Why should anyone be interested in an old cure that hasn't been used in the West for 50 years? It's a method that many doctors aren't even aware of today.
|
Figure 1: A single phage attached to the surface of a bacterium. |
The most telling answer to this question came when I received a call in my office from a man one Friday morning in January 2001. It was the day after my article on the Eliava Institute in Georgia had appeared in the German weekly newspaper Die Zeit. In the article I had described how this old remedy - called phage therapy - had survived in the impoverished country. Phages are viruses that attack bacteria, but not humans. In Georgia, some doctors use such phages to treat bacterial infections.
The caller explained that he had read the article. He was calling directly from the hospital and appeared to be under a great deal of pressure. Not mincing words, he explained that he had been suffering from an infection in his foot for two years. Doctors couldn't get it under control because the bacteria were resistant to all antibiotics. He was scheduled to have his foot operated on a fourth time the next day. Could I put him in touch with someone in Georgia? He was afraid that before long he would lose his foot.
More than any research I have done, his call hit me between the eyes. Never before had I been so aware of the power that bacteria continue to wield over us. We have grown up with the certainty that every bacterial infection can be cured by antibiotics. Most of us have no idea of the destruction that bacteria are capable of rendering, because our doctors prescribe drugs at the slightest symptom.
But in more and more cases doctors prescribe one antibiotic after the other without being able to eliminate the infection - because the bacteria are resistant against the drugs. One of the most notorious bugs is Staphylococcus aureus. In the UK, 39 per cent of staph infections were multi-resistant in 2004. In the US, this number is closer to 50 per cent. Only a few countries are as lucky as the Netherlands or Sweden where multi-resistant stap (or MRSA) make up only a few per cent or less.
An estimated 90,000 US patients die annually from infections contracted in the hospital, many of them caused by multi-resistant bacteria. And, in the past 4 to 5 years doctors have been alarmed to see some resistant staph strains leaving their original home - the hospitals - and conquering cities such as Los Angeles or Chicago.
There are not only resistant staph. Many hospital patients contract bugs called Clostridium difficile that can cause severe diarrhoea. Another bacterium that often has acquired a high degree of resistance is called Pseudomonas aeruginosa. Patients at risk are burn victims whose wounds are favorite dwellings for this bug. Another target are the lungs of cystis fibrosis (CF) patients - a hereditary disease that causes sticky secretions in the lung and other organs. The average life expectancy of CF patients is not much more than 30 years. And these are only two more examples of many.
A doctor from Texas who specializes in helping people with chronic wounds once to told me: "We see many wounds that do not heal, we have limbs that are being cut off, and we have people dying." All of this because bacteria develop resistance against all available drugs. Still, many pharmaceutical companies have stopped their research on antibiotics because they don't see enough profit in that market.
Because of all this, it makes perfect sense to revisit phage therapy and evaluate how it can help us all staying healthy. How does it work?
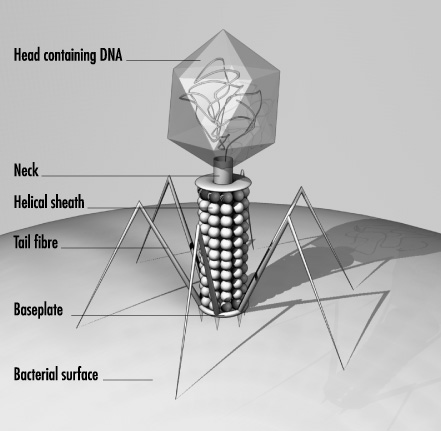
Phages (figure 1)have only one objective: their reproduction. They are so poorly equipped, however, that they can't do this on their own. Without the help of their victims, they aren't much more than a dead piece of protein with a touch of genetic material. But if the phages do hit a suitable bacterium, they multiply in a chillingly efficient cycle.
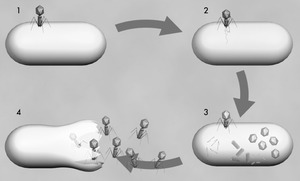
The reproductive cycle begins when the virus uses its tail fibres to attach itself to its victim (1). The details of what happens next vary according to the different phage types. But their aim is always the same: to get their genetic material, which is located in the head, inside the bacterium. T4, a well-studied phage infecting the Escherica coli bacterium, then contracts its tail sheath which pushes a tube located within the tail through the membrane of the bacterial cell (2). The phage's DNA is passed through the tube into the cell, where it takes control, brutally stops many of its vital functions and forces it to churn out new virus components - heads, tails, tail fibres - in production-line style (3). Then comes the final assembly. Finally, enzymes dissolve the wall of the bacterium from the inside and the newborn bacteriophages reach the exterior, ready to attack new victims (4). The viruses proceed very selectively as they do so. Most of them attack only a subgroup of a single bacterial species (see figure 3, below). Generally, they don't touch animal or human cells, which is why they are harmless to human beings.
Just imagine this phage multiplication going on inside the mass of bacteria that fill a boil on human skin and you get the basic message: The phages will attack the bugs and multiply as long as there are bacteria left: The drug produces itself in the body until its food - the infection - is gone.
 |
Figure 3: T4 phages on the surface of an Escherichia coli bacterium © Prof M. V. Parthasarathy, Cornell Integrated Microscopy Center, Cornell University. |
It's as simple and intriguing as that.
Already one of the two discoverers of phages has had this idea. Félix d'Herelle, a bacteriologist working at the Pasteur Institute in Paris, isolated phages in 1916 from the faeces of French soldiers struck by bacillary dysentery.
He observed that the enigmatic microbe always appeared in the stools of patients just before they began to recover from the disease. Further studies showed that these bacteriophages, as he had named them soon, dissolved even the densest cultures of dysentery bacteria (Shigella) in a spectacular manner.
D'Herelle concluded that these phages had something to do with the healing process of bacillary dysentery and he wanted to harness their power for fighting infections. First, he treated chickens with phages against an infectious disease called fowl typhoid. As he had soon discovered the picky appetite of phages he isolated for these experiments specific phages against Salmonella gallinarum, the agent of fowl typhoid.
In 1919, he thought he had done enough research and decided to start treating humans. D'Herelle's first patients were five children with bacillary dysentery. They were all cured. If the phages really were responsible remains unclear as such anecdotal evidence can never constitute a proof.
Nontheless, these pioneering experiments were the beginning of phage therapy. In the next decades, it was used in many countries to treat millions of patients against infectious disesases like cholera, bacillary dysentery and furuncles. But once doctors ushered in penicillin in the 1940s, phage therapy fell by the wayside in most places.
Only in the Eastern bloc it survived alongside antibiotics. After the fall of the Soviet Union, western scientists took note of the parallel medicine from behind the wall - their interest boosted by the growing problem of resistance.
Phage therapy has several advantages over antibiotics. One of them is that phages are very specific and do not harm the useful bacteria that live in and on the body. Antibiotics, however, also attack harmless bacteria that make a living on us. This can, for instance, lead to severe diarrhoea, especially in patients with a weak immune system. Furthermore, phages attack also bacteria that are resistant against antibiotics.
However, there are also drawbacks of phage therapy. Probably the strongest is that, despite its long history, the proponents of phage therapy never succeded in producing formal proof of efficacy in human treatment. There are many studies with positive results. But they all have one or the other weak point making definitve conclusions difficult. But several very good studies in animals do show conclusively that phages can eradicate infections in the body.
A few university researchers and small biotech companies started in the late 1990s to look into phage therapy again. Hopefully, they will be able to bring back phage therapy into medicine in the coming years. Thousands of patients that suffer from resistant infections are waiting.
- Previous How The Lymphatic System Works
- Next Lost your bottle?










Comments
Add a comment