworthy.
Post by: hamdani yusuf on 26/11/2022 12:38:13
At over 5000C what else could it be?It depends on the pressure.
Low pressure hot gases produce line emission spectra. That's not what we observe from sunlight.
Instead, what we get is line absorption spectra, which are produced by cool low pressure gases, with black body-like spectrum as the backlight.
Post by: hamdani yusuf on 26/11/2022 12:48:52
But my point is that anyone who doubts Kirchhoff's law is doubting the conservation of energy and thus not trustworthy.There must be some reasons to accept or reject an assertion if we want to make our beliefs reasonable.
There must be some reasons to accept or reject an evidence alleged to support or oppose an assertion.
Post by: alancalverd on 26/11/2022 14:41:54
*blood being cooled by evaporation in the lungs if the external skin surface is insulated.
Post by: Bored chemist on 26/11/2022 14:57:40
There must be some reasons to accept or reject an assertion if we want to make our beliefs reasonable.Yes, and, in the case of Kirchhoff's laws and the conservation of energy we have both practical experience and mathematical proof.
So, anyone who thinks it might be wrong is clearly not a good judge of things.
Not believing it is unreasonable.
That's my point.
What are you trying to argue about?
Post by: alancalverd on 26/11/2022 20:13:02
Graham's Hierarchy of DisagreementCalverd's hierarchy of disagreement
1. State case
2. set out counterarguments
3. how many lives/how much money at stake? If none, continue, else go to 8
4. agree the critical experiment
5. do the critical experiment
6. review critical experiment
7. agree - go to end
8. take the less dangerous or if equally dangerous, less costly route
9. evaluate
10 agree - go to end.
11. end - shake hands, write down and remember the answer.
If neither lives nor money are at stake, and there is no critical experiment, do something important instead of arguing.
Post by: hamdani yusuf on 27/11/2022 05:46:13
The black/white video skated over the elephant in the room! Skin temperature is regulated by perspiration and blood flow,* pretty much independent of ambient, if your clothing is reasonably insulating - which a robe certainly is because it traps a lot of air.I've seen other videos using black and white balloons. Black balloons exploded when the beam of a burning laser pointer hit them, while white balloons survived.
*blood being cooled by evaporation in the lungs if the external skin surface is insulated.
So, we have seemingly contradicting results.
Post by: Bored chemist on 27/11/2022 09:09:58
we have seemingly contradicting resultsIt only seems that way to you.
Post by: alancalverd on 27/11/2022 11:21:44
I've seen other videos using black and white balloons. Black balloons exploded when the beam of a burning laser pointer hit them, while white balloons survived.It all goes back to the basis of thermodynamics. The hotter body will always lose heat to the colder body, but
So, we have seemingly contradicting results.
the rate of radiative exchange depends on the surface "color"
the rate of conductive exchange depends on the conductivity of the intervening material
the rate of convective exchange depends on the convective heat transfer function of the carrier, which is a function of umpteen variables of chemistry and geometry.
But the result is always the same.
Laser heating was proposed in the Sixties for drilling caries out of teeth. Healthy enamel is highly reflective but decayed material tends to be brown or black and thus strongly absorbent, so you can ablate it with an optical laser.
Post by: hamdani yusuf on 28/11/2022 06:23:29
Laser heating was proposed in the Sixties for drilling caries out of teeth. Healthy enamel is highly reflective but decayed material tends to be brown or black and thus strongly absorbent, so you can ablate it with an optical laser.It can also be done using sunlight.
Post by: hamdani yusuf on 28/11/2022 06:31:12
It also doesn't seem contradicting for anyone who doesn't know them, or doesn't want to think about them.we have seemingly contradicting resultsIt only seems that way to you.
Post by: Bored chemist on 28/11/2022 08:38:36
It also doesn't seem contradicting for anyone who doesn't know them, or doesn't want to think about them.It doesn't seem contradictory for anyone who thinks about it (because the explanation is obvious), or for anyone who does not think about it (because they don't notice anything odd).
So, it seems that you are the only one who sees a contradiction.
Can you tell us what that contradiction is?
Post by: hamdani yusuf on 28/11/2022 13:27:17
The clothes aren't felt different, but the balloons reacted differently. At least an explanation should be provided to have caused the difference.It also doesn't seem contradicting for anyone who doesn't know them, or doesn't want to think about them.It doesn't seem contradictory for anyone who thinks about it (because the explanation is obvious), or for anyone who does not think about it (because they don't notice anything odd).
So, it seems that you are the only one who sees a contradiction.
Can you tell us what that contradiction is?
Post by: Bored chemist on 28/11/2022 13:35:29
At least an explanation should be provided to have caused the difference.It was.
Skin temperature is regulated by perspiration and blood flow,* pretty much independent of ambient, if your clothing is reasonably insulating - which a robe certainly is because it traps a lot of air.
Now, I accept that there are other factors involved (for example, windspeed), but none of them is "unknown to science".
So there's no "contradiction" here, just different circumstances.
Post by: hamdani yusuf on 29/11/2022 05:41:33
Science is not just imagining what could cause observed results. It's called hypothesizing.At least an explanation should be provided to have caused the difference.It was.Skin temperature is regulated by perspiration and blood flow,* pretty much independent of ambient, if your clothing is reasonably insulating - which a robe certainly is because it traps a lot of air.
Now, I accept that there are other factors involved (for example, windspeed), but none of them is "unknown to science".
So there's no "contradiction" here, just different circumstances.
Scientific investigation aims to eliminate wrong hypotheses by confronting them with related observation results.
How do you think wind speed change the results for black and white balloons?
Post by: Bored chemist on 29/11/2022 08:41:20
Scientific investigation aims to eliminate wrong hypotheses by confronting them with related observation results.Do you not understand that such observations have been made?
How do you think wind speed change the results for black and white balloons?Enough air blowing past the balloon might stop it heating up enough for the rubber to soften and might stop it bursting.
Did you not realise that?
Post by: hamdani yusuf on 30/11/2022 03:24:43
Enough air blowing past the balloon might stop it heating up enough for the rubber to soften and might stop it bursting.Do you think that eliminating wind would make black clothes feel hotter than the white one?
Post by: Bored chemist on 30/11/2022 08:54:22
It depends on other things, like whether the sun is shining, how brightly, and how windy it is.Enough air blowing past the balloon might stop it heating up enough for the rubber to soften and might stop it bursting.Do you think that eliminating wind would make black clothes feel hotter than the white one?
Are you unable to understand that something may be due to a combination of factors?
But none of those is unknown to science.
There is no contradiction.
You were simply wrong when you said this
So, we have seemingly contradicting results.
Post by: hamdani yusuf on 30/11/2022 13:30:48
It depends on other things, like whether the sun is shining, how brightly, and how windy it is.Do you understand how they combine to produce observed results?
Are you unable to understand that something may be due to a combination of factors?
Post by: Bored chemist on 30/11/2022 13:32:55
Yes thanks.It depends on other things, like whether the sun is shining, how brightly, and how windy it is.Do you understand how they combine to produce observed results?
Are you unable to understand that something may be due to a combination of factors?
Post by: hamdani yusuf on 30/11/2022 13:33:05
Do you realize that words are written to convey a meaning?
But none of those is unknown to science.
There is no contradiction.
You were simply wrong when you said thisSo, we have seemingly contradicting results.
Someone somewhere may already know the answer. Do you?
Post by: hamdani yusuf on 30/11/2022 13:34:02
You can keep it for yourself, or share it with other forum members here.Yes thanks.It depends on other things, like whether the sun is shining, how brightly, and how windy it is.Do you understand how they combine to produce observed results?
Are you unable to understand that something may be due to a combination of factors?
Post by: alancalverd on 30/11/2022 17:27:44
There have been a few "solar hot air balloons" that used a black envelope to enhance lift; google Bristol 2015 Solar Balloon for a good one that also exploits "white" fabric on the downsun side to minimise heat loss. And note that the checkerboard pattern on many NASA rockets is designed to control solar heating and radiative cooling as the craft rotates.
Post by: Bored chemist on 30/11/2022 18:46:51
You can keep it for yourself, or share it with other forum members here.It's not a secret; but the answer is "it depends" which seems to upset some people.
But I'm curious.
Did you not realise that the answer was going to be "it depends"?
Post by: hamdani yusuf on 30/11/2022 23:08:12
Depends on what?You can keep it for yourself, or share it with other forum members here.It's not a secret; but the answer is "it depends" which seems to upset some people.
But I'm curious.
Did you not realise that the answer was going to be "it depends"?
Post by: Bored chemist on 01/12/2022 09:03:43
Depends on what?If you don't understand what factors are likely to affect heat transfer, you just are not bright enough to do science.
Try your hand at something else.
If, on the other hand, you can recognise what factors are likely to affect it, stop trolling.
Both options suggest you should stop posting.
Post by: hamdani yusuf on 01/12/2022 10:23:58
If you don't want to answer questions,Depends on what?If you don't understand what factors are likely to affect heat transfer, you just are not bright enough to do science.
Try your hand at something else.
If, on the other hand, you can recognise what factors are likely to affect it, stop trolling.
Both options suggest you should stop posting.
Quote
you should stop posting.
Post by: hamdani yusuf on 31/03/2024 05:52:19
Quote
Teaching thermal physics,
is as easy as a song:
You think you make it simpler,
When you make it slightly wrong!
---Mark Zemansky
Post by: hamdani yusuf on 26/07/2024 07:35:07
This video can give some reality checks for our current understanding of temperature.
Post by: Bored chemist on 26/07/2024 14:46:11
Magnifying The World's Brightest Flashlight (200,000 Lumens)Are you using the "Royal we" ?
This video can give some reality checks for our current understanding of temperature.
BTW, this still applies.
It doesn't show that they are different in a relevant way, does it?So... you do understand that the heating effect you from from focussing sunlight is nothing to do with the "laser" cooling effect, don't you?You said that photon from laser is indistinguishable from other sources, wich make them equally good for cooling. The video shows that they are different.
You could still use a non-laser light source for cooling. (I didn't say they were "good" for it, did I?. I said they were not good.You made that up. Why did you do that?)
The point I was making was that laser cooling has nothing to do with the negative electronic temperature in a laser.
Do you understand that?
Incidentally, the video is wrong. The "explanation" about lenses is irrelevant if you use non-imaging optics.
Post by: alancalverd on 26/07/2024 22:43:45
Post by: hamdani yusuf on 27/07/2024 10:17:07
BTW, this still applies.I asked Gemini. Is there a known alternative light source for cooling other than laser?
Quote
No Practical Alternative to Lasers for Cooling
As of now, there is no known practical alternative light source to lasers for cooling atoms or molecules.
The unique properties of lasers, such as high intensity, monochromaticity, coherence, and directionality, are essential for the precise control required in laser cooling. These properties are not readily achievable with other light sources.
While theoretical explorations and experimental attempts continue, lasers remain the dominant and most effective tool for this purpose.
Post by: alancalverd on 27/07/2024 10:27:45
Post by: Bored chemist on 27/07/2024 11:13:26
So, the chat bot understands the word "practical", but you don't.BTW, this still applies.I asked Gemini. Is there a known alternative light source for cooling other than laser?QuoteNo Practical Alternative to Lasers for Cooling
As of now, there is no known practical alternative light source to lasers for cooling atoms or molecules.
The unique properties of lasers, such as high intensity, monochromaticity, coherence, and directionality, are essential for the precise control required in laser cooling. These properties are not readily achievable with other light sources.
While theoretical explorations and experimental attempts continue, lasers remain the dominant and most effective tool for this purpose.
An interesting experiment in its way, with an unsurprising conclusion.
Today, nobody in their right mind would try to make (visible light) holograms without using a laser.
But the guy who first made them did so- because there were no lasers.
The use of a filtered mercury lamp would be considered impractical today.
Post by: hamdani yusuf on 27/07/2024 23:40:44
You could still use a non-laser light source for cooling. (I didn't say they were "good" for it, did I?. I said they were not good.You made that up. Why did you do that?)Your claim above contradicts Gemini. You sound like non-laser cooling were already widely known, despite their inefficiency.
Post by: hamdani yusuf on 27/07/2024 23:42:06
The phrase "magnifying 200,000 lumens" is, I think, meaningless, so I won't bother to watch the video.What should be changed to make it more meaningful?
Post by: hamdani yusuf on 28/07/2024 01:20:10
Quote
This is the second, and longer, "solution" video to my "new brainteaser" video about compressing air, losing ALL the energy used to compress it, and still having compressed air that can be used to run my pneumatic tools.The unit of entropy reveals its connection between temperature and energy.
I want to thank Dr. Michelsen for his generosity. He also has an excellent document he's posted on-line that you can see here:
"Statistical Mechanifesto or Funky Statistical Mechanics Concepts"
https://elmichelsen.physics.ucsd.edu/...
If you search the document for "brain teaser" you can see what he's written about this problem.
I encourage people to check it out. If you want to forward the document, he requests that you forward the link rather than a copy of the document. This is simply because he updates the document fairly regularly.
Post by: hamdani yusuf on 28/07/2024 01:23:51
If you want to skip past all the nonsense (i.e. background), jump to 7:50.
Post by: Bored chemist on 28/07/2024 10:29:05
You could still use a non-laser light source for cooling. (I didn't say they were "good" for it, did I?. I said they were not good.You made that up. Why did you do that?)Your claim above contradicts Gemini. You sound like non-laser cooling were already widely known, despite their inefficiency.
Bollocks.
What I said (and you quoted) was " I said they were not good".
And that statement is obviously correct.
It's not my fault if you didn't understand it. Everyone else seems to have known what I meant.
I did not suggest that anyone would be using them, given that lasers are good at it (and would be cheaper etc).
Gemini also said that the only practical option is a laser.
I was pointing out that an atom hit by a photon does not know if that photon came from a laser or a camp fire. So, in general, if you can do things with laser light, you can do them with light from other sources.
Post by: Bored chemist on 28/07/2024 10:30:30
They should have said "putting a lens in front of a torch".The phrase "magnifying 200,000 lumens" is, I think, meaningless, so I won't bother to watch the video.What should be changed to make it more meaningful?
Because that's what they did.
Post by: Bored chemist on 28/07/2024 10:42:51
I come home and I think my room (roughly 3m by 3m by 2.5 m ; I'm ignoring the volume of the fireplace) is too cold; it's only 10 C in here (283K) , so I switch on the heating- a 1 KW electric fire.
After 1000 seconds it is warm enough and I switch the heater off.
How much more energy is there in the air in the room?
(Making the usual (unrealistic) assumption that there's no energy loss to the walls and furniture or any gain from me being in there metabolising food.
We are also assuming air is an ideal gas)
Post by: hamdani yusuf on 28/07/2024 11:19:47
" I said they were not good".You need to be more specific. Do they work, but have weaknesses or unwanted side effects?
Or they only work in theory, with no real life demonstration?
Post by: Bored chemist on 28/07/2024 11:27:48
How to cool things using light other than laser?The same way that you would do it using a laser, but using a different light source.
The fact that you ask this proves that you don't understand how laser cooling works.
I do not need to be more specific. I pointed out that you could use a different light source." I said they were not good".You need to be more specific. Do they work, but have weaknesses or unwanted side effects?
Or they only work in theory, with no real life demontration?
That statement is true.
I wasn't attempting to describe the history of all uses of light.
I was pointing out that you didn't understand laser cooling.
Post by: hamdani yusuf on 28/07/2024 11:28:49
They should have said "putting a lens in front of a torch".What's the consequences of "putting a lens in front of a torch"?
Because that's what they did.
What's the intensity of the focused light? Is it the same as the unfocused one?
Post by: hamdani yusuf on 28/07/2024 11:30:51
So, it only works in theory, then. Perhaps the theory that you believe hasn't completely describe physical reality yet.How to cool things using light other than laser?The same way that you would do it using a laser, but using a different light source.
The fact that you ask this proves that you don't understand how laser cooling works.
Post by: Bored chemist on 28/07/2024 11:31:20
Watch the video and find out.They should have said "putting a lens in front of a torch".What's the consequences of "putting a lens in front of a torch"?
Because that's what they did.
What's the intensity of the focused light? Is it the same as the unfocused one?
Or do teh experiemnt yourself.
Post by: Bored chemist on 28/07/2024 11:34:03
Could you read a book by sunlight if you were on Mars (In a suitable space-suit)?So, it only works in theory, then. Perhaps the theory that you believe hasn't completely describe physical reality yet.How to cool things using light other than laser?The same way that you would do it using a laser, but using a different light source.
The fact that you ask this proves that you don't understand how laser cooling works.
Nobody has tried.
Any answer would be theoretical.
But would you expect the theory to be wrong.
I did, in fact, explain why my prediction is correct.
It's just that you didn't understand it.
an atom hit by a photon does not know if that photon came from a laser or a camp fire
Post by: alancalverd on 28/07/2024 17:15:24
What's the intensity of the focused light?And there's the second problem with the video! The lumen is a psychophysical unit as it multiplies the actual spectral radiance by the very nonlinear photopic luminous efficiency function of the average human eye, so focussing the output of a non-monochromatic source of n lumens onto any other receptor does not produce a calculable result.
If, for instance, the source is entirely infrared, you could fry a steak with zero lumens but lots of watts. I used to use this simple phenomenon to demonstrate the hazards of surgical CO2 lasers.
Post by: alancalverd on 28/07/2024 17:21:42
Your claim above contradicts Gemini. You sound like non-laser cooling were already widely known, despite their inefficiency.No contradiction. The theory of photonic cooling predates the demonstration and is based on photons with no reference to their source. The practical application uses lasers as the most efficient source of coherent radiation.
Post by: hamdani yusuf on 29/07/2024 08:00:38
an atom hit by a photon does not know if that photon came from a laser or a camp fireBut laser cooling doesn't only work with single atom and single photon. The temporal relationship with subsequent photons are critical in determining whether the interaction ends up with net cooling or heating instead.
Non-laser sources generally lack of coherence to do the job.
Quote
Gemini
Laser cooling is a counterintuitive process where lasers are used to cool atoms or molecules to incredibly low temperatures, often approaching absolute zero. This might seem strange because lasers are typically associated with heat and energy, but the underlying physics is quite fascinating.
How Does it Work?
The principle is based on the interaction between light and matter. When an atom absorbs a photon (a particle of light), it gains momentum in the direction of the photon's travel. However, when the atom re-emits the photon, it does so in a random direction.
By carefully tuning the laser's frequency and direction, scientists can ensure that atoms moving towards the laser are more likely to absorb photons than those moving away. This results in a net slowing down of the atoms, effectively cooling them.
Post by: alancalverd on 29/07/2024 10:17:25
The gap between theory and practice is often closed by an invention from a very different field: witness the impact of the internal combustion engine, originally intended for road transport, on the development of human flight.
Post by: Bored chemist on 29/07/2024 14:15:14
But laser cooling doesn't only work with single atom and single photon.If you look really carefully, you will see that nobody said it did.
I'm pretty sure I have pointed out that "temperature" is only well defined for an ensemble of particles.
Also, if you look carefully here
"The principle is based on the interaction between light and matter. When an atom absorbs a photon (a particle of light), it gains momentum in the direction of the photon's travel. However, when the atom re-emits the photon, it does so in a random direction.
By carefully tuning the laser's frequency and direction, scientists can ensure that atoms moving towards the laser are more likely to absorb photons than those moving away. This results in a net slowing down of the atoms, effectively cooling them."
you will see they talk about frequency and direction, but not coherence.
That's because coherence is not required.
Which is why you don't need a laser. (Though , as I have pointed out, you can get coherent light without using a laser- just like Gabor did)
Sure, using a laser is the only practical option but, in principle it's not the only option.
So the "negative temperature" inside a laser is not the reason it can cool stuff
Post by: hamdani yusuf on 30/07/2024 05:13:15
If one atom has no knowledge of the source of the photon, it is reasonable to assume that all the others are equally ignorant.Then they would fail to produce interference pattern in a double slit experiment.
The gap between theory and practice is often closed by an invention from a very different field: witness the impact of the internal combustion engine, originally intended for road transport, on the development of human flight.
Post by: hamdani yusuf on 30/07/2024 05:23:41
That's because coherence is not required.Here's what Wikipedia says:
Which is why you don't need a laser. (Though , as I have pointed out, you can get coherent light without using a laser- just like Gabor did)
Quote
Optical holography needs a laser light to record the light field.
https://en.wikipedia.org/wiki/Holography
Quote
The Hungarian-British physicist Dennis Gabor invented holography in 1948 while he was looking for a way to improve image resolution in electron microscopes.[6][7][8] Gabor's work was built on pioneering work in the field of X-ray microscopy by other scientists including Mieczysław Wolfke in 1920 and William Lawrence Bragg in 1939.[9] The formulation of holography was an unexpected result of Gabor's research into improving electron microscopes at the British Thomson-Houston Company (BTH) in Rugby, England, and the company filed a patent in December 1947 (patent GB685286). The technique as originally invented is still used in electron microscopy, where it is known as electron holography. Gabor was awarded the Nobel Prize in Physics in 1971 "for his invention and development of the holographic method".[10]
Optical holography did not really advance until the development of the laser in 1960. The development of the laser enabled the first practical optical holograms that recorded 3D objects to be made in 1962 by Yuri Denisyuk in the Soviet Union[11] and by Emmett Leith and Juris Upatnieks at the University of Michigan, US.[12]
https://en.wikipedia.org/wiki/Holography#History
Post by: hamdani yusuf on 30/07/2024 05:34:20
So the "negative temperature" inside a laser is not the reason it can cool stuffI don't agree with the description of laser as negative temperature either.
I agree with Dr. Michelsen's statement that its temperature is not well defined for not being a system in equilibrium. The same situation applies to radio and microwave transmitters.
Post by: alancalverd on 30/07/2024 12:21:27
I don't agree with the description of laser as negative temperature either.It's worth reading the Wikipedia entry on the subject, to understand the concept. Nothing to do with "agreement", but a matter of definition of an unusual phenomenon.
Post by: Bored chemist on 30/07/2024 13:01:17
Here's what Wikipedia says:
Optical holography needs a laser light to record the light field.
The Hungarian-British physicist Dennis Gabor invented holography in 1948Here's what wiki also says
"On May 16, 1960, Theodore H. Maiman operated the first functioning laser
from"
https://en.wikipedia.org/wiki/Laser#History
Are you saying that Gabor time traveled forward from 1948 to 1960 in order to borrow a laser?
Do you not realise that "you need a laser to do holography" is another of the "lies we tell to children"?
Didn't it occur to you that I might simply have been correct when I said this?
"
(Though , as I have pointed out, you can get coherent light without using a laser- just like Gabor did)
Post by: Bored chemist on 30/07/2024 13:02:56
I agree with Dr. Michelsen's statement that its temperature is not well defined for not being a system in equilibrium.More pertinently, you agree with me.
I'm pretty sure I have pointed out that "temperature" is only well defined for an ensemble of particles.
Post by: hamdani yusuf on 01/08/2024 09:21:01
Are you saying that Gabor time traveled forward from 1948 to 1960 in order to borrow a laser?The Wikipedia article says that Gabor invented electron holography.
Post by: hamdani yusuf on 01/08/2024 09:25:16
Nothing to do with "agreement", but a matter of definition of an unusual phenomenon.You can agree or disagree on how other people define things.
Post by: alancalverd on 01/08/2024 14:37:03
Post by: hamdani yusuf on 01/08/2024 15:19:12
Not in science. Words have precise meanings assigned to them by the inventor or discoverer, or by bodies such as the International Standards Organisation. That way we can communicate and build rather than argue or philosophise.We disagreed on how Feynman defined diffraction.
Post by: Bored chemist on 01/08/2024 15:43:33
If he was still alive, you could resolve that..But he's not and it's hard to see how it matters.Not in science. Words have precise meanings assigned to them by the inventor or discoverer, or by bodies such as the International Standards Organisation. That way we can communicate and build rather than argue or philosophise.We disagreed on how Feynman defined diffraction.
Post by: alancalverd on 01/08/2024 20:26:39
We disagreed on how Feynman defined diffraction.Feynman was an individual, not a consensus, and did not discover diffraction.
Post by: hamdani yusuf on 02/08/2024 10:01:51
The one who discovers a phenomenon isn't always the same one who gives it the name that ends up as consensus.We disagreed on how Feynman defined diffraction.Feynman was an individual, not a consensus, and did not discover diffraction.
Post by: alancalverd on 02/08/2024 23:23:57
Post by: hamdani yusuf on 04/08/2024 13:58:01
Post by: alancalverd on 04/08/2024 17:38:46
Post by: hamdani yusuf on 06/08/2024 13:11:09
Post by: hamdani yusuf on 06/08/2024 17:47:04
Quote
That's a great question! While heat capacity and entropy both have the same units (J/K), they represent fundamentally different concepts.Is it just a coincidence?
Heat Capacity
* Measures: The amount of energy required to raise the temperature of a substance by one kelvin.
* Focus: A property of a substance that relates energy to temperature change.
Entropy
* Measures: A measure of the disorder or randomness of a system.
* Focus: A thermodynamic property that relates to the number of microstates available to a system.
So, why the same units?
The connection lies in the mathematical relationship between the two. Entropy change (ΔS) is related to heat (Q) and temperature (T) by the equation:
* ΔS = Q / T
Since heat (Q) is energy, and temperature (T) is in kelvins, the units for entropy become J/K.
In essence, while they measure different things, their mathematical relationship leads to the same units.
Gemini
Is there a deeper relationship between the two?
Post by: hamdani yusuf on 06/08/2024 17:53:28
Quote
Heat capacity and entropy, while having the same units (J/K), represent different concepts:
| Property | Description |
|---|---|
| Heat Capacity | Measures the amount of energy required to raise the temperature of a substance by one kelvin. |
| Entropy | Measures a system's disorder or randomness. |
The connection between their units lies in the mathematical relationship:
* ΔS = Q / T
where:
* ΔS is the change in entropy
* Q is the heat transferred (energy)
* T is the temperature (in kelvin)
Since heat (Q) is energy, and temperature (T) is in kelvins, the units for entropy become J/K. Therefore, even though they measure different aspects, their relationship leads to the same units.
Post by: Bored chemist on 06/08/2024 20:05:35
Is it just a coincidence?No, it's an error.
Post by: hamdani yusuf on 06/08/2024 23:30:48
What's the error?Is it just a coincidence?
No, it's an error.
I asked it to Gemini as well.
Quote
A Coincidence of Units, Not Concepts
The shared unit of J/K for heat capacity and entropy is indeed a coincidence.
While the mathematical relationship ΔS = Q/T links these two quantities, it's crucial to remember that:
* Heat capacity is a property of a substance that relates energy to temperature change.
* Entropy is a measure of the system's disorder or randomness.
These are fundamentally different concepts. The shared unit arises from the inclusion of energy and temperature in their definitions, but it doesn't imply a deeper connection between the two properties.
To summarize:
* The unit similarity is a consequence of the mathematical formulation.
* The underlying physical meanings of heat capacity and entropy are distinct.
Post by: hamdani yusuf on 07/08/2024 03:12:17
Quote
You're absolutely right!
That's a great observation. The formula for entropy change, ΔS = Q/T, does indeed lead to a mathematical issue at absolute zero (T = 0 K).
This is why the Third Law of Thermodynamics is a postulate, not derived from the equation. It's a fundamental assumption based on experimental observations and theoretical considerations.
The equation ΔS = Q/T is valid for finite temperature changes. When approaching absolute zero, the mathematical description of entropy becomes more complex and requires advanced statistical mechanics.
In summary:
* The formula ΔS = Q/T breaks down at absolute zero.
* The Third Law of Thermodynamics provides a foundational principle for entropy at this limit.
Post by: hamdani yusuf on 07/08/2024 13:20:11
Quote
Excellent question! There are several other physical quantities that share the same units, even though they represent different concepts. Here are a few examples:While the other points are basically pairs of the same measures, the second point tells a different story. There is something missing here.
1. Energy and Work
* Both measured in joules (J).
* Energy is the capacity to do work, while work is the transfer of energy.
2. Torque and Energy
* Both measured in newton-meters (Nm) or joules (J).
* Torque is a rotational force, while energy is the capacity to do work.
3. Pressure and Force per Unit Area
* Both measured in pascals (Pa) or newtons per square meter (N/m?).
* Pressure is the force exerted per unit area, while force per unit area is a direct definition of pressure.
4. Power and Energy per Unit Time
* Both measured in watts (W) or joules per second (J/s).
* Power is the rate at which energy is transferred, while energy per unit time is a direct definition of power.
These examples highlight the importance of understanding the underlying concepts behind physical quantities, even when they share the same units.
Post by: alancalverd on 07/08/2024 16:30:26
Post by: hamdani yusuf on 08/08/2024 11:20:07
I asked Gemini for other Physical Measures with the Same UnitsI told Gemini that energy is torque times the angle of rotation.QuoteExcellent question! There are several other physical quantities that share the same units, even though they represent different concepts. Here are a few examples:
...
2. Torque and Energy
* Both measured in newton-meters (Nm) or joules (J).
* Torque is a rotational force, while energy is the capacity to do work.
...
While the other points are basically pairs of the same measures, the second point tells a different story. There is something missing here.
Quote
You're absolutely right!It means that the proper unit for torque is Joule/radian, or Newton meter/radian.
Energy = Torque x Angle of Rotation
This equation is a fundamental relationship in rotational mechanics. Here's a breakdown:
* Torque: A measure of the force that can cause an object to rotate around an axis. It's analogous to force in linear motion.
* Angle of rotation: The amount of rotation an object undergoes around an axis.
When you multiply torque by the angle of rotation, you get the work done, which is a form of energy.
Key points:
* This equation holds true for constant torque. If the torque varies, integration is required.
* The units of torque (Newton-meter) multiplied by the unit of angle (radians) give the unit of energy (joules).
I found many people being confused about measurement units involving radian, which is often omitted for being a dimensionless quantity.
The unit radian for measuring angle of rotation is convenient since the traveling distance of a point due to 1 radian rotation equals the radius of rotation.
Quote
https://en.wikipedia.org/wiki/Radian
(https://upload.wikimedia.org/wikipedia/commons/thumb/4/4e/Circle_radians.gif/330px-Circle_radians.gif)
An arc of a circle with the same length as the radius of that circle subtends an angle of 1 radian. The circumference subtends an angle of 2π radians.
Post by: alancalverd on 08/08/2024 11:35:09
Torque per unit angle is (ideally) a measure of the elasticity of the bolt you are tightening plus the frictional loss of the tightening process.
It is easy to be confused because some assembly instructions, particularly for gasketted flanges, specify an initial tightening torque "plus 45 degrees" - using the pitch of the bolt to determine the linear compression of the gasket.
Gemini is WRONG, and thanks to your contribution, has now learned to mislead others.
Post by: hamdani yusuf on 08/08/2024 11:49:51
No. Newton.meter is a unit of energy (dimension ML2T-2) or torque - your standard torque wrench is calibrated by hanging a weight on the end!Let's say I have a standard torque wrench with 1 meter length. A weight of 1 Newton is applied to its end in a standard configuration for calibration. When the wrench doesn't rotate at all, no work has been done, thus 0 Joule, because the angle of rotation is 0.
Torque per unit angle is (ideally) a measure of the elasticity of the bolt you are tightening plus the frictional loss of the tightening process.
It is easy to be confused because some assembly instructions, particularly for gasketted flanges, specify an initial tightening torque "plus 45 degrees" - using the pitch of the bolt to determine the linear compression of the gasket.
Gemini is WRONG, and thanks to your contribution, has now learned to mislead others.
If it rotates by 1 milli radian, then the energy of 1 milli Joule has been transferred.
Post by: hamdani yusuf on 08/08/2024 14:12:45
Gemini is WRONG, and thanks to your contribution, has now learned to mislead others.I started a new conversation with Gemini, and asked what's the unit of torque.
Quote
The unit of torque is the Newton-meter (N.m).It seems that it doesn't learn from our previous conversation, so don't worry.
It's important to note that while the units of torque and energy (Joules) are the same, they represent different physical quantities and should not be confused.
Or should we worry instead?
Post by: alancalverd on 08/08/2024 22:24:04
Let's say I have a standard torque wrench with 1 meter length. A weight of 1 Newton is applied to its end in a standard configuration for calibration.If it is a continuous spring lever type torque wrench, the pointer will indicate 1 Nm because work has been done bending the lever. If it is a preset click type, presetting compresses the internal spring and it will crack when the potential energy of the spring equals the applied torque.
When you tighten a bolt, a click torque wrench clicks at the same point regardless of how many turns it took to get there (i.e. the length of the bolt and the pitch of the thread) so angle is irrelevant.
Post by: hamdani yusuf on 09/08/2024 03:20:53
Limitations of the particular measuring device that you use should not affect the definition of the quantity being measured.Let's say I have a standard torque wrench with 1 meter length. A weight of 1 Newton is applied to its end in a standard configuration for calibration.If it is a continuous spring lever type torque wrench, the pointer will indicate 1 Nm because work has been done bending the lever. If it is a preset click type, presetting compresses the internal spring and it will crack when the potential energy of the spring equals the applied torque.
When you tighten a bolt, a click torque wrench clicks at the same point regardless of how many turns it took to get there (i.e. the length of the bolt and the pitch of the thread) so angle is irrelevant.
Let's say a sensitive sensor is installed for the torque measuring device which can produce a reliable signal with only 1 micro-Joule input. If the torque stop as soon as the sensor is active, then the energy transferred is only 1 micro-Joule. But if it continuously applied until it turns by 1 radian, then the energy transferred is 1 Joule.
I think the discussion about torque can be more focused if it's done in a separated thread.
https://www.thenakedscientists.com/forum/index.php?topic=87006.msg730897#msg730897
Post by: hamdani yusuf on 09/08/2024 09:34:22
Quote
Did you know there's a whole branch of material sciences taking advantage of certain quirks of physics to create passive cooling devices? Because I didn't. But hopefully you'll find the science behind it as exciting as I did!According to some physicists, this radiative cooling is not part of thermodynamics.
High-performance subambient radiative cooling
enabled by optically selective and thermally insulating
polyethylene aerogel - https://doi.org/10.1126/sciadv.aat9480
Hierarchical-morphology metafabric for scalable passive daytime radiative cooling - https://doi.org/10.1126/science.abi5484
Subambient daytime radiative cooling textile based on nanoprocessed silk - https://doi.org/10.1038/s41565-021-00...
Circle larger than the wavelength - http://www.met.reading.ac.uk/clouds/m...
FTIR Spectra of Polymers - http://www.ftir-polymers.com/soon.htm
0:37 The Greenhouse Effect
1:16 How Does Radiation Influence Temperature
2:18 Electron Cloud
5:03 Inverse Greenhouse
7:08 Aerogel
9:12 Infrared Cooling Fabrics
10:11 Silk
Post by: Bored chemist on 09/08/2024 14:00:57
According to some physicists...Name them.
Post by: hamdani yusuf on 10/08/2024 11:53:10
According to some physicists...Name them.
The Long Answer - Everything You Ever Wanted To Know About EntropyQuoteThis is the second, and longer, "solution" video to my "new brainteaser" video about compressing air, losing ALL the energy used to compress it, and still having compressed air that can be used to run my pneumatic tools.The unit of entropy reveals its connection between temperature and energy.
I want to thank Dr. Michelsen for his generosity. He also has an excellent document he's posted on-line that you can see here:
"Statistical Mechanifesto or Funky Statistical Mechanics Concepts"
https://elmichelsen.physics.ucsd.edu/...
If you search the document for "brain teaser" you can see what he's written about this problem.
I encourage people to check it out. If you want to forward the document, he requests that you forward the link rather than a copy of the document. This is simply because he updates the document fairly regularly.
Post by: alancalverd on 10/08/2024 17:37:16
This is the second, and longer, "solution" video to my "new brainteaser" video about compressing air, losing ALL the energy used to compress it, and still having compressed air that can be used to run my pneumatic tools.Written by someone who has no concept of physics.
Anyone who had actually built a perpetual motion machine wouldn't waste his time making "educational" videos about it.
Post by: hamdani yusuf on 12/08/2024 08:59:11
Anyone who had actually built a perpetual motion machine wouldn't waste his time making "educational" videos about it.What perpetual motion machine are you referring to?
Post by: Bored chemist on 12/08/2024 09:58:03
The hypothetical one.Anyone who had actually built a perpetual motion machine wouldn't waste his time making "educational" videos about it.What perpetual motion machine are you referring to?
Did you not recognise that?
Post by: hamdani yusuf on 12/08/2024 10:35:29
No. Can you help me out?The hypothetical one.Anyone who had actually built a perpetual motion machine wouldn't waste his time making "educational" videos about it.What perpetual motion machine are you referring to?
Did you not recognise that?
Post by: Bored chemist on 12/08/2024 15:47:07
/wʊd/
verb
1.
past of will1, in various senses.
"he said he would be away for a couple of days"
2.
(expressing the conditional mood) indicating the consequence of an imagined event or situation.
"he would lose his job if he were identified" "
Alan didn't say "the person who did make a PM machine didn't waste his time making "educational" videos about it." as he would have done if the event was actually real.
Post by: alancalverd on 12/08/2024 17:01:36
What perpetual motion machine are you referring to?The supposed system that allows you to compress air, remove all the energy you used to compress it, and still have compressed air. You could use the removed energy to heat something, and use the remaining compressed air to compress more air, ad infinitum.
Post by: hamdani yusuf on 13/08/2024 14:19:34
I think I know where he got it wrong. When the compressed air has cooled down to ambient temperature, there's still potential energy left in it in the form of higher pressure than initial condition.What perpetual motion machine are you referring to?The supposed system that allows you to compress air, remove all the energy you used to compress it, and still have compressed air. You could use the removed energy to heat something, and use the remaining compressed air to compress more air, ad infinitum.
Post by: alancalverd on 13/08/2024 18:11:16
losing ALL the energy used to compress it, and still having compressed air that can be used to run my pneumatic tools.
What an idiot!
Post by: hamdani yusuf on 14/08/2024 10:19:35
What a surprise!Everyone makes mistakes sometimes.
losing ALL the energy used to compress it, and still having compressed air that can be used to run my pneumatic tools.
What an idiot!
He came to prominence as physicist after the Blackbird project, and popularized by Veritasium later on.
CNN Early Start - Blackbird upwind cart
Quote
CNN aired this segment on our wind powered cart. Nice segment, but it seems they forgot to mention that the vehicle was designed to go directly upwind (at about 2X wind speed) and directly downwind (at about 3X wind speed).
Wind powered direct upwind vehicle - DUWFTTW
Quote
This is a wind powered vehicle that goes directly into the wind at more than 2X wind speed. This video was taken on 16 June 2012 at the New Jerusalem airstrip in Northern California. The vehicle was originally designed and built to go directly downwind faster than the wind, and set a world record by doing so on 2 July 2010, when it went 2.8X wind-speed directly downwind. The data from one of the runs on this video will be submitted to the North American Land Sailing Association for consideration for a direct upwind record. The vehicle is called the "Blackbird".
Post by: Bored chemist on 14/08/2024 10:30:39
Here's another, similar brain teaser.
I come home and I think my room (roughly 3m by 3m by 2.5 m ; I'm ignoring the volume of the fireplace) is too cold; it's only 10 C in here (283K) , so I switch on the heating- a 1 KW electric fire.
After 1000 seconds it is warm enough and I switch the heater off.
How much more energy is there in the air in the room?
(Making the usual (unrealistic) assumption that there's no energy loss to the walls and furniture or any gain from me being in there metabolising food.
We are also assuming air is an ideal gas)
Post by: alancalverd on 14/08/2024 17:54:08
Lots of things defy arrogant folk who call themselves physicists.
The answer to BC's question is obviously 1 megajoule. Knowing the density (about 1.23 kg/m3 )and specific heat capacity (about 0.72 kJ/kg.K) of air, you can calculate the final temperature.
Post by: Bored chemist on 14/08/2024 20:40:17
The correct answer is zero.
Now work out why.
Post by: alancalverd on 14/08/2024 23:02:58
(a) because your electric fire was powered by a battery that was "in the air" or
(b) it was powered from an external source (the mains) and the laws of physics do not apply in your part of the world or
(c) when you say "it is warm enough", you actually mean "I am warm enough" because you just heated yourself with a radiant rather than a convective heater. But you will eventually equilibrate by convection.
Post by: Bored chemist on 15/08/2024 11:05:18
EitherNope.
(a) because your electric fire was powered by a battery that was "in the air" or
(b) it was powered from an external source (the mains) and the laws of physics do not apply in your part of the world or
(c) when you say "it is warm enough", you actually mean "I am warm enough" because you just heated yourself with a radiant rather than a convective heater. But you will eventually equilibrate by convection.
It's simpler than that and it's related to the observation that was made in the video about energy and compressed air.
It's also related to why this is certainty not correct.
Knowing the density (about 1.23 kg/m3 )
Post by: paul cotter on 15/08/2024 12:02:51
Post by: hamdani yusuf on 15/08/2024 13:45:18
Post by: alancalverd on 15/08/2024 15:22:43
the observation that was made in the video about energy and compressed air.See my reply #865 above.
Having just landed from an "interesting" flight, I can assure you that on Planet East Anglia the air density is indeed around 1.23 kg/m3, which is why the wings and engine worked exactly as calculated.
And I can't believe that a sensible scientist like BC would not have draught-sealed doors and windows.
Post by: Bored chemist on 15/08/2024 16:16:57
If the room is not sealed we are dealing with a constant pressure process and the air will expand leading to a loss to the external environment. Whether it all adds to zero would involve too much calculation for me.The calculation is that PV is a constant.
And I can't believe that a sensible scientist like BC would not have draught-sealed doors and windows.
I'm ignoring the volume of the fireplace
Buildings leak; they have to or atmospheric pressure variations would make them unworkable.
It must go somewhere in the room.No. It leaves the room and raises the atmosphere by a tiny amount, doing work against gravity.
The air expands to an extent which is proportional to the change in absolute temperature. So the amount of air left falls by exactly the same ratio.
Each molecule has an energy that is proportional to the absolute temperature so the effects cancel out and the energy remains the same.
Post by: Bored chemist on 15/08/2024 16:18:08
the air density is indeed around 1.23 kg/m3Whatever the air density was at the start, it was different at the end.
Post by: alancalverd on 15/08/2024 21:18:03
Now if all the heat had been absorbed by the air, you would have raised your 27.7 kg of air by about 50K - dangerously unbreathable, whether or not you allowed some to escape.
Post by: Bored chemist on 15/08/2024 22:10:14
Now if all the heat had been absorbed by the air, you would have raised your 27.7 kg of air by about 50KAnd mighty glad I was too- it started at -30 C.
That temperature rise would create an increase in pressure of roughly 1/5 of an atmosphere and exerting a force of over two tons on the window or door.
So now you know why I said "
Buildings leak; they have to or atmospheric pressure variations would make them unworkable.
BTW, why did you think I mentioned the fireplace?
Post by: alancalverd on 16/08/2024 14:06:24
The first one had a starting temperature of 283K and we were told to ignore the volume of the fireplace so it was reasonable to assume that the chimney was blocked.
0.18 atmospheres is about the pressure of a 100 mph wind on a bluff body. The internal doors might object but your front door and any modern double-glazed windows won't be too fussed. Come to think of it, most internal doors open into the room so it wouldn't even blow open.
Not that it's a problem as you would have switched off the heater and/or opened the window after about 200 seconds to avoid death.
Moral: wear a woolly sweater or a fur coat in cold weather. It works for every other mammal and people who work outdoors.
Post by: hamdani yusuf on 16/08/2024 14:15:49
No. It leaves the room and raises the atmosphere by a tiny amount, doing work against gravity.OK. I think the results depend on which factors can be safely ignore, and which factors can't.
Assuming that the air is an ideal gas, its energy = pressure x volume = n. R. T
As long as the pressure and volume is constant, the energy is constant.
But the air temperature has increased, thus the number of air molecules must have decreased to make the equation balanced.
How far can the temperature increase without changing volume of the room?
Post by: Bored chemist on 16/08/2024 15:40:18
Must be a different room.I told you there was a fireplace (and, therefore a hole, called a chimney).
The first one had a starting temperature of 283K and we were told to ignore the volume of the fireplace so it was reasonable to assume that the chimney was blocked.
0.18 atmospheres is about the pressure of a 100 mph wind on a bluff body. The internal doors might object but your front door and any modern double-glazed windows won't be too fussed. Come to think of it, most internal doors open into the room so it wouldn't even blow open.
Not that it's a problem as you would have switched off the heater and/or opened the window after about 200 seconds to avoid death.
Moral: wear a woolly sweater or a fur coat in cold weather. It works for every other mammal and people who work outdoors.
You assumed this was grounds to think the room was sealed.
That's on you.
In reality, a 5 degree temperature change would raise the air pressure in a sealed room to such an extent that it would make it hard to open the door.
People who require oxygen do not seal their houses.
Stop pretending that you were "right".
The answer is that there's no more energy in the room.
How far can the temperature increase without changing volume of the room?Bricks expand by about 6 parts per million per degree C.
So the volume change is about 18 ppm/C
How much Alan's room would expand by under a pressure of something like 2 tons per square metre is anyone's guess.
Post by: hamdani yusuf on 17/08/2024 12:38:12
Post by: alancalverd on 17/08/2024 14:24:50
How much Alan's room would expand by under a pressure of something like 2 tons per square metre is anyone's guess.Not a lot. It's pretty close to the pressure difference between an airliner cabin and the ambient at 30,000 ft, and those structures rarely fall apart.
Post by: hamdani yusuf on 19/08/2024 11:23:17
According to some physicists, this radiative cooling is not part of thermodynamics.https://youtube.com/shorts/WerKkrkuwHg?feature=shared
The way they cook the hot dog is not covered by thermodynamics either.
Post by: alancalverd on 19/08/2024 11:33:52
The current flow through the resistive hot dog generates heat which disperses through conduction, convection and radiation. At the primary contact points it gets hot enough (due to high local current density) to evaporate the sausage and generate a plasma which radiates all sorts of e.m. including visible light and possibly some ultraviolet.
Post by: alancalverd on 19/08/2024 11:36:12
People who require oxygen do not seal their houses.We all live in a yellow submarine....
chorus:
...and we keep the windows open....
Post by: hamdani yusuf on 19/08/2024 12:35:38
Oh yes it is.Which thermodynamics formula you used?
The current flow through the resistive hot dog generates heat which disperses through conduction, convection and radiation. At the primary contact points it gets hot enough (due to high local current density) to evaporate the sausage and generate a plasma which radiates all sorts of e.m. including visible light and possibly some ultraviolet.
Where does the heat come from and go to?
Post by: Bored chemist on 19/08/2024 12:43:42
Have you seen how they build the doors on airliners?How much Alan's room would expand by under a pressure of something like 2 tons per square metre is anyone's guess.Not a lot. It's pretty close to the pressure difference between an airliner cabin and the ambient at 30,000 ft, and those structures rarely fall apart.
Even in spite if that, when they do fail, it's not good news.
https://www.bbc.co.uk/news/business-67930977
You are in a hole Alan. Stop digging.
Post by: Bored chemist on 19/08/2024 12:48:59
Really?People who require oxygen do not seal their houses.We all live in a yellow submarine....
chorus:
...and we keep the windows open....
Where?
[Verse 1: Ringo Starr]
In the town where I was born
Lived a man who sailed to sea
And he told us of his life
In the land of submarines
[Verse 2: Ringo Starr]
So we sailed on to the sun
Till we found the sea of green
And we lived beneath the waves
In our yellow submarine
[Chorus: Ringo Starr, Paul McCartney, John Lennon & George Harrison]
We all live in a yellow submarine
Yellow submarine, yellow submarine
We all live in a yellow submarine
Yellow submarine, yellow submarine
[Verse 3: Ringo Starr]
And our friends are all aboard
Many more of them live next door
And the band begins to play
[Chorus: Ringo Starr, Paul McCartney, John Lennon & George Harrison]
We all live in a yellow submarine
Yellow submarine, yellow submarine
We all live in a yellow submarine
Yellow submarine, yellow submarine
[Bridge: Paul McCartney & John Lennon]
Full speed ahead, Mister Boatswain, full speed ahead
Full speed ahead it is, Sergeant
Cut the cable! Drop the cable!
Aye-aye, sir, aye-aye
Captain! Captain!
[Verse 4: Ringo Starr & John Lennon]
As we live a life of ease
Every one of us (Every one of us) has all we need (Has all we need)
Sky of blue (Sky of blue) and sea of green (Sea of green)
In our yellow (In our yellow) submarine (Submarine, ha-ha!)
[Chorus: Ringo Starr, Paul McCartney, John Lennon & George Harrison]
We all live in a yellow submarine
Yellow submarine, yellow submarine
We all live in a yellow submarine
Yellow submarine, yellow submarine
We all live in a yellow submarine
Yellow submarine, yellow submarine
We all live in a yellow submarine
Yellow submarine, yellow submarine
Post by: alancalverd on 19/08/2024 13:47:44
Sky of blue (Sky of blue) and sea of greenHow do you know if you haven't got windows?
Post by: hamdani yusuf on 19/08/2024 14:24:06
According to some physicists, this radiative cooling is not part of thermodynamics.https://youtube.com/shorts/WerKkrkuwHg?feature=shared
The way they cook the hot dog is not covered by thermodynamics either.
Magnifying The World's Brightest Flashlight (200,000 Lumens)In this case, the flashlight is cooler than the burning paper. Yet the energy flows from the flashlight to the paper.
This video can give some reality checks for our current understanding of temperature.
Post by: alancalverd on 19/08/2024 14:56:00
Post by: Bored chemist on 19/08/2024 15:11:57
Periscope.Sky of blue (Sky of blue) and sea of greenHow do you know if you haven't got windows?
Or just a good memory.
Do you realise the point of windows is that you can see through them even if they are closed?
Post by: Bored chemist on 19/08/2024 15:14:20
It's not an equilibrium system. It doesn't really have a well defined temperature.According to some physicists, this radiative cooling is not part of thermodynamics.https://youtube.com/shorts/WerKkrkuwHg?feature=shared
The way they cook the hot dog is not covered by thermodynamics either.Magnifying The World's Brightest Flashlight (200,000 Lumens)In this case, the flashlight is cooler than the burning paper. Yet the energy flows from the flashlight to the paper.
This video can give some reality checks for our current understanding of temperature.
It's not an equilibrium system. It doesn't really have a well defined temperature.
It's not an equilibrium system. It doesn't really have a well defined temperature.
It's not an equilibrium system. It doesn't really have a well defined temperature.
I said it a few more times in the hope that you will read it and remember what it means.
Post by: alancalverd on 19/08/2024 19:56:25
Thermodynamics is all about heat flow, and you don't get flow in an equilibrium system....
Post by: hamdani yusuf on 20/08/2024 03:09:12
It's not an equilibrium system. It doesn't really have a well defined temperature.Some physicists think that these cases are not covered by thermodynamics. Some others disagree.
I'd like to know the arguments from each sides.
No. The color temperature of the photons is about 4000 K.
The body of LED as well as the lens have definitive melting points. While they are functioning, we can safely say that their temperatures are below these points.
Post by: hamdani yusuf on 20/08/2024 05:15:15
Thermodynamics is all about heat flow, and you don't get flow in an equilibrium system....Adiabatic processes are part of thermodynamics.
Quote
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics, which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology.
https://en.m.wikipedia.org/wiki/Thermodynamics
Post by: alancalverd on 20/08/2024 10:58:34
Thermodynamics tells us that ΔT is proportional to ΔE, so what's the problem?
I spent many happy years measuring ΔT induced by x- and γ-rays absorbed in carbon and water - it's how we define radiation dose.
Post by: hamdani yusuf on 20/08/2024 15:20:57
Thermodynamics tells us that ΔT is proportional to ΔE, so what's the problem?Which law is it?
Isothermic process can change the energy without changing temperature.
Post by: alancalverd on 20/08/2024 16:17:48
Post by: hamdani yusuf on 20/08/2024 23:22:59
You need to combine 0, 1 and 2.How do they explain isothermic process?
Thermodynamics tells us that ΔT is proportional to ΔE, so what's the problem?
Post by: alancalverd on 21/08/2024 08:15:32
Post by: hamdani yusuf on 21/08/2024 09:41:04
Quote
Simply, we can say that in an isothermal process
T = constant
https://en.m.wikipedia.org/wiki/Isothermal_process
Example
(https://upload.wikimedia.org/wikipedia/commons/f/fc/Isothermal_expansion_of_an_ideal_gas.png)
Post by: hamdani yusuf on 21/08/2024 09:43:08
Thermodynamics tells us that ΔT is proportional to ΔE, so what's the problem?The problem is it's wrong.
Only some portion of the energy of an object is manifested as its temperature.
Post by: paul cotter on 21/08/2024 10:51:20
Post by: alancalverd on 21/08/2024 12:18:06
Only some portion of the energy of an object is manifested as its temperature.Note the conventional use of Δ.
Post by: hamdani yusuf on 21/08/2024 13:04:04
You can have ΔT = 0 while ΔE ≠ 0 and vice versa.Only some portion of the energy of an object is manifested as its temperature.Note the conventional use of Δ.
Post by: alancalverd on 21/08/2024 15:13:48
Post by: Bored chemist on 21/08/2024 20:29:47
Thermodynamics tells us that ΔT is proportional to ΔE, so what's the problem?What do you call the constant of proportionality?
Post by: alancalverd on 22/08/2024 10:55:57
Post by: Bored chemist on 23/08/2024 10:51:43
(Specific) heat capacity. Assuming no phase change, of course.So... this sort of "constant".
[ Invalid Attachment ]
Post by: alancalverd on 23/08/2024 12:32:30
And IIRC the special air in your room was an ideal gas.
Post by: hamdani yusuf on 23/08/2024 12:37:53
Over the humanly survivable temperature and pressure range, say -40 to + 150F and 14.5 psi, the curve looks flat enough to me.Since when was human survivability taken into account in fundamental physics?
Post by: alancalverd on 23/08/2024 12:55:22
Fundamental physics my well address intolerable ambients, but BC's graph, and engineering in general, is about answering the question "well enough".
Post by: hamdani yusuf on 01/09/2024 02:47:08
I asked ChatGPT again, Why Heat Capacity and Entropy Share the Same Units?
Quote
Heat capacity and entropy share the same units because they are both derived from the fundamental relationship between energy and temperature in thermodynamic processes.One obvious difference is the position of symbol Delta.
### Units of Heat Capacity
- **Heat Capacity (C)** is defined as the amount of heat required to change the temperature of a substance by one degree. Mathematically, it is expressed as:
\[
C = \frac{Q}{\Delta T}
\]
where \( Q \) is the heat added, and \( \Delta T \) is the temperature change.
Since \( Q \) is measured in joules (J) and \( \Delta T \) in kelvins (K), the unit of heat capacity is joules per kelvin (J/K).
### Units of Entropy
- **Entropy (S)** is a measure of the disorder or randomness of a system. In thermodynamics, it is related to the amount of heat exchanged in a reversible process at a constant temperature. The change in entropy is given by:
\[
\Delta S = \frac{Q_{\text{rev}}}{T}
\]
where \( Q_{\text{rev}} \) is the reversible heat exchange, and \( T \) is the absolute temperature.
Here, \( Q_{\text{rev}} \) is in joules (J), and \( T \) is in kelvins (K), making the unit of entropy also joules per kelvin (J/K).
### Why They Share the Same Units
Both heat capacity and entropy involve the relationship between energy (heat) and temperature. The similarity in units arises because both quantities describe how energy (measured in joules) is distributed with respect to temperature (measured in kelvins):
1. **Heat Capacity** measures how much energy is needed to change the temperature of a substance, indicating the energy absorption efficiency.
2. **Entropy** measures how energy is distributed at a specific temperature, representing the disorder or randomness of the system.
Although they describe different physical concepts?heat capacity deals with energy absorption, while entropy deals with energy distribution?they both fundamentally relate energy to temperature, leading to the same units (J/K).
Post by: Bored chemist on 01/09/2024 10:38:36
BC was talking about heating a room in his house because it was uncomfortably cold.I was talking about a theoretical case with the numbers chosen to make the maths easy (perhaps deceptively so).
Post by: Bored chemist on 01/09/2024 10:43:21
Over the humanly survivable temperature and pressure range, say -40 to + 150F and 14.5 psi, the curve looks flat enough to me.OK. That's the circumstances I specified in my post about heating a room.
And IIRC the special air in your room was an ideal gas.
And here's your post
Anyway, apropos setting fire to something with light beam, you are adding energy to the target by irradiating it with electromagnetic radiation. What isn't scattered or reflected either induces a chemical change or just heats the target.
Thermodynamics tells us that ΔT is proportional to ΔE, so what's the problem?
I spent many happy years measuring ΔT induced by x- and γ-rays absorbed in carbon and water - it's how we define radiation dose.
Where you specify setting fire to things so we know that the temperature is much higher and there's a chemical reaction going on.
So, is " ΔT is proportional to ΔE" in that situation?
Post by: Bored chemist on 01/09/2024 10:47:24
I asked ChatGPT again, Why Heat Capacity and Entropy Share the Same Units?A real (educated) intelligence would tell you that you are mistaken.
They do not share the same units.
You should stop asking AI about stuff.
As an aside, I was chatting to a friend of mine who is doing a PhD in computer science and she pointed out that chat bots are not programmed to be accurate; they are programmed to "sound correct".
If you want your bots to pass a Turing test, you have to teach them to make the same dumb mistakes that people do.
Never rely on them for anything important.
Post by: paul cotter on 01/09/2024 13:42:12
Post by: hamdani yusuf on 01/09/2024 15:11:30
They do not share the same units.What's the difference between their respective units?
Post by: hamdani yusuf on 01/09/2024 15:18:25
Hi BC, I have repeatedly told him to forget these bots and pop-sci videos and obtain some good textbooks. Alas, this has been in vain and I predict confusion ad infinitum.Which textbooks? What's their answer to my questions?
Post by: hamdani yusuf on 01/09/2024 15:21:46
As an aside, I was chatting to a friend of mine who is doing a PhD in computer science and she pointed out that chat bots are not programmed to be accurate; they are programmed to "sound correct".There are many models of chatbots. Some of them are even open sourced. New chatbots can be customized according to our needs.
If you want your bots to pass a Turing test, you have to teach them to make the same dumb mistakes that people do.
Post by: Bored chemist on 01/09/2024 15:22:51
Practically they don't, because (for heat capacity) you almost always add an amount so
J/K mol or J/K Kg
But for entropy, the "unit" is often "per molecule/ atom " Those entities are countable, and a number is dimensionless.
On the other hand, "mole" is also a number.
It's a perennial problem in metrology. How do you measure "how much stuff you have".
If I make an apple and blackberry pie and I want it to be 50:50 apples and blackberries, do I mix a pound of apples with a pound of blackberries, or do I count out the same number of each?
You are left to figure it out from context.
Post by: Bored chemist on 01/09/2024 15:29:24
Hi BC, I have repeatedly told him to forget these bots and pop-sci videos and obtain some good textbooks. Alas, this has been in vain and I predict confusion ad infinitum.Bots are cheap, easy and "convincing".
The fact that they don't believe there are three Rs in strawberry is, apparently, less important.
https://www.inc.com/kit-eaton/how-many-rs-in-strawberry-this-ai-cant-tell-you.html
That's not a problem because anyone can just count them and laugh at the stupid bot.
But it's a massive problem if you are trying to find the answer to something where you don't already know.
It's not really a new problem. Google, Lycos, AskJeves etc all screwed up royally in their early days. They got better but they are still not perfect.
Post by: alancalverd on 01/09/2024 22:33:23
Where you specify setting fire to things so we know that the temperature is much higher and there's a chemical reaction going on.
So, is " ΔT is proportional to ΔE" in that situation?
Up to the ignition point, yes. From there on, it's chemistry, not physics!
Post by: alancalverd on 01/09/2024 22:39:38
If I make an apple and blackberry pie and I want it to be 50:50 apples and blackberries, do I mix a pound of apples with a pound of blackberries, or do I count out the same number of each?
Old music hall joke:
I got rich selling chicken pies for ten cents
How can you make a profit at that price?
I dilute the chicken with horse meat
How much horse meat?
50/50. One horse, one chicken
Post by: Bored chemist on 02/09/2024 13:15:29
The graphs I posted show that it's distinctly not constant up to "Fahrenheit 451".Where you specify setting fire to things so we know that the temperature is much higher and there's a chemical reaction going on.
So, is " ΔT is proportional to ΔE" in that situation?
Up to the ignition point, yes. From there on, it's chemistry, not physics!
Post by: alancalverd on 02/09/2024 15:02:06
And HY's video seems to be about setting fire to paper, not air.
Post by: paul cotter on 02/09/2024 19:03:34
Post by: Bored chemist on 02/09/2024 20:16:25
In engineering it is normal to differentiate between Cv and Cp, constant volume and constant pressure specific heats. But then i'm sure you all know this(well, mostly all).Most of us do.
Some still seem to be struggling with the idea that you need air for a fire, and if it's cool enough the fire won't start.
Post by: Bored chemist on 02/09/2024 20:18:11
And HY's video seems to be about setting fire to paper, not air.Oddly enough, so is "Fahrenheit 451"- among other things.
Post by: paul cotter on 02/09/2024 20:57:42
Post by: alancalverd on 02/09/2024 22:35:06
In reply #900 I was discussing the temperature rise of the (paper?) target in the optical ignition video that HY posted. BC then threw in a graph of specific heat capacity of air over a huge range of temperatures and pressures, of dubious relevance since the video clearly shows humans in a tolerable ambient. Once any water has evaporated from the target, the specific heat capacity of most common solids (including dry paper) doesn't change much until they melt or ignite.
Post by: Bored chemist on 03/09/2024 11:09:08
Alan calls my post " of dubious relevance" because it shows his was wrong.
AC
Thermodynamics tells us that ΔT is proportional to ΔE, so what's the problem?
BC
What do you call the constant of proportionality?
AC
(Specific) heat capacity. Assuming no phase change, of course.
BC
So... this sort of "constant".
[graph of heat capacity being not constant]
Post by: alancalverd on 03/09/2024 15:35:29
in order for the paper to burn in air, the air in contact with the paper also has to be hot.So no forest fires in Canada.
Post by: Bored chemist on 03/09/2024 19:08:54
None which is started by air at 20 C.in order for the paper to burn in air, the air in contact with the paper also has to be hot.So no forest fires in Canada.
Ever blown out a candle?
Post by: alancalverd on 03/09/2024 23:01:08
None which is started by air at 20 C.Nor is the ignition of paper by photons. Air being virtually transparent to visible light, the air around the paper will be a lot colder than 451F when the paper ignites.
Post by: Bored chemist on 03/09/2024 23:25:56
(Air which is not near the paper can't react with it anyway.)
Post by: alancalverd on 04/09/2024 10:51:53
Post by: hamdani yusuf on 07/09/2024 06:03:24
Formally they have the same units. J/KJ/(K mol) is the unit for molar heat capacity
Practically they don't, because (for heat capacity) you almost always add an amount so
J/K mol or J/K Kg
But for entropy, the "unit" is often "per molecule/ atom " Those entities are countable, and a number is dimensionless.
J/(K Kg) is the unit for specific heat capacity
Entropy is an extensive quantity.
Quote
Entropy is an extensive quantity.
This means that the total entropy of a system is directly proportional to its size or mass. In other words, if you double the size of a system, you double its entropy. This is in contrast to intensive properties, which do not depend on the size of the system.
Here's a simple example:
* Imagine two identical boxes of gas. Each box has the same temperature, pressure, and volume.
* Now combine the two boxes. The resulting system has double the volume, double the mass, and double the entropy.
This extensive property of entropy is a fundamental principle in thermodynamics. It reflects the idea that larger systems have more ways to distribute energy and matter among their constituent particles, leading to a higher degree of disorder.
Post by: hamdani yusuf on 07/09/2024 09:51:17
Entropy change (ΔS) is related to heat (Q) and temperature (T) by the equation:
ΔS = Q / T
T is an intensive quantity, while ΔS and Q are both extensive quantities.
The equation above only tells about change in entropy, not the absolute entropy of the system itself.
S = S0 + ∫dS
S = S0 + ∫(dQ / T)
Meanwhile,
Quote
(https://wikimedia.org/api/rest_v1/media/math/render/svg/4422a72c7a3c220fba255df2cdc2466a34b1d62e)The equation implies that T = dQ / dS
https://en.wikipedia.org/wiki/Second_law_of_thermodynamics#Introduction
(https://upload.wikimedia.org/wikipedia/commons/thumb/0/02/Heat_flow_hot_to_cold.png/170px-Heat_flow_hot_to_cold.png)
Heat flowing from hot water to cold water
If two systems have different temperature come into contact, energy will naturally flow from the system with higher temperature to the system with lower temperature.
Post by: hamdani yusuf on 07/09/2024 22:47:07
Quote
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making up a substance.The article above also mention kinetic temperature and internal temperature.
https://en.m.wikipedia.org/wiki/Temperature
In contrast,
Quote
Thermodynamic temperature is a quantity defined in thermodynamics as distinct from kinetic theory or statistical mechanics.Historically, temperature was our attempt to determine the direction of heat transfer between objects. Heat flows naturally from objects with higher temperature to objects with lower temperature.
Historically, thermodynamic temperature was defined by Lord Kelvin in terms of a macroscopic relation between thermodynamic work and heat transfer as defined in thermodynamics, but the kelvin was redefined by international agreement in 2019 in terms of phenomena that are now understood as manifestations of the kinetic energy of free motion of microscopic particles such as atoms, molecules, and electrons. From the thermodynamic viewpoint, for historical reasons, because of how it is defined and measured, this microscopic kinetic definition is regarded as an "empirical" temperature. It was adopted because in practice it can generally be measured more precisely than can Kelvin's thermodynamic temperature.
(https://upload.wikimedia.org/wikipedia/commons/f/fa/Argon_atom_at_1E-12_K.gif)
This simulation illustrates an argon atom as it would appear through a 400-power optical microscope featuring a reticle graduated with 50-micron (0.05 mm) tick marks. This atom is moving with a velocity of 14.43 microns per second, which gives the atom a kinetic temperature of one-trillionth of a kelvin. The atom requires 13.9 seconds to travel 200 microns (0.2 mm). Though the atom is being invisibly jostled due to zero-point energy, its translational motion seen here comprises all its kinetic energy.
Strictly speaking, the temperature of a system is well-defined only if it is at thermal equilibrium. From a microscopic viewpoint, a material is at thermal equilibrium if the quantity of heat between its individual particles cancel out. There are many possible scales of temperature, derived from a variety of observations of physical phenomena.
Loosely stated, temperature differences dictate the direction of heat between two systems such that their combined energy is maximally distributed among their lowest possible states. We call this distribution "entropy".
https://en.m.wikipedia.org/wiki/Thermodynamic_temperature
Post by: hamdani yusuf on 08/09/2024 02:58:42
Quote
Two physical systems are in thermal equilibrium if there is no net flow of thermal energy between them when they are connected by a path permeable to heat. Thermal equilibrium obeys the zeroth law of thermodynamics. A system is said to be in thermal equilibrium with itself if the temperature within the system is spatially uniform and temporally constant.
Systems in thermodynamic equilibrium are always in thermal equilibrium, but the converse is not always true. If the connection between the systems allows transfer of energy as 'change in internal energy' but does not allow transfer of matter or transfer of energy as work, the two systems may reach thermal equilibrium without reaching thermodynamic equilibrium.
https://en.m.wikipedia.org/wiki/Thermal_equilibrium
Post by: hamdani yusuf on 08/09/2024 07:23:01
Quote
"There is an important distinction between thermal and thermodynamic equilibrium. According to M?nster (1970), in states of thermodynamic equilibrium, the state variables of a system do not change at a measurable rate. Moreover, "The proviso 'at a measurable rate' implies that we can consider an equilibrium only with respect to specified processes and defined experimental conditions." Also, a state of thermodynamic equilibrium can be described by fewer macroscopic variables than any other state of a given body of matter. A single isolated body can start in a state which is not one of thermodynamic equilibrium, and can change till thermodynamic equilibrium is reached. Thermal equilibrium is a relation between two bodies or closed systems, in which transfers are allowed only of energy and take place through a partition permeable to heat, and in which the transfers have proceeded till the states of the bodies cease to change.[22]"
https://en.wikipedia.org/wiki/Thermal_equilibrium
An explicit distinction between 'thermal equilibrium' and 'thermodynamic equilibrium' is made by C.J. Adkins. He allows that two systems might be allowed to exchange heat but be constrained from exchanging work; they will naturally exchange heat till they have equal temperatures, and reach thermal equilibrium, but in general, will not be in thermodynamic equilibrium. They can reach thermodynamic equilibrium when they are allowed also to exchange work.[23]
Another explicit distinction between 'thermal equilibrium' and 'thermodynamic equilibrium' is made by B. C. Eu. He considers two systems in thermal contact, one a thermometer, the other a system in which several irreversible processes are occurring. He considers the case in which, over the time scale of interest, it happens that both the thermometer reading and the irreversible processes are steady. Then there is thermal equilibrium without thermodynamic equilibrium. Eu proposes consequently that the zeroth law of thermodynamics can be considered to apply even when thermodynamic equilibrium is not present; also he proposes that if changes are occurring so fast that a steady temperature cannot be defined, then "it is no longer possible to describe the process by means of a thermodynamic formalism. In other words, thermodynamics has no meaning for such a process."[24]
Post by: alancalverd on 08/09/2024 09:41:14
Post by: Bored chemist on 08/09/2024 13:05:41
so you could define a quasi-instantaneous equilibrium during a nuclear explosion. What would be the point?Answering the question "How hot is a nuclear explosion?".
I'd have thought that anyone with an interest in MRI would be well aware of relaxation times.
And until a system has relaxed, it doesn't have a well defined temperature.
Post by: hamdani yusuf on 08/09/2024 13:44:43
That's all a bit weak. "measurable rate" and "time scale of interest" can be whatever you choose, so you could define a quasi-instantaneous equilibrium during a nuclear explosion. What would be the point?They show that the concept of temperature is not as well defined as some of us might have thought.
Post by: hamdani yusuf on 08/09/2024 13:53:52
The question is, which object is the hottest? Which is the coolest? Which direction is the energy transfered?
Post by: alancalverd on 09/09/2024 11:11:12
Post by: Bored chemist on 09/09/2024 11:29:55
The question is, which object is the hottest?The electrons in the LED with an energy of a few electron volts, corresponding to a temperature of tens of thousands of kelvin.
Post by: Bored chemist on 09/09/2024 11:34:13
They show that the concept of temperature is not as well defined as some of us might have thought.That's a bit like saying that, because you can't divide by zero, we don't understand division.
We can't say what the temperature of a non thermal system is.
That doesn't mean we don't understand temperature.
It means that we understand that there are things to which it does not apply.
We can't tell you the thermodynamic temperature of the inside of a fluorescent light bulb when it's running.
We can't tell you the temperature of love or Thursday either.
That's not because we don't understand temperature.
It's because we understand it well enough to realise when it does not apply.
Post by: alancalverd on 09/09/2024 12:02:52
They show that the concept of temperature is not as well defined as some of us might have thought.No. It shows that the source of the quotation doesn't understand the definition. Probably a "philosopher of science".
Post by: hamdani yusuf on 09/09/2024 16:28:39
Does it melt?The question is, which object is the hottest?The electrons in the LED with an energy of a few electron volts, corresponding to a temperature of tens of thousands of kelvin.
Post by: hamdani yusuf on 09/09/2024 16:30:42
That doesn't mean we don't understand temperature.Can you describe what those things are?
It means that we understand that there are things to which it does not apply.
Post by: hamdani yusuf on 09/09/2024 17:42:17
They show that the concept of temperature is not as well defined as some of us might have thought.No. It shows that the source of the quotation doesn't understand the definition. Probably a "philosopher of science".
The articles I quoted show different versions of temperature.
What's temperature?The same object may have different temperature, based on which method is used to measure it.
Quote
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making up a substance.
https://en.m.wikipedia.org/wiki/Temperature
The article above also mention kinetic temperature and internal temperature.
In contrast,
Quote
Thermodynamic temperature is a quantity defined in thermodynamics as distinct from kinetic theory or statistical mechanics.
Post by: Bored chemist on 09/09/2024 23:06:37
How the F*** do you expect an electron to melt?Does it melt?The question is, which object is the hottest?The electrons in the LED with an energy of a few electron volts, corresponding to a temperature of tens of thousands of kelvin.
Post by: Bored chemist on 09/09/2024 23:08:20
Things where the different ways in which energy can be stored are not at equilibrium.That doesn't mean we don't understand temperature.Can you describe what those things are?
It means that we understand that there are things to which it does not apply.
How many times must I point out that the vibrational, rotational and electronic energies are also involved?
If they don't all agree (i.e. if the equipartition principle isn't obeyed) then the temperature is not defined.
Post by: hamdani yusuf on 10/09/2024 08:13:30
How could you think that it referred to electron instead of LED?How the F*** do you expect an electron to melt?Does it melt?The question is, which object is the hottest?The electrons in the LED with an energy of a few electron volts, corresponding to a temperature of tens of thousands of kelvin.
Post by: alancalverd on 10/09/2024 09:18:11
Post by: Bored chemist on 10/09/2024 12:18:59
How could you think that it referred to electron instead of LED?Because it's totally obvious to anyone that the LED doesn't melt.
Post by: hamdani yusuf on 10/09/2024 13:15:45
What's the temperature of the LED?How could you think that it referred to electron instead of LED?Because it's totally obvious to anyone that the LED doesn't melt.
Why it's different from the temperature of the electrons?
Post by: alancalverd on 10/09/2024 14:10:06
The working temperature of the chip represents the kinetic energy of the nuclei, which account for most of the mass of the substrate material and hence most of the thermal energy of the LED itself. The heat sink and casing are (or should be) in near-equilibrium with the substrate.
Within the chip, the active electrons responsible for light emission obviously have mean energies of the order of visible photons, 2 - 4 eV. 1 eV ≈ 11,605 K
Post by: Bored chemist on 10/09/2024 18:29:17
FFS!What's the temperature of the LED?How could you think that it referred to electron instead of LED?Because it's totally obvious to anyone that the LED doesn't melt.
Why it's different from the temperature of the electrons?
How many times?
The electrons do not, in this instance, have a well defined temperature.
Post by: hamdani yusuf on 10/09/2024 22:19:19
The electrons do not, in this instance, have a well defined temperature.Then why did you write this statement?
The electrons in the LED with an energy of a few electron volts, corresponding to a temperature of tens of thousands of kelvin.
Post by: hamdani yusuf on 10/09/2024 22:22:56
Within the chip, the active electrons responsible for light emission obviously have mean energies of the order of visible photons, 2 - 4 eV. 1 eV ≈ 11,605 KWhy don't they cause the LED to melt down?
Post by: alancalverd on 11/09/2024 10:40:07
A poor analogy: collect the sparks from a grinding wheel in a bucket of water. The sparks are at about 800 - 1000 K but you won't get the water to boil.
Post by: Bored chemist on 11/09/2024 10:54:27
To remind you that not all energy is temperature.The electrons do not, in this instance, have a well defined temperature.Then why did you write this statement?The electrons in the LED with an energy of a few electron volts, corresponding to a temperature of tens of thousands of kelvin.
Did you not notice that I said the energy corresponded to a temperature, not that the electrons were at that temperature?
Post by: Bored chemist on 11/09/2024 10:57:59
Because someone has cleverly arranged things so that it's more likely that they will lose that energy by emitting light than that they will transfer it to thermal energy,Within the chip, the active electrons responsible for light emission obviously have mean energies of the order of visible photons, 2 - 4 eV. 1 eV ≈ 11,605 KWhy don't they cause the LED to melt down?
Post by: hamdani yusuf on 11/09/2024 17:11:08
Did you not notice that I said the energy corresponded to a temperature, not that the electrons were at that temperature?How are they correlated?
Is it proportionally? Or inversely proportional? Or another type of correlation?
Post by: hamdani yusuf on 11/09/2024 17:40:50
How is it done?Because someone has cleverly arranged things so that it's more likely that they will lose that energy by emitting light than that they will transfer it to thermal energy,Within the chip, the active electrons responsible for light emission obviously have mean energies of the order of visible photons, 2 - 4 eV. 1 eV ≈ 11,605 KWhy don't they cause the LED to melt down?
Post by: alancalverd on 11/09/2024 18:43:01
https://electronics.howstuffworks.com/led.htm
Post by: hamdani yusuf on 12/09/2024 14:37:40
Try
https://electronics.howstuffworks.com/led.htm
Quote
LEDs generate very little heat, relatively speaking. A much higher percentage of the electrical energy is going directly to generating light, which cuts down the electricity demands considerably.But when the light produced by LED hit the paper, it burns.
In the video, there is a segment where the light is reflected back to LED using a mirror. The LED breakdown as a result.
Post by: Bored chemist on 12/09/2024 14:44:18
I'm sure I already told you about the equipartition principle.Did you not notice that I said the energy corresponded to a temperature, not that the electrons were at that temperature?How are they correlated?
Is it proportionally? Or inversely proportional? Or another type of correlation?
https://en.wikipedia.org/wiki/Equipartition_theorem
Try reading it again.
Maybe take notes if that helps you remember stuff.
Post by: Bored chemist on 12/09/2024 14:46:11
That's really not a subject that is suitable for a discussion site. It's a lot of technical information.How is it done?Because someone has cleverly arranged things so that it's more likely that they will lose that energy by emitting light than that they will transfer it to thermal energy,Within the chip, the active electrons responsible for light emission obviously have mean energies of the order of visible photons, 2 - 4 eV. 1 eV ≈ 11,605 KWhy don't they cause the LED to melt down?
Are you able to use a search engine and ask how LEDs are made?
(Don't use AI; they produce dross)
Post by: Bored chemist on 12/09/2024 14:46:46
But when the light produced by LED hit the paper, it burns.Why would it not?
Post by: alancalverd on 12/09/2024 16:44:08
But when the light produced by LED hit the paper, it burns.What do you expect? Photons deliver energy to the absorber.
In the video, there is a segment where the light is reflected back to LED using a mirror. The LED breakdown as a result.
Post by: hamdani yusuf on 15/09/2024 11:12:06
The question is, why the materials in the LED didn't absorb the light during the emission, but did absorb the light after reflection by the mirror?But when the light produced by LED hit the paper, it burns.What do you expect? Photons deliver energy to the absorber.
In the video, there is a segment where the light is reflected back to LED using a mirror. The LED breakdown as a result.
Post by: hamdani yusuf on 17/09/2024 07:31:21
Do you think that Equipartition theorem is relevant in the case of radiation and absorption by LED?I'm sure I already told you about the equipartition principle.Did you not notice that I said the energy corresponded to a temperature, not that the electrons were at that temperature?How are they correlated?
Is it proportionally? Or inversely proportional? Or another type of correlation?
https://en.wikipedia.org/wiki/Equipartition_theorem
Try reading it again.
Maybe take notes if that helps you remember stuff.
Post by: Bored chemist on 17/09/2024 13:40:14
Yes.Do you think that Equipartition theorem is relevant in the case of radiation and absorption by LED?I'm sure I already told you about the equipartition principle.Did you not notice that I said the energy corresponded to a temperature, not that the electrons were at that temperature?How are they correlated?
Is it proportionally? Or inversely proportional? Or another type of correlation?
https://en.wikipedia.org/wiki/Equipartition_theorem
Try reading it again.
Maybe take notes if that helps you remember stuff.
It's what tells you that the electrons don't have a well defined temperature.
Post by: hamdani yusuf on 18/09/2024 14:56:24
Yes.Do you think that Equipartition theorem is relevant in the case of radiation and absorption by LED?I'm sure I already told you about the equipartition principle.Did you not notice that I said the energy corresponded to a temperature, not that the electrons were at that temperature?How are they correlated?
Is it proportionally? Or inversely proportional? Or another type of correlation?
https://en.wikipedia.org/wiki/Equipartition_theorem
Try reading it again.
Maybe take notes if that helps you remember stuff.
It's what tells you that the electrons don't have a well defined temperature.
Here's a quote from the article.
Quote
Although the equipartition theorem makes accurate predictions in certain conditions, it is inaccurate when quantum effects are significant, such as at low temperatures. When the thermal energy kBT is smaller than the quantum energy spacing in a particular degree of freedom, the average energy and heat capacity of this degree of freedom are less than the values predicted by equipartition. Such a degree of freedom is said to be "frozen out" when the thermal energy is much smaller than this spacing. For example, the heat capacity of a solid decreases at low temperatures as various types of motion become frozen out, rather than remaining constant as predicted by equipartition. Such decreases in heat capacity were among the first signs to physicists of the 19th century that classical physics was incorrect and that a new, more subtle, scientific model was required. Along with other evidence, equipartition's failure to model black-body radiation?also known as the ultraviolet catastrophe?led Max Planck to suggest that energy in the oscillators in an object, which emit light, were quantized, a revolutionary hypothesis that spurred the development of quantum mechanics and quantum field theory.
I asked ChatGPT if LED can be explained by classical physics theory.
Quote
No, the functioning of LEDs cannot be fully explained using classical physics theory. Classical physics, particularly concepts from electromagnetism and thermodynamics, can describe the flow of electric current and heat generation in a simple conductor, but it falls short in explaining the specific quantum processes that occur in an LED.
Here?s why classical physics is inadequate:
1. Quantum Mechanics: The behavior of electrons in a semiconductor (which is central to how LEDs work) can only be explained by quantum mechanics. In classical physics, electrons are treated as particles with definite paths and energies. However, in an LED, the process of electron-hole recombination?which leads to the emission of photons (light)?requires understanding quantized energy levels and band theory, concepts that come from quantum physics.
2. Band Theory: Classical physics does not account for the energy band structure in semiconductors, which is essential to understanding how electrons move between the valence band and the conduction band in an LED. Quantum mechanics explains how electrons can exist in discrete energy levels within a solid, which directly leads to the emission of photons at specific wavelengths (colors of light) in LEDs.
3. Electroluminescence: The phenomenon of electroluminescence (the emission of light in response to an electric current) is inherently quantum mechanical. It involves the release of energy when an electron drops from a higher energy state to a lower energy state, a process that classical theories cannot explain in terms of photon emission.
Thus, while classical physics can describe the basic flow of current in a circuit with resistors and capacitors, the working of an LED is rooted in quantum theory. Quantum mechanics gives us the necessary framework to explain the specific behavior of particles in semiconductors and their interaction with light.
Post by: Bored chemist on 18/09/2024 17:15:35
I asked ChatGPTWhy?
Post by: hamdani yusuf on 19/09/2024 09:55:53
Why not?I asked ChatGPTWhy?
By comparing different answers we might find a better answer to what we currently believe. If you think ChatGPT's answer is false, you can point out where it is, and how to improve it.
I asked it to check your answer about equipartition theorem.
Yes.
It's what tells you that the electrons don't have a well defined temperature.
Does equipartition theorem tell you that the electrons don't have a well defined temperature.
Quote
The equipartition theorem states that, at thermal equilibrium, the energy of a system is equally distributed among its degrees of freedom, with each degree of freedom contributing (where is the Boltzmann constant and is the temperature) to the average energy. For classical systems, this works well, but electrons are quantum particles, and their behavior is governed by quantum mechanics.
Electrons, especially in solids, do not follow the equipartition theorem in the classical sense because they obey Fermi-Dirac statistics. Instead of being evenly distributed in energy, electrons fill available energy states up to the Fermi level at absolute zero, and at higher temperatures, only the electrons near the Fermi level gain significant energy. This leads to the concept of a Fermi temperature, which is very high compared to the actual temperature of the material.
Therefore, the equipartition theorem does not directly apply to electrons, and their temperature, which reflects the distribution of their energies, is defined differently from that of classical particles. In summary, electrons can have a well-defined temperature in a system (such as the electron temperature in metals), but the equipartition theorem doesn't describe their energy distribution accurately due to quantum effects.
Post by: Bored chemist on 19/09/2024 11:50:01
Does equipartition theorem tell you that the electrons don't have a well defined temperature.These electrons have an energy of a few eV. (We know, because they emit visible light)
The electrons, atoms etc nearby have an energy corresponding to room temperature; about 0.025 eV. (we know, because the plastic doesn't melt).
So we know that it's a (very) non-equilibrium state.
And we know that temperatures are only well defined if the equipartition theory is true
Post by: hamdani yusuf on 24/09/2024 07:42:22
These electrons have an energy of a few eV. (We know, because they emit visible light)In your system,
The electrons, atoms etc nearby have an energy corresponding to room temperature; about 0.025 eV. (we know, because the plastic doesn't melt).
T = room temperature = 20 C = 293 K
E = 0.025 eV = 4.005441584E-21 Joule
ΔS = 4.005441584E-21 Joule / 293 K =1.36704E-23 J/K
Where did you get these numbers from?
Post by: Bored chemist on 24/09/2024 12:22:31
E = kT
k = 1.380649 ? 10^-23 m2 kg s-2 K-1
T= 297
E= 4 x 10^-21 J
Strictly speaking I got the number from remembering that the energy of thermal neutrons is about 0.025eV
But that's the joy of the equipartition theory.
I could have done this calculation instead.
The speed of sound in a gas is comparable with the speed of the molecules or atoms in that gas.
I know the speed of sound in air- it's about 330 m/s
And I know that the density of air is abut 1.2 grams per litre
And I know that a mole of gas occupies about 24 litres.
And I know Avogadro's constant is about 6 x 10^23.
So I can work out that the mass of an air molecule is about 5 x 10^26 Kg
So I can calculate the energy from 1/2 M V^2
and get about 5X10^-21 Joules
And I can convert that to eV
0.031 eV.
Close enough.
I can pick any particle with a well defined temperature and if I know its energy, then I know the energy of any other particle at that temperature.
In principle, I can measure the energy of some visible light. (It's a high school expt using the photoelectric effect).
And then I can measure the spectrum of the IR emitted by my floor or wall.
And, from that, I can measure the energy of the radiation in equilibrium with the wall + floor. (It's a simple ratio; if the peak wavelength is 100 times longer then the energy is 100 times lower).
So, I can measure the energy of a typical particle.
And, using that, and the known mass of an air molecule, I can calculate the approximate speed of sound.
Clever, isn't it?
Post by: hamdani yusuf on 25/09/2024 09:59:51
Strictly speaking I got the number from remembering that the energy of thermal neutrons is about 0.025eVWhat makes you think that equipartition theory is still valid to conclude that energy of thermal neutrons at room temperature is the same as electrons?
But that's the joy of the equipartition theory.
Post by: hamdani yusuf on 25/09/2024 10:21:47
I can pick any particle with a well defined temperature and if I know its energy, then I know the energy of any other particle at that temperature.We'll need a different theory to explain the works of induction heater, microwave oven, and infrared stoves.
In principle, I can measure the energy of some visible light. (It's a high school expt using the photoelectric effect).
And then I can measure the spectrum of the IR emitted by my floor or wall.
And, from that, I can measure the energy of the radiation in equilibrium with the wall + floor. (It's a simple ratio; if the peak wavelength is 100 times longer then the energy is 100 times lower).
Post by: Bored chemist on 25/09/2024 12:46:26
What makes you think that equipartition theory is still valid to conclude that energy of thermal neutrons at room temperature is the same as electrons?Because it's a consequence of the conservation of energy.
Post by: Bored chemist on 25/09/2024 12:46:54
We'll need a different theory to explain the works of induction heater, microwave oven, and infrared stoves.You might; the rest of us don't.
Post by: hamdani yusuf on 26/09/2024 09:10:04
(It's a simple ratio; if the peak wavelength is 100 times longer then the energy is 100 times lower)This only works for black body radiation.
Post by: Bored chemist on 26/09/2024 11:00:28
This only works for black body radiation.Guess what the spectrum looks like if you apply the equipartition principle to photons...
So once again.
If that principle doesn't apply, you don't have a well defined temperature.
Did you not think about that?
Maybe, next time, at the start of your post you should add a line or two that shows that you have considered the effect of equipartition and what the outcome is.
Because I'm frankly getting tired of reminding you about it.
Post by: hamdani yusuf on 26/09/2024 11:46:11
conservation of energy can still apply even when equipartition theorem doesn't hold.What makes you think that equipartition theory is still valid to conclude that energy of thermal neutrons at room temperature is the same as electrons?Because it's a consequence of the conservation of energy.
Post by: hamdani yusuf on 26/09/2024 11:47:42
Guess what the spectrum looks like if you apply the equipartition principle to photons...Ultraviolet catastrophe.
Post by: hamdani yusuf on 26/09/2024 11:48:53
So once again.Does it imply that we can't predict where the energy will naturally flow?
If that principle doesn't apply, you don't have a well defined temperature.
Post by: Bored chemist on 26/09/2024 11:52:35
conservation of energy can still apply even when equipartition theorem doesn't hold.Nobody suggested otherwise.
Ultraviolet catastrophe.Was resolved by the quantisation of em radiation- which is implicit in the use of the word "photons".
Does it imply that we can't predict where the energy will naturally flow?No.
Do you not recognise that this sort of exchange is a very inefficient way of you learning science- especially when you fail to pay attention to important things like equipartition?
Post by: hamdani yusuf on 27/09/2024 03:23:37
conservation of energy can still apply even when equipartition theorem doesn't hold.Nobody suggested otherwise.
Previously, you concluded that energy of thermal neutrons at room temperature is the same as electrons because you thought that equipartition theory is still valid in this case. You thought that its validity is a consequence of the conservation of energy.conservation of energy can still apply even when equipartition theorem doesn't hold.What makes you think that equipartition theory is still valid to conclude that energy of thermal neutrons at room temperature is the same as electrons?Because it's a consequence of the conservation of energy.
Post by: hamdani yusuf on 27/09/2024 03:27:23
Which implies that the em radiation is not equally partitioned.Ultraviolet catastrophe.Was resolved by the quantisation of em radiation- which is implicit in the use of the word "photons".
Post by: hamdani yusuf on 27/09/2024 03:30:34
How do you predict where the energy will naturally flow between two objects if their temperatures are not well defined?Does it imply that we can't predict where the energy will naturally flow?No.
Post by: Bored chemist on 27/09/2024 12:07:34
I think you may have failed to spot the word "average" in the definition.Which implies that the em radiation is not equally partitioned.Ultraviolet catastrophe.Was resolved by the quantisation of em radiation- which is implicit in the use of the word "photons".
Post by: Bored chemist on 27/09/2024 12:08:39
It generally flows towards equipartition.How do you predict where the energy will naturally flow between two objects if their temperatures are not well defined?Does it imply that we can't predict where the energy will naturally flow?No.
Post by: Bored chemist on 27/09/2024 12:10:21
Previously, you concluded that energy of thermal neutrons at room temperature is the same as electrons because you thought that equipartition theory is still valid in this case. You thought that its validity is a consequence of the conservation of energy.The type of error that you have made here is comparable with assuming that, since all poodles are dogs, all dogs must be poodles.
Post by: hamdani yusuf on 28/09/2024 13:01:35
But you don't know which way, for they are not well defined.It generally flows towards equipartition.How do you predict where the energy will naturally flow between two objects if their temperatures are not well defined?Does it imply that we can't predict where the energy will naturally flow?No.
Post by: hamdani yusuf on 28/09/2024 13:22:40
Ultraviolet catastrophe is a scenario already accounting for average.I think you may have failed to spot the word "average" in the definition.Which implies that the em radiation is not equally partitioned.Ultraviolet catastrophe.Was resolved by the quantisation of em radiation- which is implicit in the use of the word "photons".
Post by: hamdani yusuf on 28/09/2024 13:25:04
What makes you think that equipartition theory is still valid to conclude that energy of thermal neutrons at room temperature is the same as electrons?Previously, you concluded that energy of thermal neutrons at room temperature is the same as electrons because you thought that equipartition theory is still valid in this case. You thought that its validity is a consequence of the conservation of energy.The type of error that you have made here is comparable with assuming that, since all poodles are dogs, all dogs must be poodles.
Post by: Bored chemist on 29/09/2024 12:08:03
It's still, as I said, the conservation of energy.What makes you think that equipartition theory is still valid to conclude that energy of thermal neutrons at room temperature is the same as electrons?Previously, you concluded that energy of thermal neutrons at room temperature is the same as electrons because you thought that equipartition theory is still valid in this case. You thought that its validity is a consequence of the conservation of energy.The type of error that you have made here is comparable with assuming that, since all poodles are dogs, all dogs must be poodles.
Ultraviolet catastrophe is a scenario already accounting for average.But, as I said, not for quantisation.
But you don't know which way, for they are not well defined.You might not think they are well enough defined.
But, as I said, the rest of us do.
Why do you keep repeating teh same questions after that have been answered?
Do you have a learning disability?
Post by: hamdani yusuf on 30/09/2024 10:08:54
You might not think they are well enough defined.You are the one who said that the temperature wasn't well defined.
But, as I said, the rest of us do.
Post by: hamdani yusuf on 30/09/2024 10:16:23
Why do you keep repeating teh same questions after that have been answered?Because you keep giving contradicting or circular answers.
Post by: Bored chemist on 30/09/2024 10:32:16
Temperature isn't well defined. The state of the system is well defined.You might not think they are well enough defined.You are the one who said that the temperature wasn't well defined.
But, as I said, the rest of us do.
This is not meant to be difficult.
You really should stop wasting time here and go and learn some physics.
Post by: hamdani yusuf on 17/10/2024 14:47:56
Quote
You may have heard temperature is just the kinetic energy of molecules, but that's a subtle lie. In this video, Nick Lucid explains those subtlies in the only way he knows how: CRAZY!
This old video describes what became my concern pretty well.
Post by: hamdani yusuf on 17/10/2024 15:06:30
Post by: hamdani yusuf on 17/10/2024 15:36:05
Quote
This video explains the concepts of temperature and heat, their units of measurement, instruments used to measure them and their main characteristics and behavior with graphic examples.Average kinetic energy alone isn't enough to describe temperature, a concept that's supposed to predict heat flow when two objects are allowed to exchange heat. The missing ingredients are what I was trying to identify.
Post by: Bored chemist on 17/10/2024 16:16:04
Misconception about temperature.Yes; the misconception of people who were never properly taught about it.
If you asked the same questions at a science conference you would get laughed at.
All that video does is show that some people didn't study physics.
And we already know that.
Post by: Bored chemist on 17/10/2024 16:17:24
This old video describes what became my concern pretty welWell... he's wrong.
He failed to explain that, the various modes have to be in equilibrium.
But, he got sort of close when he said something about averages sometimes not making much sense.
Post by: Bored chemist on 17/10/2024 16:24:33
Difference Between Temperature and HeatAnd, once again, you are listening to the "lies we tell to children".QuoteThis video explains the concepts of temperature and heat, their units of measurement, instruments used to measure them and their main characteristics and behavior with graphic examples.Average kinetic energy alone isn't enough to describe temperature, a concept that's supposed to predict heat flow when two objects are allowed to exchange heat. The missing ingredients are what I was trying to identify.
Consider this picture and what the writig says
Post by: Bored chemist on 17/10/2024 16:26:09
But the average velocity of a vibrating molecule is zero- it moves left to right, them it moves right to left.
On average, it doesn't move at all.
He goes on to say that temperature only makes sense if you look at a large group of molecules.- so, at least he gets that part right.
He goes wrong at about 6:55 because he fails to point out that he's talking about spontaneous, macroscopic heat transfer.
Seriously. What "point" do you think you are making?
Post by: hamdani yusuf on 18/10/2024 12:44:30
"the faster the molecules vibrate".If you use speed instead of velocity, the average is not zero.
But the average velocity of a vibrating molecule is zero- it moves left to right, them it moves right to left.
On average, it doesn't move at all.
Post by: Bored chemist on 18/10/2024 12:56:03
Please yourself."the faster the molecules vibrate".If you use speed instead of velocity, the average is not zero.
But the average velocity of a vibrating molecule is zero- it moves left to right, them it moves right to left.
On average, it doesn't move at all.
But remember, on average half the stored energy is in the spring, not the movement.
So the average kinetic energy of an oscillating spring/ mass system is not the average energy of that system.
The point remains; he's close but wrong.
And with other systems, you may need to consider electronic excitation. (Possibly even nuclear if it's hot enough).
Post by: hamdani yusuf on 18/10/2024 17:14:22
Quote
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making up a substance.Kinetic energy is 1/2 m.v^2
https://en.m.wikipedia.org/wiki/Temperature
For oscillating motion, the average velocity might be zero, but the kinetic energy is not.
Post by: Bored chemist on 18/10/2024 17:43:28
What's the potential energy?
What about when it's at the bottom?
What's the total energy of the swinging pendulum?
Does the total energy change with time (in an idealised case)?
What is the average total energy?
Is the average total energy of the pendulum the same as the average kinetic energy of the pendulum?
Post by: alancalverd on 18/10/2024 17:54:22
Kinetic energy is 1/2 m.v^2Possible misunderstanding here. k.e. is not (m vxv)/2 (a vector) but (m |v|2)/2 - a scalar multiple of speed, not velocity. The average speed of a pendulum is not zero.
For oscillating motion, the average velocity might be zero, but the kinetic energy is not.
Post by: Bored chemist on 19/10/2024 11:28:16
Sorry if I threw in a bit of a red herring earlier. I was making the point that the velocity isn't the only thing happening.
The system still has energy, even when the velocity (or speed) is zero.
Post by: hamdani yusuf on 20/10/2024 13:29:43
When a pendulum is at the top of its swing, what is its kinetic energy?0
What's the potential energy?
What about when it's at the bottom?
What's the total energy of the swinging pendulum?
Does the total energy change with time (in an idealised case)?
What is the average total energy?
Is the average total energy of the pendulum the same as the average kinetic energy of the pendulum?
Maximum
Maximum, 0
Kinetic+potential
No
=average of Kinetic+potential
No
Post by: hamdani yusuf on 20/10/2024 13:31:40
i'm hoping that hamdani yusuf has caught on to the fact that the average energy of a vibrating system is NOT the average kinetic energy.Temperature is more related to kinetic energy instead of the total energy.
Sorry if I threw in a bit of a red herring earlier. I was making the point that the velocity isn't the only thing happening.
The system still has energy, even when the velocity (or speed) is zero.
Post by: hamdani yusuf on 20/10/2024 13:36:53
Where do you get that cross product from?Kinetic energy is 1/2 m.v^2Possible misunderstanding here. k.e. is not (m vxv)/2 (a vector) but (m |v|2)/2 - a scalar multiple of speed, not velocity. The average speed of a pendulum is not zero.
For oscillating motion, the average velocity might be zero, but the kinetic energy is not.
Quote
In classical mechanics, the kinetic energy of a non-rotating object of mass m traveling at a speed v isIn the derivation section, you'll find the use of dot product of vectors, which results in a scalar.
1
2
m
v
2
{\textstyle {\frac {1}{2}}mv^{2}}.[2]
https://en.m.wikipedia.org/wiki/Kinetic_energy
Post by: Bored chemist on 20/10/2024 13:54:45
average of Kinetic+potential... and since Kinetic+potential is a constant, you don't need to average it.
Temperature is more related to kinetic energy instead of the total energy.So, a fast moving snow ball (like a comet) is hot...
Well. that's one approach.
Post by: alancalverd on 20/10/2024 18:32:38
Where do you get that cross product from?It represents the square of velocity.
Whilst the average velocity of a pendulum is zero, its average speed is not.
Post by: hamdani yusuf on 21/10/2024 09:42:12
... and since Kinetic+potential is a constant, you don't need to average it.Only in ideal case.
Post by: hamdani yusuf on 21/10/2024 09:45:28
So, a fast moving snow ball (like a comet) is hot...You seem to forget about entropy.
Well. that's one approach.
Post by: alancalverd on 21/10/2024 09:50:05
Only in ideal case.Physics is about ideal gases, weightless strings, etc. So you can assume your pendulum is swinging in a vacuum, suspended from a frictionless pivot.
Engineering is about the real world and uses lots of physics to approximate frictional losses, viscous drag, etc. But the conservation of energy is always observed.
Post by: hamdani yusuf on 21/10/2024 10:54:47
In physics, the symbol x is typically used for vector cross product, which is not what you were trying to describe.Where do you get that cross product from?It represents the square of velocity.
Whilst the average velocity of a pendulum is zero, its average speed is not.
Quote
The square of a vector typically refers to the square of its magnitude (or length), not the vector itself. Mathematically, if v is a vector with components , the square of its magnitude is given by:
|\mathbf{v}|^2 = v_1^2 + v_2^2 + \dots + v_n^2
This is essentially the dot product of the vector with itself:
\mathbf{v} \cdot \mathbf{v} = |\mathbf{v}|^2
So, squaring a vector usually means taking the sum of the squares of its components, yielding a scalar value (not another vector).
ChatGPT
Post by: Bored chemist on 21/10/2024 13:57:17
The "ideal" case where energy is conserved.... and since Kinetic+potential is a constant, you don't need to average it.Only in ideal case.
LOL
Post by: hamdani yusuf on 21/10/2024 15:01:17
Physics is about ideal gases, weightless strings, etc. So you can assume your pendulum is swinging in a vacuum, suspended from a frictionless pivot.It depends on the level of complexity. High school physics already takes friction into account.
Post by: alancalverd on 21/10/2024 17:37:37
In physics, the symbol x is typically used for vector cross product, which is not what you were trying to describe.But it has the property that you wanted, i.e. an average of zero for harmonic motion.
Post by: Bored chemist on 22/10/2024 11:45:58
Do you think that the zero point vibration of a lattice has friction?Physics is about ideal gases, weightless strings, etc. So you can assume your pendulum is swinging in a vacuum, suspended from a frictionless pivot.It depends on the level of complexity. High school physics already takes friction into account.
Do you know what an analogy is?
Post by: hamdani yusuf on 24/10/2024 13:38:45
Do you know what an analogy is?a comparison between two things, typically for the purpose of explanation or clarification.
a process of arguing from similarity in known respects to similarity in other respects.
Post by: Bored chemist on 24/10/2024 14:24:32
And do you realise that, in this context, there's no energy loss?
Post by: hamdani yusuf on 02/11/2024 22:31:51
OK, so, do you recognise that the pendulum was an analogy for the ZPE?Where did you make that statement?
And do you realise that, in this context, there's no energy loss?
My previous comment was a response to Alan's statement on the scope of physics.
Post by: hamdani yusuf on 02/11/2024 22:43:37
Quote
In this installment of "Thermal Shorts", I explain why metal feels colder than wood, even though they are at the same temperature.
Content:
0:00 Intro - We cannot feel temperature
1:00 The Mechanics behind Heat Conduction
2:43 The Description of Heat Conduction
5:05 The Ice Cube Experiment
Post by: Bored chemist on 03/11/2024 13:38:21
Where did you make that statement?
Do you think that the zero point vibration of a lattice has friction?
Post by: Bored chemist on 03/11/2024 13:39:04
We Cannot Feel TemperatureThis issue is interesting physiology; it is not physics.QuoteIn this installment of "Thermal Shorts", I explain why metal feels colder than wood, even though they are at the same temperature.
Content:
0:00 Intro - We cannot feel temperature
1:00 The Mechanics behind Heat Conduction
2:43 The Description of Heat Conduction
5:05 The Ice Cube Experiment
Post by: hamdani yusuf on 04/11/2024 06:47:02
Where did you make that statement?Do you think that the zero point vibration of a lattice has friction?
Quote
https://en.wikipedia.org/wiki/Friction
Friction is the force resisting the relative motion of solid surfaces, fluid layers, and material elements sliding against each other.[2][3] Types of friction include dry, fluid, lubricated, skin, and internal -- an incomplete list. The study of the processes involved is called tribology, and has a history of more than 2000 years.[4]
Quote
https://en.wikipedia.org/wiki/Friction#Internal_friction
Internal friction is the force resisting motion between the elements making up a solid material while it undergoes deformation.
Plastic deformation in solids is an irreversible change in the internal molecular structure of an object. This change may be due to either (or both) an applied force or a change in temperature. The change of an object's shape is called strain. The force causing it is called stress.
Quote
https://en.wikipedia.org/wiki/Friction#Radiation_frictionConsidering the types of friction above, I think the answer is yes.
As a consequence of light pressure, Einstein[73] in 1909 predicted the existence of "radiation friction" which would oppose the movement of matter. He wrote, "radiation will exert pressure on both sides of the plate. The forces of pressure exerted on the two sides are equal if the plate is at rest. However, if it is in motion, more radiation will be reflected on the surface that is ahead during the motion (front surface) than on the back surface. The backward-acting force of pressure exerted on the front surface is thus larger than the force of pressure acting on the back. Hence, as the resultant of the two forces, there remains a force that counteracts the motion of the plate and that increases with the velocity of the plate. We will call this resultant 'radiation friction' in brief."
This resistive force by Lenz' law below also acts like friction.
Quote
A magnet is dropped down a conducting copper pipe and feels a resistive force. The falling magnet induces a current in the copper pipe and, by Lenz's Law, the current creates a magnetic field that opposes the changing field of the falling magnet. Thus, the magnet is "repelled" and falls more slowly.
Post by: alancalverd on 04/11/2024 09:26:41
This issue is interesting physiology; it is not physics.Beg to differ slightly on this quite complex issue.
We sense temperature differences quite well, with sensitivity 0.5K or less, either between hands (the most frequently used sensor) or between body and ambient, but sensation depends on the rate of heat transfer as well as its direction. Whilst cold metal may give a stronger immediate touch sensation than cold wood, the same is also true of hot metal and hot wood.
If the sample is small and thermally isolated, both metal and wood will equilibrate to your skin temperature and the sensation will disappear, but if they are large and maintained at constant temperature, the thermal gradient across your skin will depend on the conductivity of the material. The epidermis is effectively dead, and the active sensors are in the dermis, so if the object's conductivity is low the sensor will gradually return towards body temperature as the blood flow tries to regulate and nullify the sensation (which is why we have the sensors in the first place) but if the object conductivity is high you won't be able to compensate for the heat loss.
In summary: yes, we do sense temperature, but even before we get to neurophysiology, the physics is more complicated and dynamic than an ideal thermometer.
Post by: Bored chemist on 04/11/2024 09:57:00
Considering the types of friction above, I think the answer is yes.Then you are wrong.
The ZPE can't be lost to anything (including any sort of friction) because that would mean that the molecules stopped vibrating and that would violate uncertainty.
Post by: hamdani yusuf on 07/11/2024 07:15:44
How do you completely block radiation from the environment?Considering the types of friction above, I think the answer is yes.Then you are wrong.
The ZPE can't be lost to anything (including any sort of friction) because that would mean that the molecules stopped vibrating and that would violate uncertainty.
Post by: hamdani yusuf on 07/11/2024 08:20:45
In summary: yes, we do sense temperature, but even before we get to neurophysiology, the physics is more complicated and dynamic than an ideal thermometer.The invention of temperature as a concept was a necessity since it's essential to our survival. Earliest objective measurement of temperature was conveniently done by using conduction as heat transfer method, which requires mechanical contact between the measured object and the measuring device.
Non-contact temperature measurement using radiation was invented much later.
Post by: Bored chemist on 07/11/2024 10:43:20
How do you completely block radiation from the environment?Why did you think that question was relevant?
Post by: hamdani yusuf on 07/11/2024 11:22:04
Because you expect that friction would cause molecules to stop vibrating while ignoring the fact that they are continuously perturbed by various radiations from the environment.How do you completely block radiation from the environment?Why did you think that question was relevant?
Post by: Bored chemist on 09/11/2024 16:15:29
Because you expect that friction would cause molecules to stop vibratingThat's the exact opposite of what I said.
Post by: hamdani yusuf on 09/11/2024 23:13:16
What do you mean in this post?Because you expect that friction would cause molecules to stop vibratingThat's the exact opposite of what I said.
My interpretation was you think that friction, if it exists in molecular level would stop vibration. It makes you concluded that friction doesn't exist in molecular level.Considering the types of friction above, I think the answer is yes.Then you are wrong.
The ZPE can't be lost to anything (including any sort of friction) because that would mean that the molecules stopped vibrating and that would violate uncertainty.
Post by: Bored chemist on 10/11/2024 11:31:30
I meant "The ZPE can't be lost to anything (including any sort of friction) because that would mean that the molecules stopped vibrating and that would violate uncertainty.".
My conclusion is that the motion associated with the zpe is frictionless (strictly, lossless).
Post by: hamdani yusuf on 12/11/2024 15:09:28
My conclusion is that the motion associated with the zpe is frictionless (strictly, lossless).It seems like our disagreement stems on what counts as friction.
Here's what Chatgpt says.
Quote
At the molecular level, motion isn?t exactly "frictionless," but it operates differently than friction at the macroscopic level.
In a traditional sense, friction arises from physical interactions and resistance between surfaces. On a molecular scale, however, molecules experience interactions like collisions, van der Waals forces, and electrostatic attractions, which affect their movement. While these interactions cause energy dissipation and slow down molecules, it?s different from what we typically consider friction.
In a vacuum at extremely low temperatures, where molecules have minimal energy, they might appear to move "freely" in the sense that they aren?t slowed down by other particles. However, at typical conditions, molecules still experience resistance due to their interactions with other molecules, leading to effects like viscosity in fluids or drag in gases. This is why we can see molecular motion as being "resisted," but calling it friction doesn?t fully capture the behavior at such a small scale.
Post by: Bored chemist on 12/11/2024 16:18:31
Here's what Chatgpt says.Can you get it into your head that ChatGPT is monumentally stupid?
https://community.openai.com/t/incorrect-count-of-r-characters-in-the-word-strawberry/829618
Your post is like saying "I asked a five-year-old and they said..."
Stop using it.
Post by: alancalverd on 12/11/2024 17:21:45
ChatGPT is monumentally stupidHardly surprising. In science there is usually one correct answer and an infinity of wrong ones. If you have a bot that scans the internet to form a consensus, it will at best give you a fuzzy answer, hedged about with "maybes", and at worst settle on popular nonsense rather than a little-known or counterintuitive truth.
Fact is that friction is a combination of geometry and chemistry, which is why we like to make bearings with dissimilar metals: highly polished clean surfaces of the same metal tend to stick together, which is quite a problem in high-vacuum systems.
Post by: hamdani yusuf on 14/11/2024 02:35:45
Last time I checked it gave the correct answer. Fierce competition forces AI developers to continuously improve their models. The rate of improvement is exponential.Here's what Chatgpt says.Can you get it into your head that ChatGPT is monumentally stupid?
https://community.openai.com/t/incorrect-count-of-r-characters-in-the-word-strawberry/829618
Your post is like saying "I asked a five-year-old and they said..."
Stop using it.
That aside, can you point out which part of its answer is false?
Post by: hamdani yusuf on 14/11/2024 06:10:38
Hardly surprising. In science there is usually one correct answer and an infinity of wrong ones. If you have a bot that scans the internet to form a consensus, it will at best give you a fuzzy answer, hedged about with "maybes", and at worst settle on popular nonsense rather than a little-known or counterintuitive truth.Some AI models are strictly trained using articles in encyclopedia and peer reviewed papers in reputable science journals.
Post by: alancalverd on 14/11/2024 08:17:27
Post by: Bored chemist on 14/11/2024 10:01:15
That aside, can you point out which part of its answer is false?None of it is relevant.
GIGO
I said "The ZPE can't be lost to anything (including any sort of friction) because that would mean that the molecules stopped vibrating and that would violate uncertainty.".
And that's still true.
The GPT poetry doesn't even refer to ZPE, but that may not be its fault.
What question did you ask it?
Post by: hamdani yusuf on 14/11/2024 11:00:21
And the result is still crap. See above.It reflects current understanding of mainstream science.
Post by: Bored chemist on 14/11/2024 13:20:56
It reflects current understanding of mainstream science.Whose?
What question did you ask it?
Post by: hamdani yusuf on 14/11/2024 14:25:50
What question did you ask it?Is molecular motion frictionless ?
Post by: alancalverd on 14/11/2024 17:41:24
See reply #1055 above for an explanation.
Post by: Bored chemist on 14/11/2024 23:33:26
So... you missed out the important bit.What question did you ask it?Is molecular motion frictionless ?
Why did you ignore the fact tat I was talking about zpe?
If you ask a better question, you get a better answer. (Even with Chatgpt.)
Post by: hamdani yusuf on 15/11/2024 09:45:10
So, it's OK to use ChatGPT now?So... you missed out the important bit.What question did you ask it?Is molecular motion frictionless ?
Why did you ignore the fact tat I was talking about zpe?
If you ask a better question, you get a better answer. (Even with Chatgpt.)ZPE.JPG (37.46 kB . 805x236 - viewed 398 times)
Post by: hamdani yusuf on 15/11/2024 09:46:42
The question is similar to asking whether the motion of the moon is caused by tides.No. It's like asking whether the motion of the moon is frictionless.
See reply #1055 above for an explanation.
Post by: Bored chemist on 15/11/2024 10:42:25
So, it's OK to use ChatGPT now?If you are using it to explain why it's not much use, yes.
Did you forget to answer my question?
Why did you ignore the fact that I was talking about zpe?
Post by: hamdani yusuf on 15/11/2024 11:38:06
Why did you ignore the fact that I was talking about zpe?Because it's irrelevant to my respond to Alan's assertion.
Why did you bring out ZPE into discussion?
Physics is about ideal gases, weightless strings, etc. So you can assume your pendulum is swinging in a vacuum, suspended from a frictionless pivot.It depends on the level of complexity. High school physics already takes friction into account.
Post by: hamdani yusuf on 15/11/2024 11:43:27
It's where the system doesn't exchange energy with the outside. It can happen through friction or radiation.The "ideal" case where energy is conserved.... and since Kinetic+potential is a constant, you don't need to average it.Only in ideal case.
LOL
Post by: Bored chemist on 15/11/2024 15:17:30
That's true whether the energy is kinetic or potential.
There are no losses.
So, if you wanted to ask a bot about the energy losses from ZPE, you need to tell it that's what you are referring to.
A better question might be why did Alan bring friction into it?
Post by: hamdani yusuf on 15/11/2024 22:31:57
Average kinetic energy alone isn't enough to describe temperature, a concept that's supposed to predict heat flow when two objects are allowed to exchange heat. The missing ingredients are what I was trying to identify.Modern study for temperature was developed during early industrial revolution to optimize steam engines. Its measurements were mostly based on conductive heat transfer from the measured objects to the thermometer.
Here's a temperature measurement based on energy transfer through radiation.
Antenna Part 13. How to Calculate Antenna Noise Temperature. How Ant Temp Affects the Noise Floor.
Quote
Antenna Temperature is a measure of the noise generated by an antenna in a given environment, also referred to as Antenna Noise Temperature. This is not the physical temperature of the antenna itself; rather, it depends on the antenna's gain, radiation pattern and the noise picked up from the surrounding environment.
To define the environment (and thus provide a complete definition of antenna temperature), we will introduce a temperature distribution, which represents the temperature in every direction away from the antenna in spherical coordinates. For example, the temperature of the night sky is approximately 4 Kelvin, while the temperature in the direction of the Earth's surface corresponds to the physical temperature of the ground.
This equation shows that the antenna temperature is calculated by integrating over the entire sphere, based on the radiation pattern of the antenna and the temperature distribution of the antenna.
This states that the temperature surrounding the antenna is integrated over the entire sphere and weighted by the antenna's radiation pattern. Thus, an isotropic antenna would have a noise temperature that represents the average of all temperatures around it. For a perfectly directional antenna (such as one with a pencil beam), the antenna temperature will depend only on the temperature in the direction it is "pointing." Consequently, an antenna's temperature will vary depending on whether it is directional and aimed into space or directed toward the sun.
Here?s how antenna temperature works and is calculated:
Measurement in Kelvin: Antenna temperature is usually measured in Kelvin (K), where a higher temperature indicates more power from the radiation field being received.
Power Relation: The antenna temperature (Ta) is proportional to the power density received by the antenna. The total received power Pr is calculated as:
Pr = k TA B
where:
k is the Boltzmann constant, k = 1.38 X 10-23 J/K
TA is the antenna temperature in Kelvin,
B is the bandwidth in Hz.
The receiver has a temperature TR & total system temperature (antenna plus receiver) has a combined temperature given by Tsys = TA + TR.
A parameter often encountered in antenna specification sheets for operation in certain environments is the ratio of the antenna gain to the antenna temperature (or system temperature, if a receiver is specified). This parameter is denoted as G/T and has units of dB/Kelvin [dB/K].
Additionally, many RF engineers use the term Noise Figure (or Noise Factor, NF) to describe system performance. Noise Figure is the ratio of the input SNR (signal-to-noise ratio) to the output SNR. Essentially, all RF devices (such as mixers and amplifiers) introduce some noise. However, antenna temperature is not directly related to Noise Figure, as the power level of the signal input can vary significantly depending on the desired signal's direction of arrival, while the noise contribution remains constant.
In summary, antenna temperature is a key concept in understanding the amount of radiation an antenna receives, which can represent either signal or noise, depending on the application. It helps quantify the effective power of this radiation by linking it to the temperature concept, facilitating better analysis and system design in both astronomy and communications.
Post by: alancalverd on 16/11/2024 09:16:23
A better question might be why did Alan bring friction into it?I don't recall doing so. I did point out that friction between mesoscopic objects has a strong chemical (i.e. molecular) component, but, being a physicist, all my pendulums are of course point masses on weightless strings in vacuo. Or, if you really insist, uniform rigid bars suspended on frictioness pivots.
Post by: hamdani yusuf on 16/11/2024 09:52:55
You narrowed down the scope of your physics to a level to be simpler than high school text books.A better question might be why did Alan bring friction into it?I don't recall doing so. I did point out that friction between mesoscopic objects has a strong chemical (i.e. molecular) component, but, being a physicist, all my pendulums are of course point masses on weightless strings in vacuo. Or, if you really insist, uniform rigid bars suspended on frictioness pivots.
Post by: hamdani yusuf on 16/11/2024 10:20:48
Modern study for temperature was developed during early industrial revolution to optimize steam engines. Its measurements were mostly based on conductive heat transfer from the measured objects to the thermometer.In both cases, temperature determines the direction of energy transfer when two systems are allowed to exchange energy. They are also related to kinetic energy of each system, as well as entropy, which is shown by the Boltzmann constant in the equations.
Here's a temperature measurement based on energy transfer through radiation.
Post by: Bored chemist on 16/11/2024 11:05:28
Here's a temperature measurement based on energy transfer through radiation.The purpose of antennae is not that they produce black body radiation.
It's obviously very far from equilibrium and does not have a well defined temperature. (For example, if you consider a simple dipole antenna, the electrons going "up and down" are not in equilibrium with the electros going "side to side")
Why did you bring it up?
Post by: Bored chemist on 16/11/2024 11:06:47
That's entirely reasonable when communicating with someone who does not understand high school physics.You narrowed down the scope of your physics to a level to be simpler than high school text books.A better question might be why did Alan bring friction into it?I don't recall doing so. I did point out that friction between mesoscopic objects has a strong chemical (i.e. molecular) component, but, being a physicist, all my pendulums are of course point masses on weightless strings in vacuo. Or, if you really insist, uniform rigid bars suspended on frictioness pivots.
Post by: hamdani yusuf on 16/11/2024 11:38:43
The purpose of antennae is not that they produce black body radiation.Who says it is?
Post by: Bored chemist on 16/11/2024 11:40:58
OK, so what antennae do is nothing to do with temperature.The purpose of antennae is not that they produce black body radiation.Who says it is?
Post by: hamdani yusuf on 16/11/2024 11:42:13
It's obviously very far from equilibrium and does not have a well defined temperature. (For example, if you consider a simple dipole antenna, the electrons going "up and down" are not in equilibrium with the electros going "side to side")Antenna temperature is well defined.
Why did you bring it up?
Quote
Power Relation: The antenna temperature (Ta) is proportional to the power density received by the antenna. The total received power Pr is calculated as:
Pr = k TA B
where:
k is the Boltzmann constant, k = 1.38 X 10-23 J/K
TA is the antenna temperature in Kelvin,
B is the bandwidth in Hz.
Post by: alancalverd on 16/11/2024 13:27:58
You narrowed down the scope of your physics to a level to be simpler than high school text books.
AFAIK high school physics still deals with ideal pendulums. I don't recall the chemical and crystallographic aspects of vacuum tribology being part of the syllabus, though some of my contemporaries did study it for their PhDs and as of this week it remains an important topic for electron microscopists in a well-known laboratory down the road from here.
Post by: Bored chemist on 17/11/2024 00:17:47
Antenna temperature is well defined.
Antenna Temperature is a measure of the noise generated by an antenna in a given environment,... This is not the physical temperature of the antenna itself; rather, it depends on ....
Then tell me, is the temperature of the electrons going up and down, the same as that of the electrons going side to side?
Do you realise that a noise temperature or antenna temperature isn't a real temperature of anything?
e.g.
"C-band LNB
Here is an example of a North American C-band LNB:
Local oscillator: 5.15 GHz
Frequency: 3.40?4.20 GHz
Noise temperature: 25?100 kelvins"
From
https://en.wikipedia.org/wiki/Low-noise_block_downconverter#C-band_LNB
Post by: alancalverd on 17/11/2024 09:45:37
Post by: Bored chemist on 17/11/2024 17:01:24
The Boltzmann equation actually derives from considerations of thermal noise in a conductor.This was particularly clever of him since he derived the eqn in 1872 and the electron was not discovered until 1897.
Post by: alancalverd on 18/11/2024 08:09:12
It is the mark of greatness that people name things for you after you are dead.
IIRC Einstein currently holds the record for posthumous nomenclature in physics and chemistry, including an element and a derived unit among other things, but Boltzmann's constant and distribution are more widely used in engineering.
PS only two people have colleges named after them in both Oxford and Cambridge. Good pub quiz question!
Post by: Bored chemist on 18/11/2024 10:32:01
Jesus (AKA Corpus Christi) and
Mary Magdalen
each have a college.
Are there two Saints Catherine and two Saints John?
Post by: alancalverd on 18/11/2024 12:08:41
Meanwhile, here's an old chestnut:
Tourist in Cambridge: Hey, buddy, where's "Cayus"?
Student: It's pronounced "Keys" and on your map it is spelled "Gon...."
(note for mystified non-Brits: Gonville and Caius College, usually referred to Caius , was founded in 1348 by Edmund Gonville, ran out of money, and was refounded in 1557 by John Caius.)
Post by: hamdani yusuf on 18/11/2024 15:11:24
AFAIK high school physics still deals with ideal pendulums. I don't recall the chemical and crystallographic aspects of vacuum tribology being part of the syllabus, though some of my contemporaries did study it for their PhDs and as of this week it remains an important topic for electron microscopists in a well-known laboratory down the road from here.Some textbooks have advanced sections or more detailed problems addressing damped pendulums or other oscillatory motions like vibrating mass on a spring or a bouncing ball. They show how energy is lost due to non-ideal conditions.
Post by: hamdani yusuf on 18/11/2024 15:22:45
Then tell me, is the temperature of the electrons going up and down, the same as that of the electrons going side to side?It depends on how it's measured. If the antenna is diagonally polarized, or circularly polarized, they can be the same.
Although the values can be different, they are still well defined.
Post by: hamdani yusuf on 18/11/2024 15:25:02
Do you realise that a noise temperature or antenna temperature isn't a real temperature of anything?How do you define real temperature?
How do you measure it?
Post by: Bored chemist on 18/11/2024 20:07:27
There are subtle differences between Catherine/CatharineSo subtle that both colleges have crests featuring Catherine wheels.
and Magdalen/Magdalene.Again, the difference is spelling.
Both refer to Mary.
Post by: alancalverd on 18/11/2024 22:08:44
Post by: Bored chemist on 18/11/2024 22:22:28
:-)
How many Saints Catherine was the firework named after?
Anyway...
Back to the less important matter of reminding HY of stuff he's already been told...
How do you define real temperature?The temperature corresponds to the average energy in the various forms available to the system.
If there isn't one- as in this case for example
It depends on how it's measured.then there isn't a real temperature.
Temperature is important if you are looking at things in thermal equilibrium. But if they are not even at equilibrium with themselves, that's not going to work.
How do you measure it?With a thermometer that is at equilibrium with the thing you are measuring, which it can't be if the things is not, in itself, at equilibrium.
Post by: hamdani yusuf on 19/11/2024 22:36:44
The temperature corresponds to the average energy in the various forms available to the system.This might be necessary, but inadequate to distinguish temperature from other forms of energy.
Post by: Bored chemist on 19/11/2024 22:49:14
Anyway...
Back to the less important matter of reminding HY of stuff he's already been told...
The energy of a moving object like a car isn't thermal energy for two reasons.
1) there's only 1 car and temperature is defined for an ensemble of particles.
2) the various forms of energy within the car (rotation, vibration, translation, electronic, etc) are not all in equilibrium.
Post by: hamdani yusuf on 20/11/2024 00:57:09
Temperature is important if you are looking at things in thermal equilibrium. But if they are not even at equilibrium with themselves, that's not going to work.Temperature in water column of the ocean, or atmospheric air column, or water being boiled are not in equilibrium. But their temperature can be measured.
Post by: hamdani yusuf on 20/11/2024 02:24:41
With a thermometer that is at equilibrium with the thing you are measuring, which it can't be if the things is not, in itself, at equilibrium.What kind of thermometer is the most accurate to measure the real temperature? What makes it better than the others?
Post by: Bored chemist on 20/11/2024 14:10:51
OK, so we can start with water being boiled. That, by definition, is the temperature at which the liquid and gas phases are at equilibrium at the local external pressure.Temperature is important if you are looking at things in thermal equilibrium. But if they are not even at equilibrium with themselves, that's not going to work.Temperature in water column of the ocean, or atmospheric air column, or water being boiled are not in equilibrium. But their temperature can be measured.
It really is at equilibrium.
The water column etc have average temperatures.
But the temperature of the bottom is different to that at the top.
So what?
What kind of thermometer is the most accurate to measure the real temperature? What makes it better than the others?It depends, and it depends.
Post by: hamdani yusuf on 20/11/2024 21:14:23
OK, so we can start with water being boiled. That, by definition, is the temperature at which the liquid and gas phases are at equilibrium at the local external pressure.At equilibrium with what?
It really is at equilibrium.
Post by: hamdani yusuf on 20/11/2024 21:32:54
When were the term temperature defined in scientific community?
Quote
The concept of temperature has been studied and refined over centuries, with its formal scientific definition taking shape in the 17th and 18th centuries as thermodynamics and kinetic theory developed.
Key Milestones in the Definition of Temperature:
1. Ancient Roots (Pre-17th Century):
Early cultures understood the qualitative idea of "hot" and "cold" but had no precise measurement.
Ancient Greek philosophers like Aristotle (4th century BCE) described temperature in terms of the "qualities" of heat and cold, but these ideas were purely philosophical.
2. Invention of Temperature Scales (17th Century):
Galileo Galilei (1593): Invented a rudimentary thermoscope to measure temperature changes qualitatively.
Fahrenheit Scale (1724): Gabriel Fahrenheit developed a mercury-in-glass thermometer and defined a temperature scale.
Celsius Scale (1742): Anders Celsius introduced a scale based on the freezing and boiling points of water.
3. Thermodynamic Definition (19th Century):
The scientific definition of temperature was linked to energy and the motion of particles.
William Thomson (Lord Kelvin, 1848): Proposed the absolute temperature scale, now called the Kelvin scale, rooted in thermodynamic principles.
Kelvin defined temperature based on the second law of thermodynamics, making it independent of the properties of specific substances.
4. Kinetic Theory (19th Century):
James Clerk Maxwell and Ludwig Boltzmann connected temperature to the average kinetic energy of particles in a system:
T ~ < KE >
Summary:
Pre-1600s: Temperature understood qualitatively.
17th?18th Century: Development of temperature scales and thermometers.
19th Century: Temperature defined scientifically in terms of thermodynamics and particle motion.
Today, temperature is formally defined as a measure of the average kinetic energy of particles in a system, with the Kelvin being the SI unit.
Post by: hamdani yusuf on 20/11/2024 21:36:10
Quote
The concept of "energy" has evolved over centuries, but its formal definition in the scientific community began to take shape in the 17th and 18th centuries during the development of classical mechanics.
Key Milestones:
1. Aristotle (4th century BCE): The idea of "energeia," meaning "activity" or "actuality," was introduced in philosophy but was not a scientific concept.
2. Gottfried Wilhelm Leibniz (1676?1689): Introduced the concept of vis viva (living force), an early precursor to kinetic energy, defining it as proportional to the mass and the square of the velocity.
3. Isaac Newton (1687): His work in Principia Mathematica developed foundational principles of motion and introduced concepts that contributed to the later understanding of energy.
4. Thomas Young (1807): First used the term "energy" in its modern sense in a lecture, relating it to the capacity to perform work.
5. William Rankine and James Prescott Joule (19th century): Formalized the concept of energy as a measurable quantity and connected it to the principle of conservation of energy.
Joule's experiments (1840s) demonstrated the equivalence of mechanical energy and heat, a cornerstone of the first law of thermodynamics.
By the mid-19th century, energy was universally recognized in science as the capacity to perform work, with multiple forms like kinetic, potential, thermal, and chemical energy systematically classified.
Post by: hamdani yusuf on 20/11/2024 21:37:07
Quote
Thermal energy refers to the total internal energy of a system due to the random motion of its particles, such as atoms and molecules. It is associated with the temperature of the system and arises from the kinetic and potential energies of these particles.
Key Points:
1. Particle Motion:
In solids: Particles vibrate around fixed positions.
In liquids: Particles move more freely but still interact strongly.
In gases: Particles move rapidly and independently.
2. Relation to Temperature:
Temperature is a measure of the average kinetic energy of particles in a system.
Thermal energy depends on both temperature and the number of particles in the system.
3. Forms of Thermal Energy:
Kinetic energy of particles: Due to their movement.
Potential energy of particles: Due to intermolecular forces.
4. Units: Thermal energy is measured in joules (J) in the SI system.
5. Not Heat: Thermal energy is not the same as heat.
Thermal energy is the energy contained within a system.
Heat is the transfer of thermal energy from one body to another due to a temperature difference.
Examples:
Boiling water has high thermal energy due to the rapid motion of water molecules.
A heated metal rod has more thermal energy than a cold one because its atoms vibrate more intensely.
Post by: hamdani yusuf on 20/11/2024 21:38:12
Quote
The term entropy was first defined in the scientific community in the mid-19th century as a concept related to the second law of thermodynamics. Its development is primarily credited to Rudolf Clausius, a German physicist.
Key Milestones:
1. Rudolf Clausius (1850s-1865):
In 1850, Clausius formulated the second law of thermodynamics, introducing the idea that energy transformations involve a loss of usable energy.
In 1865, Clausius coined the term entropy from the Greek word trope (transformation) to describe the measure of energy unavailable for work during a thermodynamic process.
He mathematically defined entropy () and introduced the famous relation:
dS = dQ / T
2. Ludwig Boltzmann (1870s):
Boltzmann provided a statistical interpretation of entropy, linking it to the microscopic properties of systems.
He introduced the equation:
S = k_B ln W
3. Josiah Willard Gibbs (1870s):
Extended the concept of entropy to chemical thermodynamics and equilibrium, laying the groundwork for its broader applications in physics and chemistry.
Summary:
1865: Clausius defines entropy in thermodynamics.
1870s: Boltzmann and Gibbs refine and expand the concept to statistical mechanics.
Entropy is now recognized as a central concept in thermodynamics, statistical mechanics, and information theory.
Post by: Bored chemist on 21/11/2024 11:04:54
The water at the bottom is not boiling.OK, so we can start with water being boiled. That, by definition, is the temperature at which the liquid and gas phases are at equilibrium at the local external pressure.At equilibrium with what?
It really is at equilibrium.
It is, therefore irrelevant to any discussion of "boiling water".
Why did you mention it?
Post by: Bored chemist on 21/11/2024 11:06:40
ChatGPT's answer to my questionWhy are you still asking ChatGPT and wasting bandwidth with it?
Post by: hamdani yusuf on 22/11/2024 10:15:35
The system is not in equilibrium. But the temperature can still be defined and measured.The water at the bottom is not boiling.OK, so we can start with water being boiled. That, by definition, is the temperature at which the liquid and gas phases are at equilibrium at the local external pressure.At equilibrium with what?
It really is at equilibrium.
It is, therefore irrelevant to any discussion of "boiling water".
Why did you mention it?
Post by: alancalverd on 22/11/2024 11:34:36
But the temperature can still be defined and measured.The temperature of what, where? Obviously the ice is at 273K or less, and the water at anything between ambient and 373K depending on where you measure it. The outside of the test tube may be close to 273K at the bottom and anything up to 1500K in the flame.
Post by: alancalverd on 22/11/2024 11:39:56
What kind of thermometer is the most accurate to measure the real temperature?An ideal gas thermometer defines kelvin over the range of its gas phase, but (a) it is clumsy and (b) it only exists in textbooks. For most applications a platinum resistance thermometer gives a very good approximation to ideal linear performance but a calibrated thermistor or radiation pyrometer is usually more practical over a short range.
Post by: paul cotter on 22/11/2024 13:12:17
Post by: Bored chemist on 22/11/2024 16:47:33
The system is not in equilibrium. But the temperature can still be defined and measured.You can't sensibly talk about that tube full of water having "A temperature".
It has at least three. The water at the top is near 100C The water at the bottom is near 0C and there's a range in between.
So, it has many "temperatures".
A thermal camera would make that obvious.
Post by: hamdani yusuf on 22/11/2024 21:58:50
You've answered your own question. Every small part of the system have defined and measurable temperature.But the temperature can still be defined and measured.The temperature of what, where? Obviously the ice is at 273K or less, and the water at anything between ambient and 373K depending on where you measure it. The outside of the test tube may be close to 273K at the bottom and anything up to 1500K in the flame.
But how small the subsystem can be while still having defined temperature when isolated?
Post by: alancalverd on 22/11/2024 22:25:56
Post by: Bored chemist on 23/11/2024 15:50:09
But how small the subsystem can be while still having defined temperature when isolated?If you isolate some matter and then wait, the equipartition principle will shuffle the energy around until you have an equilibrium.
I dare say Alan knows more about relaxation times than I do.
Post by: hamdani yusuf on 24/11/2024 05:32:55
If you define temperature as the mean kinetic energy of a confined ensemble, then one molecule in a box can have a temperature. Though that does raise the question: what is the box made of?The definition in dictionary is
Quote
the degree or intensity of heat present in a substance or object, especially as expressed according to a comparative scale and shown by a thermometer or perceived by touch.While Wikipedia says
Quote
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making up a substance.The last sentence put a constraint to objects made up of atoms. Hence temperature of a collection of free electrons or positrons, or vacuum of space can't be defined.
The word "reflect" there implies that there are other factor(s) not mentioned yet in the sentence.
The Wikipedia article contains definition of temperature as Intensive variability.
Quote
Intensive variability
In thermodynamic terms, temperature is an intensive variable because it is equal to a differential coefficient of one extensive variable with respect to another, for a given body. It thus has the dimensions of a ratio of two extensive variables. In thermodynamics, two bodies are often considered as connected by contact with a common wall, which has some specific permeability properties. Such specific permeability can be referred to a specific intensive variable. An example is a diathermic wall that is permeable only to heat; the intensive variable for this case is temperature. When the two bodies have been connected through the specifically permeable wall for a very long time, and have settled to a permanent steady state, the relevant intensive variables are equal in the two bodies; for a diathermal wall, this statement is sometimes called the zeroth law of thermodynamics.
In particular, when the body is described by stating its internal energy U, an extensive variable, as a function of its entropy S, also an extensive variable, and other state variables V, N, with U = U (S, V, N), then the temperature is equal to the partial derivative of the internal energy with respect to the entropy:
T = (dU/dS) V, N
Quote
Local thermodynamic equilibrium
Real-world bodies are often not in thermodynamic equilibrium and not homogeneous. For the study by methods of classical irreversible thermodynamics, a body is usually spatially and temporally divided conceptually into 'cells' of small size. If classical thermodynamic equilibrium conditions for matter are fulfilled to good approximation in such a 'cell', then it is homogeneous and a temperature exists for it. If this is so for every 'cell' of the body, then local thermodynamic equilibrium is said to prevail throughout the body.
Post by: hamdani yusuf on 24/11/2024 09:08:54
How Hot is Light? How Lasers Bend the Rules of Heat Transfer
Quote
made a video before explaining that lasers can heat things to any positive temperature due to the fact that they have a population inversion which gives them a negative absolute temperature which is hotter than any positive temperature. But the problem with that explanation is that a temperature can only be defined in a system that is in thermal equilibrium. So technically you can't assign a laser a negative absolute temperature. Only a "psuedo" negative temperature. But it turns out that explanation isn't necessary since magnetrons can do it and they don't have a negative temperature. Really the best explanation of why a laser can heat things hotter than itself is that you are inputting energy into the system and that energy turns into heat as I explained in this video. All of the mystery fades away when you think of it this way.
Post by: Bored chemist on 24/11/2024 11:29:43
All of the mystery fades away when you think of it this way.What mystery?
Post by: Eternal Student on 24/11/2024 22:49:42
This bit worried me:
If you define temperature as the mean kinetic energy of a confined ensemble, then one molecule in a box can have a temperature.
I might be missing the context, is this a hypotehtical retort to some older post perhaps?
Anyway, I would have asked "Can you really do this?"
Typically the bulk translational motion of an object is excluded as a mode of energy that might be called "thermal energy" or contribute to the temperature we can assign to the object. It's still an important form of energy that the object can have - but just not one which contributes to its temperature or "hotness".
Simple example: Heat a metal rod to 100 deg. C while at rest in the lab frame. Now set the rod moving at 100 mph (or 1000, or 10 000 mph) relative to the lab frame. Has that changed the temperature of the rod? Does a thermometer used on the moving rod record a temperature of 100 deg C, or slightly more than 100 deg C now that the rod has some more translational k.e. ? If you put some good lubricating (0 friction) but also heat conducting gel between the moving rod and a thermometer you keep static in the lab frame, surely the thermometer still reports the temperature as being 100 deg C exactly? Or... do you not think so?
As mentioned earlier, the bulk translational motion of the metal rod is an important form of energy that the object does have and there are certainly ways you could convert this into extra heat delivered to some other object (e.g. forget about using the lubricating gel) but the point is that we don't have to and there is no reason why this form of energy would be considered to contribute to the temperature of the object. As a more extreme example, we don't consider the mass of an object to contribute to its temperature. You could annhilate the mass in the rod and generate a whole lot more heat energy if you wanted but that isn't anything that is counted as making a contribution to the temperature or hotness of the object.
In your example, the entire object under consideration is just one molecule and you'd want to exclude the bulk translational motion of that object when assigning a temperature to it. Or... do you not think so?
For additional consideration please keep in mind that there's no reason why the bulk translational k.e. of an object would have to be in equilibrium with other modes of storing energy within that object (your object is one molecule so modes of storing energy which will contribute to temperature can include the vibration of atoms within the molecule).
Best Wishes.
Post by: alancalverd on 25/11/2024 08:55:20
The atoms within your rod have no idea how fast the rod is travelling in space if the speed is constant, but they do know what happens when they bash into each other. If you decelerate the rod by crashing into a brick wall, the energy transferred to the wall will be the 0.5mv2 component of the rod plus its heat content, but we are no longer talking about the original bounded ensemble.
Post by: Eternal Student on 25/11/2024 13:20:30
Thanks for your comments @alancalverd . I think I can see what you're saying.
Although there isn't anything resembling an ensemble of particles inside your box, there is only one particle in the box, we can theoretically treat it as a confined ensemble of particles. That theoretical ensemble of particles could have a temperature much as you described.
I don't often sympathise with a lot of stuff that @hamdani yusuf talks about. However, temperature isn't the simple or completely objective thing that we may have thought it was after sutdying some school level science and there's not much wrong with discussing this in a science forum like this. It can be fun to re-examine some of the stuff that you (we) thought we knew and understood. All the words you may have heard at school like "we are only presenting some models and as you study more, you'll find that many of these models will be replaced with something else or possibly that the model is just wrong and fails to explain some things but there's no better model available at the moment" are actually true but the problem is that people do actually leave school and then there's no school reunion event to actually follow up with the discussion about this. A forum like this one can usefully provide a place for such discussion.
If you (we) end up teaching some young people then we'd better make sure we teach the syllabus but at least we can say those words in red (.... we are only presenting some models..... ) with some genuine conviction.
One of the things about "temperature" or "hotness" of an object that I don't think has been mentioned much so far is that it can depend on the place where the object is put. I hope @hamdani yusuf won't mind too much if I spend a moment discussing something about this here.
Let's start here:
Temperature is a measure of a quality of a state of a material. The quality may be regarded as a more abstract entity than any particular temperature scale that measures it, and is called "hotness" by some writers. The quality of hotness refers to the state of material only in a particular locality, and in general, apart from bodies held in a steady state of thermodynamic equilibrium, hotness varies from place to place....
[Taken from Wikipedia under "Basic Theory" for temperature. https://en.wikipedia.org/wiki/Temperature#Basic_theory ]
What does it mean? Well it could mean that some things, like a long rod, have a temperature of 100 deg C at one end and a temperature of 80 deg C at the other end - temperature can be locally defined and vary from place to place. However, that is almost certainly NOT what was meant. They probably meant that even if the whole rod had a temperature of 100 deg C while it's measured in one place then if we pick the whole rod up and just put it in a different place, it's quality of hotness or temperature can change.
An example: The Zeeman effect describes how the energy levels occupied by electrons can split in the presecence of a magnetic field.
We consider a theoretical object, a sink for all thermal energy, that we identify as being at 0 Kelvin (absolute zero). If we put a test object into thermal contact with this sink then energy will flow from our test object to the sink if and only if the test object has a temperature above 0 kelvin. So we have a way to know if our test object is at absolute zero.
Now we have a test object that is a bit like a group I metal, with a single valence electron in its outer shell. We'll have this test object in its lowest possible energy state. In a place with no magnetic field, the electrons have no lower energy level they can fall to, we can make thermal contact with out idealised thermal sink and no energy will flow to that sink. Our test object seems to have a temperature of 0 kelvin.
If we move our test object to a region with a uniform but non-zero magnetic field, without deliberately adding or removing any energy to the test object, then the electrons wave functions become a superposition of the two energy levels available from the splitting by the Zeeman effect. Alternatively, if you prefer to think of the electrons individually and more classically then you could say half of the electrons go into the lower energy level, half go into the higher energy level and overall the energy of the test object remains the same. Either way you wish to consider it, there are some electrons capable of falling into a lower energy state. So when we make thermal contact with the sink, some energy can flow into the sink and out of our test object. It seems that the test object has a temperature above 0 kelvin now that it's in a place with a non-zero magnetic field.
There's quite a few things about temperature that are strange and not as clear cut as we may have thought. In General Relativity we don't often consider temperature as an important thing, it may not be fundamental or objective enough. For example, the temperature of an ideal fluid doesn't appear in the stress-energy tensor but the pressure of that fluid does. Similarly, cosmological "equations of state" relate energy density to pressure and don't involve temperature unlike the typical "equatons of state" that you may encounter in thermodynamics. Pressure may be a bit more fundamental, objective, or useful as a property that an object can have rather than temperature. I don't know.... it's all just a model but maybe a model where we recognise temperature as just an approximate and emergent property is a small step closer to the underlying nature of things.
In that respect, I would differ from @hamdani yusuf 's line of attack: I'm not sure there is a lot of point trying to make a better or more precise definition for temperature. It's just an approximation that is useful sometimes.
Best Wishes.
Post by: hamdani yusuf on 26/11/2024 13:37:50
Typically the bulk translational motion of an object is excluded as a mode of energy that might be called "thermal energy" or contribute to the temperature we can assign to the object. It's still an important form of energy that the object can have - but just not one which contributes to its temperature or "hotness".Bulk rotational motion is also usually excluded as a mode of thermal energy.
But a freely spinning magnet inside a vacuum glass container can transfer it's energy to a nearby metal block and raise its temperature. Then the next question is what is thermal energy?
Post by: alancalverd on 26/11/2024 13:38:12
Post by: Bored chemist on 26/11/2024 14:01:06
Then the next question is what is thermal energy?The same as it was when you started this thread 4 years ago.
What have you learned in the meantime?
Post by: paul cotter on 26/11/2024 14:29:00
Post by: paul cotter on 26/11/2024 14:55:18
Post by: hamdani yusuf on 26/11/2024 21:49:40
ES's theoretical test object at 0K raises some interesting questions. Since theory predicts that you can't bring an object to 0K, it rather looks like dividing 0 by 0 with a predetermined idea of the result instead of admitting that the result is undefined. In other words, ES has devised a "thought experiment" that can't be done and is therefore not an experiment!What is 0 in numerator, and 0 in denominator refer to?
Post by: hamdani yusuf on 26/11/2024 21:50:33
What is it?Then the next question is what is thermal energy?The same as it was when you started this thread 4 years ago.
What have you learned in the meantime?
Do you have any information that ChatGPT doesn't?
Post by: alancalverd on 26/11/2024 23:09:18
Then the next question is what is thermal energy?The kinetic energy of the particles inside a boundary.
Post by: hamdani yusuf on 27/11/2024 06:06:39
That would include rotational motion of a spinning magnet.Then the next question is what is thermal energy?The kinetic energy of the particles inside a boundary.
Post by: hamdani yusuf on 27/11/2024 06:48:01
Following up on the test tube with water being heated, here is something NOT to do and I repeat NOT. Faced with a dirty pyrex boiling tube which resisted cleaning I added water to a depth of about 2 inches and added conc sulphuric acid to a depth of about 1 inch and applied vigorous heating at the base. Some time later I was surprised that boiling had not ensued and I gave the tube a slight shake and whoosh, the entire contents of hot acid and boiling water exploded out of the tube. The acid had not mixed significantly with the water and with a boiling point in the region of 300c it was not going to boil for quite some time and shaking the tube led to mixing and a subsequent runaway reaction. My mistake was a failure to thoroughly mix acid and water before heating.Thanks for sharing your experience. People say that experience is the best teacher. But we should not rely on our own experience in learning about safety issues. We need to learn from someone else's mistakes. We won't have enough time to make them all ourselves.
Post by: hamdani yusuf on 27/11/2024 07:15:01
Thermodynamics temperature is mostly measured by heat conduction between the measured object and the thermometer. While antenna temperature is specifically measured by electromagnetic radiation between the measured object and the receiving antenna. It's an analogy to thermodynamics temperature, which was introduced first.
Post by: hamdani yusuf on 27/11/2024 08:02:29
How the system will deplete its internal energy?
What happens to its angular momentum? Will it be conserved?
Post by: hamdani yusuf on 27/11/2024 08:17:33
The atoms within your rod have no idea how fast the rod is travelling in space if the speed is constant, but they do know what happens when they bash into each other. If you decelerate the rod by crashing into a brick wall, the energy transferred to the wall will be the 0.5mv2 component of the rod plus its heat content, but we are no longer talking about the original bounded ensemble.Perhaps some variations of this experiment can improve our understanding.
We can use cooled magnet. Or a superconductor as the stopper.
Post by: Bored chemist on 27/11/2024 11:30:14
What happens to its angular momentum? Will it be conserved?Yes.
And I didn't need to read the rest of your post.
So, we can work back from that fact.
Thus we deduce that you are wrong somewhere in your argument.
Feel free to find out where.
Post by: hamdani yusuf on 27/11/2024 14:15:23
Thus we deduce that you are wrong somewhere in your argument.What makes you think that there's something wrong?
I haven't even made any conclusion.
Post by: Bored chemist on 27/11/2024 17:45:43
I haven't even made any conclusion.
How the system will deplete its internal energy?You can't ask how something happens without concluding that the thing happens.
Are you sure that energy is available to drive a laser?
Are you sure that there will be a change in angular momentum?
Post by: alancalverd on 28/11/2024 22:58:44
The gas particles move slower the closer they are from the axis of rotation, and faster the further away from the axis.Why use a gas? The molecules of any spinning wheel are moving faster at the edge, so you can extract energy by fixing a thermocouple to any rotating object. Problem is that nobody else can!
Your fallacy is in confusing gross motion of a body with thermal motion of the particles inside it..
Post by: hamdani yusuf on 29/11/2024 08:21:19
You can't ask how something happens without concluding that the thing happens.A conclusion is
Quote
a judgment or decision reached by reasoning.You are confused between conclusion and proposition.
the necessary consequence of two or more propositions taken as premises
Post by: hamdani yusuf on 29/11/2024 08:23:54
Are you sure that energy is available to drive a laser?As long as the extractable energy is non-zero, the system can be upscaled until it's enough to drive a laser.
Post by: hamdani yusuf on 29/11/2024 08:26:19
Are you sure that the laser doesn't carry away some of the system's angular momentum?What happens to its angular momentum? Will it be conserved?Yes.
And I didn't need to read the rest of your post.
Post by: hamdani yusuf on 29/11/2024 08:37:55
Because the thermometer can be stationary in the frame of reference of the tubes, while in relative motion against the gas particles to exchange their thermal energy.The gas particles move slower the closer they are from the axis of rotation, and faster the further away from the axis.Why use a gas? The molecules of any spinning wheel are moving faster at the edge, so you can extract energy by fixing a thermocouple to any rotating object. Problem is that nobody else can!
The gas is chosen for the simplicity of equation connecting its temperature and its kinetic energy.
E=nRT d/2
Post by: hamdani yusuf on 29/11/2024 08:39:46
Your fallacy is in confusing gross motion of a body with thermal motion of the particles inside it..What's the fundamental difference between gross motion and thermal motion? If you can answer this question, then this thread can be satisfactorily closed.
Post by: alancalverd on 29/11/2024 11:06:18
Post by: Bored chemist on 29/11/2024 13:48:41
Not if it's set up to point away from the system's centre of mass.Are you sure that the laser doesn't carry away some of the system's angular momentum?What happens to its angular momentum? Will it be conserved?Yes.
And I didn't need to read the rest of your post.
Post by: Bored chemist on 29/11/2024 13:50:58
You are muddling the question of "does this happen?" with the question of "how does this happen?".You can't ask how something happens without concluding that the thing happens.A conclusion isQuotea judgment or decision reached by reasoning.You are confused between conclusion and proposition.
the necessary consequence of two or more propositions taken as premises
The second only makes sense if you already concluded that the answer to the first is yes.
Post by: hamdani yusuf on 05/12/2024 13:45:58
You are muddling the question of "does this happen?" with the question of "how does this happen?".In mathematics, there's an example for this kind of argumentation.
The second only makes sense if you already concluded that the answer to the first is yes.
The question is : is there an infinite number of prime?
The first step is to assume that there is a finite number of prime.
And then describe its implications. By showing that some of those implications contradict each other, we conclude that the assumption is false.
Post by: Bored chemist on 05/12/2024 21:08:29
Post by: hamdani yusuf on 06/12/2024 11:29:23
Yes, but that's not what you were doing.Do you think the system will not deplete its energy?
Post by: hamdani yusuf on 06/12/2024 11:55:35
Thermal motion is the displacement of particles within[ a body relative to each other and (in the "one particle " case, or) the boundaries of the body. Gross motion is the displacement of the entire body relative to other bodies.How about a gas in a container vibrating when a sound wave is passing by? Will it be the same with liquid or solid?
Post by: alancalverd on 06/12/2024 12:40:36
Post by: hamdani yusuf on 07/12/2024 13:49:45
You can indeed heat things by shaking them. If the gas or liquid has nonzero viscosity, some of the kinetic energy of shaking the walls is transferred to the fluid. Friction heating of a solid is the same thing - we routinely use ultrasonic welding for plastics.Is the vibration classified as thermal energy?
Post by: alancalverd on 07/12/2024 16:19:40
Post by: hamdani yusuf on 10/12/2024 23:43:59
The result of vibration is thermal energy.Before it's converted into thermal energy, vibration wasn't thermal initially.
Post by: alancalverd on 11/12/2024 00:35:42
Post by: hamdani yusuf on 12/12/2024 00:03:23
How very true. And before you applied the brakes, the car had kinetic energy, which turned into heat in the brake discs or electrical energy via the regen system. So what?
What's the fundamental difference between gross motion and thermal motion?
Post by: Bored chemist on 12/12/2024 11:00:26
What's the fundamental difference between gross motion and thermal motion?Direction.
Post by: alancalverd on 12/12/2024 15:19:31
Post by: hamdani yusuf on 12/12/2024 20:39:27
What's the direction that makes a motion thermal, and what direction makes it non-thermal?What's the fundamental difference between gross motion and thermal motion?Direction.
Post by: alancalverd on 12/12/2024 22:51:07
Post by: paul cotter on 13/12/2024 09:35:25
Post by: hamdani yusuf on 13/12/2024 13:51:45
Thermal motion is random and uncoordinated, which is why things don't accelerate in a single direction when they get hot - that would be gross motion.How is the randomness measured?
If two identical objects have the same total kinetic energy. The first one has more random motion than the second one. Which one has higher temperature?
If they're let to exchange their kinetic energy, which one will end up with higher kinetic energy?
Post by: alancalverd on 13/12/2024 14:46:47
How is the randomness measured?By the degree of anisotropy of thermal expansion.
Post by: Bored chemist on 13/12/2024 17:59:36
It's becoming clear that you are either trolling or, I'm afraid, just not bright enough to understand science.What's the direction that makes a motion thermal, and what direction makes it non-thermal?What's the fundamental difference between gross motion and thermal motion?Direction.
I think it would be better all round if you tried something else.
If, after literally years of patient explanation, you haven't got to grips with this idea, you probably never will and maybe you should try a different field
Post by: hamdani yusuf on 14/12/2024 04:35:05
If you truly understand the topic, you should be able to answer my follow up questions.It's becoming clear that you are either trolling or, I'm afraid, just not bright enough to understand science.What's the direction that makes a motion thermal, and what direction makes it non-thermal?What's the fundamental difference between gross motion and thermal motion?Direction.
I think it would be better all round if you tried something else.
If, after literally years of patient explanation, you haven't got to grips with this idea, you probably never will and maybe you should try a different field
How is the randomness measured?
If two identical objects have the same total kinetic energy. The first one has more random motion than the second one. Which one has higher temperature?
If they're let to exchange their kinetic energy, which one will end up with higher kinetic energy?
Post by: hamdani yusuf on 14/12/2024 04:37:15
Quote
Timestamps
0:00 Celsius
2:14 Fahrenheit
4:42 Kelvin
8:00 Lightning Round (Rankinet, R?mer, Delisle, Newton, Reaumur, Wedgwood)
? DISCLAIMER ?
This video is intended for entertainment and educational purposes only. It should not be your sole source of information. Some details may be oversimplified or inaccurate. My goal is to spark your curiosity and encourage you to conduct your own research on these topics.
Post by: hamdani yusuf on 14/12/2024 04:53:49
Formally they have the same units. J/KIn this thread, at least you can learn new things, like the difference between heat capacity and specific heat capacity.
Practically they don't, because (for heat capacity) you almost always add an amount so
J/K mol or J/K Kg
But for entropy, the "unit" is often "per molecule/ atom " Those entities are countable, and a number is dimensionless.
On the other hand, "mole" is also a number.
It's a perennial problem in metrology. How do you measure "how much stuff you have".
Also, that entropy is an extensive quantity. If a system consists of an object with entropy of 1 J/K, and another identical object is added to the system, its entropy becomes 2 J/K.
In contrast, temperature is an intensive quantity. If a system consists of an object with temperature of 1 K, and another identical object is added to the system, its temperature is still 1 K.
Post by: hamdani yusuf on 14/12/2024 05:13:16
Quote
https://en.m.wikipedia.org/wiki/TemperatureFrom the underlined statement, we need to define what counts as thermal contact. Also, what counts as heat transfer and thermal equilibrium.
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making up a substance.
...
When two systems in thermal contact are at the same temperature no heat transfers between them. When a temperature difference does exist heat flows spontaneously from the warmer system to the colder system until they are in thermal equilibrium. Such heat transfer occurs by conduction or by thermal radiation.[45][46][47][48][49][50][51][52]
Experimental physicists, for example Galileo and Newton,[53] found that there are indefinitely many empirical temperature scales. Nevertheless, the zeroth law of thermodynamics says that they all measure the same quality. This means that for a body in its own state of internal thermodynamic equilibrium, every correctly calibrated thermometer, of whatever kind, that measures the temperature of the body, records one and the same temperature. For a body that is not in its own state of internal thermodynamic equilibrium, different thermometers can record different temperatures, depending respectively on the mechanisms of operation of the thermometers.
Post by: hamdani yusuf on 14/12/2024 06:02:43
Quote
No, the temperature of the xenon gas at the top of the 100 km tall isolated tube will not be the same as at the bottom due to the effects of gravity and the barometric temperature gradient.
Explanation:
1. Hydrostatic Equilibrium:
In the presence of gravity, the gas molecules are denser near the bottom and less dense at the top. The pressure decreases with height following the barometric equation:
P(h) = P_0 e^{-\frac{m g h}{k_B T}}
- P(h) is the pressure at height h,
- P_0 is the pressure at the bottom,
- m is the mass of a xenon atom,
- g is the acceleration due to gravity,
- h is the height,
- k_B is Boltzmann's constant,
- T is the temperature.
2. Temperature Gradient:
If the tube is adiabatically isolated, the temperature will vary with height due to the adiabatic lapse rate. As gas at the bottom is compressed under high pressure, it is warmer, while gas at the top expands, causing cooling.
The temperature change with height is given by:
\frac{dT}{dh} = -\frac{g M}{C_p}
is the acceleration due to gravity,
is the molar mass of xenon (),
is the specific heat capacity at constant pressure.
For xenon gas, this lapse rate will cause the temperature to decrease with increasing height.
3. Key Factors:
Isolated Tube: If there is no external heat exchange (adiabatic system), the temperature will follow the lapse rate described above.
Non-isolated Tube: If heat can be transferred between the gas and the surroundings, the temperature might equalize over time.
Conclusion:
The gas temperature at the top will generally be lower than at the bottom in an isolated system due to the adiabatic lapse rate caused by the influence of gravity. For a 100 km tall column, this difference could be significant.
Post by: hamdani yusuf on 14/12/2024 06:06:47
Quote
The gas temperature at the top and bottom would not be the same. Here's why:How atomic mass of the gas affect the temperature difference between the top and bottom of the tube?
* Pressure Variation: The weight of the gas column creates a higher pressure at the bottom of the tube compared to the top. This pressure difference can affect the gas's internal energy and temperature.
* Adiabatic Processes: As the gas moves up the tube, it experiences a decrease in pressure, leading to adiabatic expansion. This expansion causes the gas to cool slightly. Conversely, as the gas moves down the tube, it experiences adiabatic compression, which causes it to warm slightly.
While these effects might be subtle, they would still lead to a slight temperature difference between the top and bottom of the tube.
However, it's important to note that this is a highly idealized scenario. In real-world conditions, other factors like heat leaks, convection currents, and the specific properties of the gas would further influence the temperature distribution.
Quote
The atomic mass of the gas does affect the temperature difference between the top and bottom of the tube. A heavier gas, like Xenon, will create a larger pressure gradient along the height of the tube compared to a lighter gas. This larger pressure gradient will result in more significant adiabatic compression and expansion effects.
Therefore, a heavier gas will generally exhibit a larger temperature difference between the top and bottom of the tube compared to a lighter gas under the same conditions.
However, it's important to note that other factors, such as the initial temperature and pressure, the specific heat capacity of the gas, and the exact height of the tube, will also influence the magnitude of the temperature difference.
Post by: Bored chemist on 14/12/2024 13:35:23
If you truly understand the topic, you should be able to answer my follow up questions.I could answer it, but I don't see the point.
By next week you would be asking essentially the same thing again because you can't or won't learn.
I mean, if you really can't tell the difference between "this is an airplane and all its atoms and molecules are moving in the same direction at 500 meters per second" and "this is a gas and its molecules are moving in random directions with an average speed of 500 metres per second" then I don't think there's anything we can do to help you understand it.
Post by: hamdani yusuf on 14/12/2024 21:58:18
I could answer it, but I don't see the point.You can't see the point because you haven't learned.
Post by: hamdani yusuf on 14/12/2024 22:04:02
If two identical objects have the same total kinetic energy. The first one has more random motion than the second one. Which one has higher temperature?
If they're let to exchange their kinetic energy, which one will end up with higher kinetic energy?
Quote
Scenario 1: Which object has the higher temperature?
Temperature is a measure of the average kinetic energy per particle due to random motion (thermal motion).
If the first object has more random motion, it means a greater fraction of its total kinetic energy is due to the random motion of its particles, while in the second object, a larger fraction of kinetic energy is associated with ordered motion (e.g., linear or rotational motion).
Conclusion: The first object has a higher temperature, as its random kinetic energy (thermal energy) is greater.
---
Scenario 2: If they exchange kinetic energy, which one ends up with higher kinetic energy?
When two objects are allowed to exchange kinetic energy, energy tends to flow from higher temperature to lower temperature until thermal equilibrium is reached.
Since the first object has a higher temperature, it will lose some of its random kinetic energy to the second object.
However, because both objects started with the same total kinetic energy, the final distribution depends on their energy exchange mechanism:
If the energy exchange is purely thermal: The second object will gain more thermal energy, leading to a more equal temperature, but their total kinetic energies will remain the same.
If the energy exchange also involves ordered motion: The final distribution may vary, depending on whether some of the ordered motion in the second object is converted to random motion.
Conclusion: If the objects are identical and only thermal energy is exchanged, both will have the same total kinetic energy at equilibrium. If ordered motion is involved, the outcome depends on the specifics of the exchange.
Post by: hamdani yusuf on 14/12/2024 22:09:44
Quote
Temperature and Kinetic Energy: A Deeper Dive
Which object has a higher temperature?
The object with more random motion will have a higher temperature.
Temperature is directly related to the average kinetic energy of the particles in a substance. More random motion means a higher average kinetic energy, and thus a higher temperature.
What happens when they exchange kinetic energy?
When two objects with different temperatures are brought into thermal contact, they will exchange energy until they reach thermal equilibrium. In this case, the object with the higher temperature (more random motion) will transfer some of its energy to the object with the lower temperature.
Eventually, both objects will reach the same temperature. This means that the final kinetic energy of both objects will be equal, but distributed differently. The object that initially had more random motion will end up with less random motion, and vice versa.
In essence, energy will flow from the hotter object (higher average kinetic energy) to the colder object (lower average kinetic energy) until both objects reach the same temperature.
Post by: hamdani yusuf on 14/12/2024 22:11:09
Quote
In thermodynamics, temperature is directly related to the average kinetic energy of the particles in an object, not to the total kinetic energy or the randomness of the motion (which is more related to entropy).
Temperature Comparison:
Given that both objects have the same total kinetic energy but one has more random motion, the object with more random motion would generally have a higher temperature. This is because more random motion typically means a higher average kinetic energy per particle, even if the total kinetic energy is the same.
Kinetic Energy Exchange:
When two objects at different temperatures are allowed to exchange kinetic energy, heat will flow from the hotter object to the cooler one until they reach thermal equilibrium. Here:
The object with the higher temperature (more random motion) will initially have particles with higher average kinetic energy.
As they exchange energy, the heat will flow from the object with higher temperature to the one with lower temperature.
Eventually, both objects will reach the same temperature, meaning their average kinetic energies per particle will be equal.
Therefore, after the exchange:
Neither will have higher kinetic energy; they will share the total kinetic energy in such a way that both have the same average kinetic energy per particle, hence the same temperature.
So, to summarize:
The object with more random motion initially has a higher temperature.
After they exchange energy, both will end up with the same kinetic energy because they will reach thermal equilibrium.
Which one do you think has the best answer?
Post by: hamdani yusuf on 14/12/2024 22:27:16
The first object is a stationary warm neodymium magnet, while the second object is a spinning but cooler neodymium magnet. How would they exchange their kinetic energy?
Post by: Bored chemist on 14/12/2024 23:27:48
You may well be right.I could answer it, but I don't see the point.You can't see the point because you haven't learned.
I can't see the point in explaining things to you because I have yet to learn that you can not, or will not listen.
I see that, for example, you still think that quoting chatGPT is somehow useful.
Post by: alancalverd on 15/12/2024 00:07:14
How would they exchange their kinetic energy?By radiative transfer.
Post by: hamdani yusuf on 15/12/2024 07:08:59
I see that, for example, you still think that quoting chatGPT is somehow useful.By asking them the same question at different times, we can see how they learn. Or how mainstream science shift its paradigm over time.
Post by: hamdani yusuf on 15/12/2024 07:09:48
Does it count as thermal energy transfer?How would they exchange their kinetic energy?By radiative transfer.
Post by: alancalverd on 15/12/2024 10:56:28
Post by: hamdani yusuf on 15/12/2024 12:20:08
I asked them with follow up question.To keep the problem simple, let's make the stationary magnet anchored to the floor. And the whole system is isolated from the environment.
The first object is a stationary warm neodymium magnet, while the second object is a spinning but cooler neodymium magnet. How would they exchange their kinetic energy?
Some experimental videos show that induction heating can happen quickly, relative to infrared radiation heating by a warm object.
Eddy current will be generated in both magnets while the spinning magnet decelerates. It means that the stationary magnet will heat up and increase its total kinetic energy, at least initially. Only after the second magnet stops spinning, the excess of total kinetic energy from first magnet will be transferred to the second magnet through electromagnetic radiation, mainly in infrared, until eventually they have the same temperature.
Post by: Bored chemist on 15/12/2024 21:35:06
That's nothing to do with the topic, is it?I see that, for example, you still think that quoting chatGPT is somehow useful.By asking them the same question at different times, we can see how they learn. Or how mainstream science shift its paradigm over time.
Thermodynamics hasn't changed much in 50 years.
The definition of temperature is essentially even older.
Post by: hamdani yusuf on 16/12/2024 07:56:20
That's nothing to do with the topic, is it?It is. AI models will be the first reference for most people to get their information when they need it. Some of them might care enough to cross check the answers with other sources. But most of them won't, especially when the stake is not considered too high.
Post by: hamdani yusuf on 16/12/2024 08:00:41
Thermodynamics hasn't changed much in 50 years.The term temperature is also used in fields other than thermodynamics, such as statistical mechanics, telecommunications, photography, and information science. It will be important to know their differences and similarities.
The definition of temperature is essentially even older.
Post by: hamdani yusuf on 16/12/2024 09:02:04
Quote
The interpretation of probability is a disputed topic in philosophy. But shouldn't physics, as an objective study of nature, be devoid of terms like probability at the fundamental level? Surprisingly, this is not the case. Probability plays a fundamental role even at the level of classical physics. In this video, we explore how Maxwell and Boltzmann brought probability to explain thermodynamic concepts such as entropy, pressure, and temperature. Since then, physics hasn't been the same.
Timestamps:
0:00 - 0:27 Intro
0:28 - 1:07 Paradox of perfume
1:08 - 1:57 Clausius's mean free path approach
1:58 - 3:33 Maxwell's velocity probability distribution
3:44 - 5:08 Weird prediction of the theory
5:09 - 5:38 Influence on Boltzmann
5:39 - 6:09 Prof. Sean Carroll explains the arrow of time
6:10 - 6:45 Boltzmann's H theorem of entropy
6:46 - 8:50 Prof. Cedric Villani explains the formula
8:51 - 9:35 Loschmidt's paradox
9:36 - 10:02 Conclusion
Some interesting comments.
Quote
Planck's approach to Blackbody Radiation was to analyze the entropy as a function of energy. To make both high-frequency and low-frequency data consistent with the Second Law of Thermodynamics, he included an additional "guess" term proportional to the frequency (hf); this results in Planck's Law which is strictly Classical. Planck's subsequent application of Boltzmann's Statistical Mechanics to justify his guess then led to his revolutionary conclusion that the material of the walls emit and absorb radiation in discrete quanta. A paper titled "Planck?s Route to the Black Body Radiation Formula and Quantization" by Michael Fowler (7/25/2008) gives a nice discussion. "Theoretical Concepts in Physics: An Alternative View of Theoretical Reasoning in Physics" (1984) by Malcolm S. Longair contains more details.
Post by: alancalverd on 16/12/2024 09:15:12
Surprisingly, this is not the case.Nothing surprising about it at all. I suspect this video was made by a philosopher. Particularly as the rest of the synopsis is garbage.
Post by: Bored chemist on 16/12/2024 12:01:45
. AI models will be the first reference for most people to get their information when they need it.Maybe they will; but not yet.
Several people here have told you it's not a source of information.
You are the only one using it, and we keep pointing out that such behaviour is foolhardy.
Post by: Bored chemist on 16/12/2024 12:03:13
If they are not all talking about the same thing, then some of them are wrong.Thermodynamics hasn't changed much in 50 years.The term temperature is also used in fields other than thermodynamics, such as statistical mechanics, telecommunications, photography, and information science. It will be important to know their differences and similarities.
The definition of temperature is essentially even older.
Post by: hamdani yusuf on 16/12/2024 13:57:00
Earlier scientists did get surprised, or even straight out rejected the idea.Surprisingly, this is not the case.Nothing surprising about it at all. I suspect this video was made by a philosopher. Particularly as the rest of the synopsis is garbage.
Post by: hamdani yusuf on 16/12/2024 14:00:04
They are sources of information. They can be asked to show their references.. AI models will be the first reference for most people to get their information when they need it.Maybe they will; but not yet.
Several people here have told you it's not a source of information.
You are the only one using it, and we keep pointing out that such behaviour is foolhardy.
Post by: hamdani yusuf on 16/12/2024 14:03:43
If they are not all talking about the same thing, then some of them are wrong.When they are different about something, how to determine the correct one?
Post by: alancalverd on 16/12/2024 14:50:37
Post by: hamdani yusuf on 16/12/2024 15:17:55
As the term is borrowed from thermodynamics, it should be used in inverted commas and specifically redefined for any other use.The term temperature was already widely used before thermodynamics.
Post by: Bored chemist on 16/12/2024 16:08:17
Aside from the fact that you just proves that they are not sources of information (The references are the sources), why don't you ask then?They are sources of information. They can be asked to show their references.. AI models will be the first reference for most people to get their information when they need it.Maybe they will; but not yet.
Several people here have told you it's not a source of information.
You are the only one using it, and we keep pointing out that such behaviour is foolhardy.
And then you can quote those sources and we can judge them.
Post by: paul cotter on 16/12/2024 18:01:53
Post by: alancalverd on 16/12/2024 22:06:34
The term temperature was already widely used before thermodynamics.I very much doubt is its use in telecommunications derives directly from the physiological phenomenon or the etymology of the word, but has a lot more to do with statistics and entropy.
Post by: hamdani yusuf on 17/12/2024 11:02:24
Aside from the fact that you just proves that they are not sources of information (The references are the sources), why don't you ask then?A secondary source is a source.
And then you can quote those sources and we can judge them.
Post by: hamdani yusuf on 17/12/2024 14:47:59
It's used for the similar behaviors of radiation from objects with the same temperature.The term temperature was already widely used before thermodynamics.I very much doubt is its use in telecommunications derives directly from the physiological phenomenon or the etymology of the word, but has a lot more to do with statistics and entropy.
In summary, antenna temperature is a key concept in understanding the amount of radiation an antenna receives, which can represent either signal or noise, depending on the application. It helps quantify the effective power of this radiation by linking it to the temperature concept, facilitating better analysis and system design in both astronomy and communications.
Post by: Bored chemist on 17/12/2024 17:21:48
A thing that makes up nonsense is not a source.Aside from the fact that you just proves that they are not sources of information (The references are the sources), why don't you ask then?A secondary source is a source.
And then you can quote those sources and we can judge them.
Post by: hamdani yusuf on 17/12/2024 21:56:04
Humans make up nonsense everyday. What matters is whether or not the statements accurately represent objective reality.A thing that makes up nonsense is not a source.Aside from the fact that you just proves that they are not sources of information (The references are the sources), why don't you ask then?A secondary source is a source.
And then you can quote those sources and we can judge them.
Post by: alancalverd on 18/12/2024 09:16:05
Post by: Bored chemist on 18/12/2024 09:40:31
Humans make up nonsense everyday.Speak for yourself.
Oh, I see you did.
Post by: hamdani yusuf on 20/12/2024 03:37:34
By definition, nonsense does not represent reality.
Humans make up nonsense everyday.Speak for yourself.
Oh, I see you did.
Geoffrey Hinton said that remembering things is basically the same process as hallucinating, with the difference only in the multiple constraints it has to fulfill.
In many cases, witnesses remember some details of an event with deviations from what really happened. The mistakes are often honest ones, like when you confused between heat capacity and specific heat capacity.
What matters is whether or not the statements accurately represent objective reality.
Post by: hamdani yusuf on 20/12/2024 04:04:37
Every Weird Temperature Scale ExplainedPrimitive concept of temperature was to quantify a human sensory input, namely hotness. Even Newton still used this concept in his temperature scale. In this case, the concept is more closely related to the rate of heat flow between the measured object and human's thermoreceptors.Quote
Timestamps
0:00 Celsius
2:14 Fahrenheit
4:42 Kelvin
8:00 Lightning Round (Rankinet, R?mer, Delisle, Newton, Reaumur, Wedgwood)
? DISCLAIMER ?
This video is intended for entertainment and educational purposes only. It should not be your sole source of information. Some details may be oversimplified or inaccurate. My goal is to spark your curiosity and encourage you to conduct your own research on these topics.
https://en.wikipedia.org/wiki/Thermoreceptor
It was like brightness to quantify visual inputs or loudness to quantify auditory inputs.
More modern concepts for temperature are meant to overcome the limitations of the primitive concept described above, namely variations in humans' thermoreceptors among different individuals, also the limitations in their range of measurement. Instead of heat flow between the measured object and thermoreceptors, new concepts of temperature try to describe and predict the naturally occurring heat flow between two objects with different temperature, when they are in thermal contact.
When heat was discovered as a form of kinetic energy, it becomes necessary to define the distinctions between thermal and non-thermal kinetic energy, also thermal and non-thermal energy transfer.
Post by: hamdani yusuf on 20/12/2024 08:57:02
Quote
Emerging science indicates plants can benefit from cold plasma treatment, so I tested this with controlled conditions, using Grass and Radish seeds. Can plasma change our agriculture?At 0:20 he distinguishes between 2 types of plasma: thermal and non-thermal.
Post by: alancalverd on 20/12/2024 10:11:38
Primitive concept of temperature was to quantify a human sensory input, namely hotness.And for what it's worth, Fahrenheit was brilliant experimentalist who used the human body (his wife) to define his temperature scale. He defined 100 degrees as body temperature but by sheer bad luck Frau F happened to be sick at the time, so "normal" body temperature is actually in the region 97.5 to 98.5.
Realising that the freezing of water depended on its purity, he sensibly set zero to be the lowest temperature achievable with an ice/salt mixture, hence the slightly odd 32F becoming an important value in meteorology and hydrology.
Despite the weird numbers, Fahrenheit formed the basis for steam engineering which was exported over the entire planet in the 19th and 20th centuries.
Post by: hamdani yusuf on 21/12/2024 07:35:01
More modern concepts for temperature are meant to overcome the limitations of the primitive concept described above, namely variations in humans' thermoreceptors among different individuals, also the limitations in their range of measurement. Instead of heat flow between the measured object and thermoreceptors, new concepts of temperature try to describe and predict the naturally occurring heat flow between two objects with different temperature, when they are in thermal contact.It turns out that beside the temperature difference, heat flow also depends on heat conductance.
Similar cases are found in the flowrate of fluid materials which depends on pressure and fluid conductance. Likewise, electric current depends on electric potential and electrical conductance.
As long as the conductance between two nodes is not zero, there will be heat flow between them when there's a difference in temperature.
Post by: alancalverd on 21/12/2024 23:59:15
heat flow also depends on heat conductance.Let's be pedantic. Heat flow (i.e. the direction of mean energy transfer) depends on temperature difference. The rate of heat flow by conduction depends also on the thermal diffusivity of the materials and the boundary conductance.
Post by: hamdani yusuf on 22/12/2024 01:59:20
Since we are talking about fundamental definition of temperature, I try to simplify things to the absolute minimum. I referred to heat transfer between two objects. the system is modelled as 2 vertices and 1 edge connecting them.heat flow also depends on heat conductance.Let's be pedantic. Heat flow (i.e. the direction of mean energy transfer) depends on temperature difference. The rate of heat flow by conduction depends also on the thermal diffusivity of the materials and the boundary conductance.
Post by: alancalverd on 22/12/2024 09:43:12
I try to simplify thingsWhich is why you need to distinguish between direction and rate.
Then for any finite object you need to consider diffusivity. This is where a lot of geothermal energy proposals fall down: you may have found a prospective hotspot, but once you start extracting energy from it your power is limited by the rate at which it is replenished by diffusion from the adjacent rocks - similar to the internal impedance of a battery.
Post by: hamdani yusuf on 22/12/2024 09:52:15
Quote
Here are some great papers on what I talk about in this video:I bought a Newton's cradle meant to use it in videos investigating its behaviors. But since this videos has come out first, I think I won't bother anymore. It saved me time and efforts.
https://www.karlsruher-physikkurs.de/download/ball-chain_part1.pdf
https://www.karlsruher-physikkurs.de/download/ball-chain_part2.pdf
When the plastic tape is used, some kinetic energy is converted to other forms faster, including heat.
Post by: hamdani yusuf on 22/12/2024 09:55:14
Which is why you need to distinguish between direction and rate.The direction of heat transfer between two nodes can be unambiguously represented by positive or negative sign of the number for the transfer rate.
Post by: hamdani yusuf on 22/12/2024 12:38:14
I asked them with follow up question.The graph model for this case may not be the simplest one. We can add one more edge between two nodes, representing two modes/channels of energy transfer.
The first object is a stationary warm neodymium magnet, while the second object is a spinning but cooler neodymium magnet. How would they exchange their kinetic energy?
The first is EM radiation of warm objects, which is mainly in infrared spectrum. The direction of the net energy transfer in this channel is from warm magnet to the cooler one.
The other one is electromagnetic induction, which causes Eddy current, which generate heat in both magnets, but at the cost of reduction in angular velocity of the spinning magnet. The direction of the net energy transfer in this channel is from spinning magnet to the stationary one.
By splitting the energy transfer into multiple channels, we can explain the mechanism of heat transfer in various phenomena, like induction heating, radio and microwave heating, infrared and visible light heating, laser heating, also sound/acoustic heating.
Can Loud Sounds Actually Cook Things? The Sound to Heat Experiment
Quote
In this experiment I test out if you can heat things up with sound waves! I start off with low frequency 20 Hz and then turn up the frequency higher and higher to try to cook an egg just from sound! I talk about sound waves and how frequency is related to the energy of the wave.
WARNING:
If you use the information from this video for your own projects then you assume complete responsibility for the results.
IMO, the concept of temperature in thermodynamics was meant to combine all of those different modes of kinetic energy transfer into a single quantity.
Post by: alancalverd on 22/12/2024 18:54:22
James Joule published On the Mechanical Equivalent of Heat in 1845, including the conversion coefficient he measured to within 1% of the current value.
Post by: paul cotter on 22/12/2024 21:17:12
Post by: alancalverd on 22/12/2024 22:25:47
Post by: Eternal Student on 22/12/2024 22:46:05
I reckon it would disintegrate before adequate cooking
Apparently you adjust the slap depth, so that the chicken isn't turned to pulp and rely on having a very high rate of slapping. If it's the video I think it is, they built a slapping machine that managed about 10 slaps per second.
Of course, there's a limited market for chicken slapping but it it may have more potential as a steak slapping machine because then your steak should come out both cooked and very tender.
The inverse principle is important in the manufacture of sausages.Sausages need a bigger slap. They're building a special machine to deliver the bangers.
Best Wishes.
Post by: hamdani yusuf on 24/12/2024 01:29:42
IMO, the concept of temperature in thermodynamics was meant to combine all of those different modes of kinetic energy transfer into a single quantity.In practice, the multiple pathways of kinetic energy transfer can cause difference in temperature measurement by different types of thermometer. For example, a mercury thermometer and alcohol thermometer can show different values when measuring temperature of the air around a spinning magnet.
Post by: Bored chemist on 24/12/2024 23:13:46
For example, a mercury thermometer and alcohol thermometer can show different values when measuring temperature of the air around a spinning magnet.The mercury thermometer will not be showing the temperature of the air, will it?
Most bits of measuring equipment can be misused.
So what?
Post by: hamdani yusuf on 25/12/2024 11:34:26
When different types of thermometer show different values when measuring the temperature of the same object at the same time, we need to determine which one is the most accurate.For example, a mercury thermometer and alcohol thermometer can show different values when measuring temperature of the air around a spinning magnet.The mercury thermometer will not be showing the temperature of the air, will it?
Most bits of measuring equipment can be misused.
So what?
Post by: alancalverd on 25/12/2024 12:27:26
For example, a mercury thermometer and alcohol thermometer can show different values when measuring temperature of the air around a spinning magnet.No. They both show the temperature of the contents of the thermometer bulb. One is heated entirely by conduction from the air, one has an additional dynamo component.
we need to determine which one is the most accurate.which is why experimental physics is nontrivial - particularly when dealing with heat. If you get a result within 10% of the textbook answer in a school laboratory, you are doing pretty well, and I'd hope most undergraduates can get within 1%, but ?0.05% confidence demands some serious mathematics and very good engineering.
Post by: hamdani yusuf on 25/12/2024 16:12:06
No. They both show the temperature of the contents of the thermometer bulb. One is heated entirely by conduction from the air, one has an additional dynamo component.How can we measure the temperature of the air around the spinning magnet?
Post by: hamdani yusuf on 25/12/2024 16:13:59
which is why experimental physics is nontrivial - particularly when dealing with heat.I don't think I have said otherwise.
Post by: hamdani yusuf on 25/12/2024 16:16:40
Quote
Never before has a man-made object come so close to the Sun! The Parker Solar Probe will provide unique measurement data about our star - but for all the excitement about this project, people are moved by one question: why doesn't the probe melt when it's near the Sun?
Using a wrong definition might bring us undesired results.
Post by: alancalverd on 26/12/2024 12:29:54
How can we measure the temperature of the air around the spinning magnet?A thermistor or thermocouple at the focus of a concave mirror, is a very simple thermal telescope. Or you could calculate the heat dissipated into the ambient air from the drag on the spinning magnet, and model the subsequent convection. or use schlieren technique to visualise it.
Post by: Bored chemist on 26/12/2024 17:48:46
Are you asking an actual question, or do you plan to go down a pointless rabbit hole about the impossibility of measuring a system without perturbing it?No. They both show the temperature of the contents of the thermometer bulb. One is heated entirely by conduction from the air, one has an additional dynamo component.How can we measure the temperature of the air around the spinning magnet?
Post by: alancalverd on 27/12/2024 13:14:02
A thermistor dissipates heat when you measure its resistance, so if you allow it to heat up gradually you will (in theory) find an inflection point in the resistance/time curve where its temperature is actually in equilibrium with the target and no heat is being exchanged.
This proposal combines a theory of brilliant simplicity with an experiment of impossible difficulty, but it might amuse a philosopher.
Post by: hamdani yusuf on 28/12/2024 09:49:57
This technique filters out any frequency outside the reflective capacity of the mirror. It also filters out any frequency outside the absorption capacity of thermistor or thermocouple.How can we measure the temperature of the air around the spinning magnet?A thermistor or thermocouple at the focus of a concave mirror, is a very simple thermal telescope. Or you could calculate the heat dissipated into the ambient air from the drag on the spinning magnet, and model the subsequent convection. or use schlieren technique to visualise it.
It's also more likely to capture the radiation from behind the air, rather than representing the temperature of the air itself.
Post by: alancalverd on 28/12/2024 10:59:40
What level of accuracy do you want? Why? (Experimental physicist talking here)
Post by: hamdani yusuf on 28/12/2024 12:34:51
Whish is why we use a focussed array. If you want to be really clever, substitute a microwave antenna and use the analysis of Penzias and Wilson to estimate the temperature of your target, then heat up the antenna.What's the range of temperature it can measure?
What level of accuracy do you want? Why? (Experimental physicist talking here)
Typical accuracy in industrial instruments is 0.5% of measuring range. It's practical for the system to be controlled within operating condition.
Post by: alancalverd on 28/12/2024 14:09:01
Post by: hamdani yusuf on 29/12/2024 15:07:01
Thermistors work from around -50C to 300C. I have used them to measure temperature changes of 1mK to +/- 1μK. If you want a traceable calibration in the room temperature range, no real problem - use an oil bath and a platinum resistor to fix absolute points to better than 0.05KYou will need reflector in infrared range instead of microwave.
Post by: Bored chemist on 29/12/2024 22:11:13
So, for example, aluminium foil instead of aluminium foil.Thermistors work from around -50C to 300C. I have used them to measure temperature changes of 1mK to +/- 1μK. If you want a traceable calibration in the room temperature range, no real problem - use an oil bath and a platinum resistor to fix absolute points to better than 0.05KYou will need reflector in infrared range instead of microwave.
Post by: hamdani yusuf on 30/12/2024 11:11:59
So, for example, aluminium foil instead of aluminium foil.I was comparing with common microwave antenna, which Alan mentioned.
Post by: alancalverd on 30/12/2024 11:37:47
Post by: hamdani yusuf on 31/12/2024 03:23:41
I'm not sure what final antenna Penzias and Wilson were using to determine the cosmic background: AFAIK the receiver was an exponential horn waveguide.I found a Naked Science video about this.
Big Bang Discovery - Penzias & Wilson
Quote
380,000 years after the Big Bang the Universe had expanded to the size of the Milky Way. It had cooled from billions of degrees Fahrenheit to a few thousand. As it cooled the electrons slowed down. The Universe was now ready to make its first true elements.
One of the scientists who discovered this critical moment in the story of the Universe was Arno Allan Penzias. In 1963, a 30 year old Penzias and his 27 year old colleague Robert Woodrow Wilson began work on the Holmdel Horn Antenna in New Jersey. Initially they were only studying cosmic radio waves, but they would stumble upon one of the greatest discoveries of all time.
Clip taken from our documentary ?Birth of the Universe?.
Post by: alancalverd on 31/12/2024 10:37:06
Post by: Bored chemist on 31/12/2024 15:25:00
Your result would presume that the target volume was effectively a black body but I guess the behavior of air below 2.4 GHz is a good approximation.
Better guesses are available.
In particular, better guesses should be available to anyone whose field of interest includes radar which depends on microwaves travelling through air for long distances.
Post by: alancalverd on 01/01/2025 10:34:48
Post by: Bored chemist on 01/01/2025 12:43:15
Long-range radar generally works in the kHz to MHz range where water vapor absorption is minimal.In clear air, what's the typical range of microwave radar?
Post by: Bored chemist on 01/01/2025 12:50:53
No. They both show the temperature of the contents of the thermometer bulb. One is heated entirely by conduction from the air, one has an additional dynamo component.How can we measure the temperature of the air around the spinning magnet?
We get a bunch of thermometers.
It would be a dumb choice but, just to make the point, we can use a mercury thermometer, an alcohol thermometer a gallium thermometer, a water thermometer and a hexane thermometer.
And then we measure the electrical conductivity of the filling liquids.
We can plot the apparent temperature against the electrical conductivity of the filling liquid and then we can extrapolate to zero conductivity.
Science has been doing that sort of thing for centuries.
As I have said before, you should try learning it.
Post by: hamdani yusuf on 02/01/2025 04:14:59
We get a bunch of thermometers.Liquid filled thermometers like mercury and alcohol typically use glass for interface with measured object, which was chosen to allow visual observation of the temperature driven expansion of the liquids. While electronic thermometer like RTD and thermocouple use metal alloys for interface. They have different selectiveness in radiation frequencies to be accommodated in the temperature measurement. But those contact temperatures rely on heat conductance between the measured objects and the thermometer interface. Other effects like electromagnetic radiation from the measured object or its environment are considered noise. But this noise can be significant in some situations. Being unaware of these effects can lead to erroneous measurement values.
It would be a dumb choice but, just to make the point, we can use a mercury thermometer, an alcohol thermometer a gallium thermometer, a water thermometer and a hexane thermometer.
Post by: alancalverd on 02/01/2025 09:19:36
In clear air, what's the typical range of microwave radar?Depends on the radiated peak power, and is only limited by the horizon.
The problem with using the em radiation spectrum to measure the temperature of a distant target is selective absorption by the intervening air, which distorts the received spectrum - particularly if the target is air itself! So I'd prefer to concentrate on the low-frequency (sub-GHz) end of the spectrum.
This is a bit of an angels-on-pinheads argument anyway. Common sense says that the air close to the spinning magnet will be in thermal equilibrium with the magnet surface, so we can use the magnet as the target for a conventional thermistor pyrometer - much simpler and good to about +/-0.5K.
Post by: Bored chemist on 03/01/2025 22:57:00
Depends on the radiated peak power, and is only limited by the horizon.Pick one.
But...
"Common sense says that the air close to the spinning magnet will be in thermal equilibrium with the magnet surface"
Not really; but the problem lies with the definition of the problem.
If the magnet is spinning then viscous dag will heat the air near to it.
And then it won't be at equilibrium any more.
Post by: Bored chemist on 03/01/2025 23:00:36
Being unaware of these effects can lead to erroneous measurement values.And the guy who can tell you about 5 different sorts of liquid in glass thermometers is almost certainly not "unaware of these effects".
But you seem un\aware of the fact that a spinning magnet in air will heat the air, thus disturbing the equilibrium.
And, if it's not at equilibrium, it doesn't have a properly defined temperature.
What point did you think you were making, and to whom?
Post by: hamdani yusuf on 06/01/2025 11:42:43
But you seem un\aware of the fact that a spinning magnet in air will heat the air, thus disturbing the equilibrium.You seem to be unaware that as an example, I put the spinning magnet inside a vacuum glass box. There will be no friction between the air and the spinning magnet. But the spinning magnet can still generate heat in nearby metals by inducing Eddy current.
Post by: hamdani yusuf on 06/01/2025 11:44:34
And, if it's not at equilibrium, it doesn't have a properly defined temperature.Does the surface of the sun have a properly defined temperature?
Does the corona of the sun have a properly defined temperature?
Post by: Bored chemist on 07/01/2025 10:29:20
I'm unaware of many things that you just decided to make up. Mindreading isn't my forte.But you seem un\aware of the fact that a spinning magnet in air will heat the air, thus disturbing the equilibrium.You seem to be unaware that as an example, I put the spinning magnet inside a vacuum glass box. There will be no friction between the air and the spinning magnet. But the spinning magnet can still generate heat in nearby metals by inducing Eddy current.
You should have mentioned it.
You do know that oxygen is paramagnetic, don't you?
Fairly well defined. But, in principle, your spinning magnet warms it up a bit.And, if it's not at equilibrium, it doesn't have a properly defined temperature.Does the surface of the sun have a properly defined temperature?
Does the corona of the sun have a properly defined temperature?
Why do you ask?
Post by: alancalverd on 07/01/2025 15:30:30
If the magnet is spinning then viscous dag will heat the air near to it.We tend to consider the boundary layer a stationary in laminar viscous flow. And it would be a pity if Joule's determination of the mechanical equivalent of heat by stirring water was seriously wrong - his result was within 0.75% of the current value.
And then it won't be at equilibrium any more.
Post by: Bored chemist on 07/01/2025 17:52:45
Joule measured the rate of rise of temperature.If the magnet is spinning then viscous dag will heat the air near to it.We tend to consider the boundary layer a stationary in laminar viscous flow. And it would be a pity if Joule's determination of the mechanical equivalent of heat by stirring water was seriously wrong - his result was within 0.75% of the current value.
And then it won't be at equilibrium any more.
Funny looking eqm that's getting hotter with time.
Post by: alancalverd on 07/01/2025 22:18:15
The classic Callendar and Barnes continuous flow water calorimeter uses a similar principle.
Post by: hamdani yusuf on 08/01/2025 04:56:36
But, in principle, your spinning magnet warms it up a bit.The effects are much less than Eddy current generated in metals, thus doesn't significantly change the temperature measurement.
Post by: hamdani yusuf on 08/01/2025 05:55:27
I started this thread in new theory section because I found that currently available explanations are not satisfactory and contains many caveats. I wondered if it can be improved.For anyone who wonders why I started this thread, or already forget it. I discussed this with ChatGPT.Quotehttps://en.m.wikipedia.org/wiki/TemperatureFrom the underlined statement, we need to define what counts as thermal contact. Also, what counts as heat transfer and thermal equilibrium.
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making up a substance.
...
When two systems in thermal contact are at the same temperature no heat transfers between them. When a temperature difference does exist heat flows spontaneously from the warmer system to the colder system until they are in thermal equilibrium. Such heat transfer occurs by conduction or by thermal radiation.[45][46][47][48][49][50][51][52]
Experimental physicists, for example Galileo and Newton,[53] found that there are indefinitely many empirical temperature scales. Nevertheless, the zeroth law of thermodynamics says that they all measure the same quality. This means that for a body in its own state of internal thermodynamic equilibrium, every correctly calibrated thermometer, of whatever kind, that measures the temperature of the body, records one and the same temperature. For a body that is not in its own state of internal thermodynamic equilibrium, different thermometers can record different temperatures, depending respectively on the mechanisms of operation of the thermometers.
What's the common temperature of incandescent lamps?
Quote
The common temperature of incandescent lamps typically refers to their color temperature, which is measured in Kelvin (K). It usually falls in the range of:Note that objects heated by radiation from incandescent lamps can have higher temperature than the glass surface of the lamps. This pose question about thermal contact. Is exchange of kinetic energy through radiation counted as thermal contact?
2,700 K to 3,000 K: This gives off a warm white or yellowish light, resembling traditional incandescent lighting.
3,200 K: Often used in photography and studio lighting, providing a slightly cooler white light.
In terms of operating temperature, the filament inside an incandescent lamp can reach 2,500 to 3,000?C (4,500 to 5,400?F) when glowing. The surface of the bulb itself is much cooler but still quite hot, often around 150 to 250?C (300 to 480?F) depending on the wattage.
Post by: hamdani yusuf on 08/01/2025 05:57:24
Quote
Yes, the spectrum of incandescent lamps is very similar to black body radiation because the light is produced by heating a tungsten filament to high temperatures.
Here?s how they compare:
1. Black Body Radiation:
A black body is an idealized physical body that absorbs all incident radiation and emits a spectrum of radiation based solely on its temperature.
Its spectrum is continuous, with a peak wavelength determined by the Planck radiation law.
2. Incandescent Lamps:
The tungsten filament closely approximates a black body because it emits light due to being heated to 2,500?3,000 K.
The spectrum is continuous, covering visible light and extending into the infrared (IR), which is why these lamps are inefficient as a significant portion of energy is emitted as heat (IR radiation).
However, the spectrum of an incandescent lamp is not a perfect black body due to:
Material properties: The tungsten filament isn't an ideal black body, so its emissivity deviates slightly.
Glass envelope: The bulb's material can absorb some of the emitted light, especially UV radiation.
Overall, incandescent lamps are a practical approximation of black body radiation in the visible and near-IR regions.
What is the typical glass material in incandescent lamps?
Quote
The typical glass material used in incandescent lamps is soda-lime glass or borosilicate glass, depending on the application:
1. Soda-Lime Glass:
Commonly used for standard incandescent bulbs.
Composed primarily of silica (SiO₂), sodium oxide (Na₂O), and calcium oxide (CaO).
Suitable for general-purpose bulbs due to its affordability and ease of production.
Not highly resistant to heat or thermal shock but adequate for the temperatures of most incandescent lamps.
2. Borosilicate Glass:
Used in specialized bulbs (e.g., high-wattage or heat lamps).
Contains silica and boron oxide (B₂O₃), making it more resistant to heat and thermal shock.
Common in applications where higher durability and temperature resistance are required.
The choice of glass also depends on whether the bulb is designed to withstand specific conditions, such as higher operating temperatures, pressure, or exposure to chemicals.
Post by: hamdani yusuf on 08/01/2025 05:59:31
Quote
The transmission of infrared (IR) radiation through the glass of an incandescent lamp depends on the type of glass used and the wavelength of the IR radiation. Here's a general breakdown:
1. Soda-Lime Glass:
IR Transmission: Poor for wavelengths beyond 2.5 ?m (mid to far-infrared).
Glass absorbs most of the longer-wavelength IR radiation emitted by the filament.
However, shorter wavelengths in the near-infrared range (0.8?2.5 ?m) can partially pass through soda-lime glass.
2. Borosilicate Glass:
IR Transmission: Slightly better than soda-lime glass for near-infrared radiation but still limited for mid- and far-IR wavelengths.
Its improved heat resistance allows it to handle high temperatures without significant structural degradation.
Efficiency:
A significant portion (up to 85?90%) of the energy from an incandescent lamp is emitted as IR radiation, but much of this is absorbed or reflected back by the glass.
To improve IR management, special coatings (e.g., dichroic reflectors) are sometimes applied to allow visible light through while reflecting or absorbing IR radiation.
If precise IR transmission values are needed, these depend on the exact glass composition and thickness, which can vary between manufacturers and specific lamp designs.
What's the ratio of absorbed and reflected infrared radiation by the glass?
Quote
The ratio of absorbed and reflected infrared (IR) radiation by the glass in an incandescent lamp depends on the type and thickness of the glass, as well as the IR wavelength. Here's a general overview:
1. Soda-Lime Glass:
Reflection: About 4?8% of IR radiation at each surface (depending on the angle of incidence and wavelength).
For typical soda-lime glass, near-IR wavelengths (0.8?2.5 ?m) reflect less than mid- and far-IR radiation.
Absorption: The majority of IR radiation (>80?90%) is absorbed for wavelengths above 2.5 ?m.
Soda-lime glass strongly absorbs longer wavelengths in the mid-IR and far-IR range, converting them into heat.
2. Borosilicate Glass:
Reflection: Similar to soda-lime glass, reflecting about 4?8% per surface.
Absorption: Less absorption in the near-IR range compared to soda-lime glass but still absorbs strongly in the mid- and far-IR range.
Slightly better transmission in the near-IR spectrum (0.8?2.5 ?m) than soda-lime glass.
Overall Ratio:
For incandescent bulbs with soda-lime glass:
About 85?95% of IR radiation is absorbed.
About 5?15% of IR radiation is reflected.
The exact distribution depends on the thickness and coatings on the glass.
For specialized applications, glass coatings (like low-emissivity or dichroic coatings) can be applied to alter this ratio, increasing reflectance to reduce heat loss or improving IR transmission for specific uses.
Post by: hamdani yusuf on 08/01/2025 06:38:10
When two systems in thermal contact are at the same temperature no heat transfers between them. When a temperature difference does exist heat flows spontaneously from the warmer system to the colder system until they are in thermal equilibrium. Such heat transfer occurs by conduction or by thermal radiation.[45][46][47][48][49][50][51][52]The last sentence rises a question, what distinguishes thermal radiation from non-thermal radiation?
Post by: alancalverd on 08/01/2025 22:25:17
Post by: Bored chemist on 08/01/2025 23:31:32
I spent several years working on "dynamic equilibrium" calorimeters for measuring ionising radiation dose rates. Long story but it was a cunning way of measuring millidegree temperature changes to better than 0.1% precision.So... not Joule's method.
The classic Callendar and Barnes continuous flow water calorimeter uses a similar principle.
You found a method where the temperature difference between ingoing and outbound was essentially constant.
OK.
I agree it's a very clever method.
It seems to be drifting rather from the point (so far as HY's threads ever have one)
Post by: Bored chemist on 08/01/2025 23:48:53
Note that objects heated by radiation from incandescent lamps can have higher temperature than the glass surface of the lamps. This pose question about thermal contact. Is exchange of kinetic energy through radiation counted as thermal contact?
It does not pose that question.
If you put a mirror round the bulb so that all the radiation was reflected back then the glass would reach thermal equilibrium with the filament. (And it would melt)
But a bit like the Calender and Barnes continuous flow water calorimeter, the heat is continuously carried away from the glass (by the air round it, and by emission of IR) , so it never reaches the same temperature as the filament.
It never reaches equilibrium.
Paradoxically, even though quartz glass is nearly opaque to far IR radiation quartz halogen lamps were sometimes used as the "light" source for far IR spectroscopy.
The radiation used was actually that emitted from the quartz envelope.
Post by: hamdani yusuf on 09/01/2025 07:47:09
If you put a mirror round the bulb so that all the radiation was reflected back then the glass would reach thermal equilibrium with the filament. (And it would melt)You can put silver layer on the outside of the bulb glass, and additional insulation layers.
But a bit like the Calender and Barnes continuous flow water calorimeter, the heat is continuously carried away from the glass (by the air round it, and by emission of IR) , so it never reaches the same temperature as the filament.It will melt before reaching equilibrium. But the surface of the glass bulb can be measured to have temperature between 150 and 250 degree Celsius.
It never reaches equilibrium.
Post by: hamdani yusuf on 09/01/2025 07:48:51
Paradoxically, even though quartz glass is nearly opaque to far IR radiation quartz halogen lamps were sometimes used as the "light" source for far IR spectroscopy.According to Kirchhoff's law of thermal radiation, a good absorber is also a good emitter for the same radiation frequency.
The radiation used was actually that emitted from the quartz envelope.
Post by: alancalverd on 09/01/2025 10:30:35
You found a method where the temperature difference between ingoing and outbound was essentially constant.Actually not the case. We delivered electrical energy or ionising radiation into a multi-layer graphite/vacuum calorimeter and measured the time it took for the sensing thermistor in the middle to change by 1 milliohm. The clever bit was designing a bridge that reproducibly offset by 0.001 ohm to +/- 0.1%.
Post by: hamdani yusuf on 09/01/2025 12:10:13
And, if it's not at equilibrium, it doesn't have a properly defined temperature.If that's the case, the curve below won't be possible because the temperature is measured while heat is being transferred to/from the system.
(https://energyeducation.ca/wiki/images/e/e5/Water_Phase_Change_Diagram.png)
Post by: Bored chemist on 09/01/2025 12:20:12
According to Kirchhoff's law of thermal radiation, a good absorber is also a good emitter for the same radiation frequency.There's a good chance that I told you about Kirchhoff's radiation law. I have cited it quite a bit in these pages.
Post by: Bored chemist on 09/01/2025 12:31:46
If you look carefully, you will se the only bits of the line where the temperature is constant are the bits where there are two phases in equilibrium.And, if it's not at equilibrium, it doesn't have a properly defined temperature.If that's the case, the curve below won't be possible because the temperature is measured while heat is being transferred to/from the system.
(https://energyeducation.ca/wiki/images/e/e5/Water_Phase_Change_Diagram.png)
It will melt before reaching equilibrium.I know.
And it would melt
Why do you keep posting stuff that we clearly already know?
Post by: hamdani yusuf on 10/01/2025 14:57:05
The experiment in the video clearly shows this.But, in principle, your spinning magnet warms it up a bit.The effects are much less than Eddy current generated in metals, thus doesn't significantly change the temperature measurement.
Eddy Current Demo #electromagnetic induction #physics #physicsninja
Post by: hamdani yusuf on 10/01/2025 14:58:58
If you look carefully, you will se the only bits of the line where the temperature is constant are the bits where there are two phases in equilibrium.It's not an equilibrium when the energy transfer into the system is larger than the energy transfer out from the system. By your reasoning, liquid water in the graph is not well defined. You won't be able to tell when the water temperature reach 300 K, for example.
Post by: hamdani yusuf on 10/01/2025 15:08:55
Why do you keep posting stuff that we clearly already know?Because you keep saying that temperature is not well defined before equilibrium is reached. But we know that glass melts at its melting temperature.
Post by: paul cotter on 10/01/2025 16:51:26
Post by: alancalverd on 10/01/2025 16:52:39
It's not an equilibrium when the energy transfer into the system is larger than the energy transfer out from the system.Except for the case of "dynamic equilibrium". A small volume of material can be heated almost uniformly by ionising radiation or microwaves, and even in the common (and remarkably complicated) case of an egg in boiling water we can define the temperature any depth and any point in time, which is why we use roasting thermometers and variable-temperature ovens to determine the final state of food.
Post by: hamdani yusuf on 11/01/2025 04:04:23
The temperature of a system is determined by how we define its boundaries.It's not an equilibrium when the energy transfer into the system is larger than the energy transfer out from the system.Except for the case of "dynamic equilibrium". A small volume of material can be heated almost uniformly by ionising radiation or microwaves, and even in the common (and remarkably complicated) case of an egg in boiling water we can define the temperature any depth and any point in time, which is why we use roasting thermometers and variable-temperature ovens to determine the final state of food.
Post by: hamdani yusuf on 11/01/2025 04:17:20
Glass does not have an mp as such, it progressively softens as the temperature rises. Glass behaves as a supercooled liquid rather than a solid.We can use the point where the bulb starts to lose its structural integrity.
Post by: hamdani yusuf on 12/01/2025 01:51:51
Two isolated containers each with 1 cubic meter volume. Their internal surface is perfectly elastic, thus doesn't absorb energy from molecules hitting it. A small pipe equipped with a closed valve is connecting those containers. Initially, the first container contains ideal gas at STP, while the second container is at vacuum. The whole system is inside a space lab, isolated from outside world.
When the valve is opened, some gas molecules will move to the second container. Since the collision with the container walls are elastic, they maintain their kinetic energy. At equilibrium, the flow rate from the first container equals the reverse flow. The pressure of both containers become half of standard pressure. The temperature is still at standard temperature, according to ideal gas law, P.V=n.R.T
The gas, which initially occupied 1 cubic meter of volume, now occupy 2 cubic meter.
Post by: Bored chemist on 12/01/2025 14:17:09
But we know that glass melts at its melting temperature.We know that the melting point of something (when it has one- glass doesn't) is defined as the temperature where the liquid and solid phases are in equilibrium.
Post by: Bored chemist on 12/01/2025 14:18:20
That's a lot of trouble to go to in order to show that you don't know what the phrase "in principle" means.The experiment in the video clearly shows this.But, in principle, your spinning magnet warms it up a bit.The effects are much less than Eddy current generated in metals, thus doesn't significantly change the temperature measurement.
Eddy Current Demo #electromagnetic induction #physics #physicsninja
Post by: Bored chemist on 12/01/2025 14:19:52
Here's another thought experiment to check our understanding of temperature, and its relationship with entropy.
Two isolated containers each with 1 cubic meter volume. Their internal surface is perfectly elastic, thus doesn't absorb energy from molecules hitting it. A small pipe equipped with a closed valve is connecting those containers. Initially, the first container contains helium gas at STP, while the second container is at vacuum. The whole system is inside a space lab, isolated from outside world.
When the valve is opened, some gas molecules will move to the second container. Since the collision with the container walls are elastic, they maintain their kinetic energy. At equilibrium, the flow rate from the first container equals the reverse flow. The pressure of both containers become half of standard pressure. The temperature is still at standard temperature, according to ideal gas law, P.V=n.R.T
The gas, which initially occupied 1 cubic meter of volume, now occupy 2 cubic meter.
Why do you keep posting stuff that we clearly already know?
By the way, if you say "an ideal gas" rather than "helium", you can get round the problem that it isn't actually ideal.
Post by: hamdani yusuf on 13/01/2025 15:10:54
By the way, if you say "an ideal gas" rather than "helium", you can get round the problem that it isn't actually ideal.I've changed helium with ideal gas to avoid unnecessary arguments.
In this scenario, we get an increase in system entropy without any change in temperature nor internal energy.
Post by: hamdani yusuf on 13/01/2025 15:13:41
I think it's necessary, since you didn't seem to realize how insignificant it was.That's a lot of trouble to go to in order to show that you don't know what the phrase "in principle" means.The experiment in the video clearly shows this.But, in principle, your spinning magnet warms it up a bit.The effects are much less than Eddy current generated in metals, thus doesn't significantly change the temperature measurement.
Eddy Current Demo #electromagnetic induction #physics #physicsninja
Post by: Bored chemist on 16/01/2025 12:59:24
I think it's necessary, since you didn't seem to realize how insignificant it was.As I said,
you don't know what the phrase "in principle" means.
Post by: Bored chemist on 16/01/2025 13:06:22
In this scenario, we get an increase in system entropy without any change in temperature nor internal energy.Lucky us!
You can calculate it if you like.
https://en.wikipedia.org/wiki/Sackur%E2%80%93Tetrode_equation
Post by: hamdani yusuf on 17/01/2025 21:12:32
These videos show that energy transfer by radiation can be passed through material as well as being absorbed. Other experiments show that it ca also be reflected. This is like impedance matching problem in electronic engineering.
Post by: hamdani yusuf on 17/01/2025 22:14:58
Impedance matching in mechanical wave.
Post by: alancalverd on 17/01/2025 23:47:37
These videos show that energy transfer by radiation can be passed through material as well as being absorbed.Good heavens! Whatever will those crazy scientists discover next? Are air, water and glass transparent? Can x-rays pass through the human body?
Truly it is a privilege to live in the 21st century when, thanks to the miracle of the internet, we can be amazed by what our parents considered to be bloody obvious.
Post by: hamdani yusuf on 18/01/2025 11:41:43
It's understandable that scientific research started with simpler cases. But let's not forget that there are more than what earlier discovery has told us.
Post by: hamdani yusuf on 18/01/2025 11:57:20
Good heavens! Whatever will those crazy scientists discover next? Are air, water and glass transparent? Can x-rays pass through the human body?You seem to forget that water and typical glasses are not transparent in infrared spectrum.
Post by: alancalverd on 18/01/2025 13:28:00
Transparency does not imply zero attenuation at all frequencies, just usefully low values at important points on the spectrum.
Post by: Bored chemist on 18/01/2025 23:11:20
Most of us know that impedance is frequency dependent. Mathematical model for impedance is a complex number. Thermodynamics and black body radiation seem to consider only the real part of this impedance. That's because in those cases, the imaginary part is not significant. They're only special cases from the more general cases where the radiating or radiated bodies are not necessarily black body.You have that more or less exactly wrong.
It's understandable that scientific research started with simpler cases. But let's not forget that there are more than what earlier discovery has told us.
The imaginary part of the refractive index relates to absorbing light.(And thus BBR) But the real part relates to the wavelike properties of the light that carries on or is reflected.
"Light propagation in absorbing materials can be described using a complex-valued refractive index.[2] The imaginary part then handles the attenuation, while the real part accounts for refraction"
From
https://en.wikipedia.org/wiki/Refractive_index
Did you consider learning science (rather than just watching YT)?
Post by: hamdani yusuf on 21/01/2025 14:23:07
Unfortunately the makers of infrared heat lamps do not share your profound knowledge!A good absorber is also a good emitter in the same spectrum. That's where infrared heat lamp can optimize it.
But lower frequency radiation should also be considered.
Short video about the importance of grounding your heat lamp.
Post by: alancalverd on 22/01/2025 09:33:41
LED lamps obviously emit less because they are DC-powered, but if you obtain that power from the mains, you are probably emitting 100 kHz from the switch-mode power supply.
Post by: Bored chemist on 22/01/2025 12:14:15
What "point" are you trying to make here?Unfortunately the makers of infrared heat lamps do not share your profound knowledge!A good absorber is also a good emitter in the same spectrum. That's where infrared heat lamp can optimize it.
But lower frequency radiation should also be considered.
Short video about the importance of grounding your heat lamp.
Post by: paul cotter on 22/01/2025 14:31:29
Post by: Bored chemist on 22/01/2025 15:39:13
And incandescent lamp is basically a coil of wire, it's going to generate a magnetic field when you use it.
The big question is...
Who cares?
Post by: paul cotter on 22/01/2025 17:47:27
Post by: hamdani yusuf on 23/01/2025 14:16:44
What makes you fail to distinguish between impedance and refractive index?Most of us know that impedance is frequency dependent. Mathematical model for impedance is a complex number. Thermodynamics and black body radiation seem to consider only the real part of this impedance. That's because in those cases, the imaginary part is not significant. They're only special cases from the more general cases where the radiating or radiated bodies are not necessarily black body.You have that more or less exactly wrong.
It's understandable that scientific research started with simpler cases. But let's not forget that there are more than what earlier discovery has told us.
The imaginary part of the refractive index relates to absorbing light.(And thus BBR) But the real part relates to the wavelike properties of the light that carries on or is reflected.
"Light propagation in absorbing materials can be described using a complex-valued refractive index.[2] The imaginary part then handles the attenuation, while the real part accounts for refraction"
From
https://en.wikipedia.org/wiki/Refractive_index
Did you consider learning science (rather than just watching YT)?
Post by: Bored chemist on 23/01/2025 15:16:58
What makes you fail to distinguish between impedance and refractive index?The fairly close relationship between them.
The impedance is sqrt( permeability /permittivity).
And (for non magnetic materials) the dielectric constant is the square of the refractive index.
Light reflected from a window is an impedance mismatch effect.
Were you aware of this?
https://en.wikipedia.org/wiki/Clausius%E2%80%93Mossotti_relation
Post by: hamdani yusuf on 23/01/2025 22:40:18
Why? It's pretty obvious that a conductor carrying a couple of amps at 50 - 60 Hz is going to radiate em. The question is whether that actually matters in the context of all the other current carriers in the house.It depends on the shape of the conductors, whether it will act like an antenna, or more like a Faraday cage.
Quote
LED lamps obviously emit less because they are DC-powered, but if you obtain that power from the mains, you are probably emitting 100 kHz from the switch-mode power supply.That oscillation can be radiated to its surroundings. It can affect the temperature measurement of some types of thermometers, especially the electronic ones.
Post by: hamdani yusuf on 23/01/2025 23:55:43
Were you aware of this?Not the exact formula. But my experiments with microwave and metamaterials suggest that refractive index depends on the unit density of metamaterial and polarizability of the metamaterial unit.
https://en.wikipedia.org/wiki/Clausius%E2%80%93Mossotti_relation
Post by: Bored chemist on 24/01/2025 13:09:47
Why do you keep stating the obvious?Why? It's pretty obvious that a conductor carrying a couple of amps at 50 - 60 Hz is going to radiate em. The question is whether that actually matters in the context of all the other current carriers in the house.It depends on the shape of the conductors, whether it will act like an antenna, or more like a Faraday cage.QuoteLED lamps obviously emit less because they are DC-powered, but if you obtain that power from the mains, you are probably emitting 100 kHz from the switch-mode power supply.That oscillation can be radiated to its surroundings. It can affect the temperature measurement of some types of thermometers, especially the electronic ones.
Post by: hamdani yusuf on 26/01/2025 04:35:10
Why do you keep stating the obvious?Because there are someone who keep overlooking it.
Post by: Bored chemist on 27/01/2025 00:02:23
Post by: hamdani yusuf on 27/01/2025 13:58:27
Are you saying we shouldn't ignore small effects?We won't know if the effects were small until they are measured, or at least calculated.
Post by: hamdani yusuf on 28/01/2025 01:46:54
Quote
I show you how glass becomes conductive when heated
I also asked the author in the comment section.
Quote
What would happen if you use pure silicon dioxide glass?
Post by: hamdani yusuf on 28/01/2025 01:54:29
Quote
Glass is one of the most important materials humans have ever made.
00:00 Glass and our place in the universe
01:23 How Gorilla Glass works
04:35 What is glass?
05:15 Is glass a liquid?
07:29 Different types of glass
09:59 Invention of transparent glass
11:56 Why is some glass transparent?
14:54 Invention of glass lenses
15:52 Development of magnification
18:02 How to make glass more durable
Post by: hamdani yusuf on 28/01/2025 08:57:18
This is like impedance matching problem in electronic engineering.
Ultrasound gel does amazing things to sound waves
Quote
I was curious about the purpose of ultrasound gel. It's really interesting so I built this wave machine to explain how it affects sound.He also mentioned impedance and refractive index in the video.
CORRECTION: when I calculate the efficiency of the ultrasound scanner without gel I come to a figure 1/10000%. But that would only be if there was a full on air gap. When pressed against the skin the situation is much less dire. The gel ensures a better acoustic coupling by preventing air gaps etc. Thanks to a couple of comments for pointing that out.
Electrical impedance was generalization of electrical resistance in DC. Thus the real part represents the energy absorption or energy transformed into different forms.
On the other hand, refractive index was initially used to describe the behavior of light being bent by transparent media like water and glass. Thus the real part represents the material's ability to bend light.
Post by: paul cotter on 28/01/2025 12:31:35
Post by: hamdani yusuf on 29/01/2025 01:10:26
As regards #1300, quartz(silica) needs nearly 2000c to melt and it has an mp as opposed to glass.My follow up question is, does it become a good microwave absorber when it's hot?
As shown in some videos, normal glass can already absorb microwave before it melts.
Post by: Bored chemist on 29/01/2025 11:38:55
My follow up question is, does it become a good microwave absorber when it's hot?What is stopping you googling that ?
" Comparing the transmission performance of mullite, alumina (Al2O3) and fused silica ceramics, it was found that the fused silica ceramics have excellent transmission properties; especially it is at high temperature. "
from
https://www.sciencedirect.com/science/article/abs/pii/S0272884218334746
Post by: hamdani yusuf on 29/01/2025 13:41:24
What is stopping you googling that ?Asking Gemini is more convenient.
Quote
Fused silica is transparent to microwaves. This means that microwaves pass through it without being absorbed or significantly interacting with the material.
Here's why:
* Low Dielectric Loss: Fused silica has a very low dielectric loss, which means it doesn't absorb microwave energy.
* Non-Polar Material: Silica is a non-polar material, meaning its molecules don't have a permanent dipole moment. Microwaves interact with polar molecules, causing them to rotate and generate heat. Since fused silica is non-polar, it doesn't interact with microwaves in this way.
Implications:
* Microwave Ovens: You can safely use fused silica containers in microwave ovens, as they won't heat up or interfere with the cooking process.
* Microwave Applications: This transparency to microwaves makes fused silica useful in various microwave applications, such as windows for microwave ovens and components in microwave communication systems.
In summary, fused silica's transparency to microwaves is a valuable property that makes it suitable for applications where microwave interaction is undesirable or where it needs to be used in microwave environments.
Post by: Bored chemist on 29/01/2025 14:41:35
Asking Gemini is more convenient.And yet, you were still too lazy to do it.
However, Gemini's reply seems to miss the critical point.
You can microwave your food in a glass dish.
It's only when the glass is very hot that it starts to absorb significantly.
So...
as long as it isn't too hot...glass is transparent to microwaves. This means that microwaves pass through it without being absorbed or significantly interacting with the material.
Here's why:
* Low Dielectric Loss: glass has a very low dielectric loss, which means it doesn't absorb microwave energy.
* Non-Polar Material: glass is a non-polar material, meaning its molecules don't have a permanent dipole moment. Microwaves interact with polar molecules, causing them to rotate and generate heat. Since glass is non-polar, it doesn't interact with microwaves in this way.
Implications:
* Microwave Ovens: You can safely use glass containers in microwave ovens, as they won't heat up or interfere with the cooking process.
* Microwave Applications: This transparency to microwaves makes glass useful in various microwave applications, such as windows for microwave ovens and components in microwave communication systems.
In summary, glass's transparency to microwaves is a valuable property that makes it suitable for applications where microwave interaction is undesirable or where it needs to be used in microwave environments.
Post by: hamdani yusuf on 30/01/2025 07:49:56
However, Gemini's reply seems to miss the critical point.Not really. It answered my question specifically about fused silica.
You can microwave your food in a glass dish.
It's only when the glass is very hot that it starts to absorb significantly.
Post by: hamdani yusuf on 30/01/2025 08:14:35
So...The reason some glasses become electrically conductive and absorb microwave when heated is because the ions previously held tight in their position can then wiggle when the glass is hot enough.
as long as it isn't too hot...glass is transparent to microwaves. This means that microwaves pass through it without being absorbed or significantly interacting with the material.
Here's why:
* Low Dielectric Loss: glass has a very low dielectric loss, which means it doesn't absorb microwave energy.
* Non-Polar Material: glass is a non-polar material, meaning its molecules don't have a permanent dipole moment. Microwaves interact with polar molecules, causing them to rotate and generate heat. Since glass is non-polar, it doesn't interact with microwaves in this way.
Implications:
* Microwave Ovens: You can safely use glass containers in microwave ovens, as they won't heat up or interfere with the cooking process.
* Microwave Applications: This transparency to microwaves makes glass useful in various microwave applications, such as windows for microwave ovens and components in microwave communication systems.
In summary, glass's transparency to microwaves is a valuable property that makes it suitable for applications where microwave interaction is undesirable or where it needs to be used in microwave environments.
Post by: paul cotter on 30/01/2025 10:24:57
Post by: Bored chemist on 30/01/2025 21:31:36
Your question was about hot fused silica.However, Gemini's reply seems to miss the critical point.Not really. It answered my question specifically about fused silica.
You can microwave your food in a glass dish.
It's only when the glass is very hot that it starts to absorb significantly.
does it become a good microwave absorber when it's hot?
But the answer from the AI didn't mention that aspect.
Unless you specify that, there's not much difference between glass and fused quartz.
Post by: Bored chemist on 30/01/2025 21:32:59
The reason some glasses become electrically conductive and absorb microwave when heated is because the ions previously held tight in their position can then wiggle when the glass is hot enough.We know.
What was your post for?
Post by: hamdani yusuf on 31/01/2025 13:21:20
Because your previous answer in #1307 hasn't explicitly stated this information.The reason some glasses become electrically conductive and absorb microwave when heated is because the ions previously held tight in their position can then wiggle when the glass is hot enough.We know.
What was your post for?
Post by: Bored chemist on 07/02/2025 12:47:04
It also didn't explicitly state the best growing conditions for garlic.Because your previous answer in #1307 hasn't explicitly stated this information.The reason some glasses become electrically conductive and absorb microwave when heated is because the ions previously held tight in their position can then wiggle when the glass is hot enough.We know.
What was your post for?
Post by: hamdani yusuf on 09/02/2025 13:09:27
It also didn't explicitly state the best growing conditions for garlic.What does garlic have to do with this thread?
Post by: Bored chemist on 09/02/2025 20:25:20
Nothing, and that was my point.It also didn't explicitly state the best growing conditions for garlic.What does garlic have to do with this thread?
There is an enormous amount of information in the world which I didn't need to include in that post.
Post by: hamdani yusuf on 11/02/2025 14:04:57
I think my post above is necessary. Your post below seems to imply that ordinary glass doesn't contain trapped ions which would be loosened up when it's heated.The reason some glasses become electrically conductive and absorb microwave when heated is because the ions previously held tight in their position can then wiggle when the glass is hot enough.We know.
What was your post for?
* Non-Polar Material: glass is a non-polar material, meaning its molecules don't have a permanent dipole moment. Microwaves interact with polar molecules, causing them to rotate and generate heat. Since glass is non-polar, it doesn't interact with microwaves in this way.
Implications:
Post by: hamdani yusuf on 29/03/2025 04:44:06
Jim Al-Khalili explores the history of thermal energy (thermodynamics).