Pandemics, whether viral or bacterial, always come with suitable hysteria from  the media. Bacterial resistance, however, is a serious threat we have always known about yet little action has been taken. There is a risk that we could be faced with a post-apocalyptic like, post-antibiotic world, where minor infections and common injuries which have been easily treatable for generations are now life threatening. Currently in the United States 23,000 people die every year as a result of anti-biotic resistant infections1. In recent years we have made huge advances from HIV medication to transplantation, however none of this would be possible without antibiotics to prevent infections.
the media. Bacterial resistance, however, is a serious threat we have always known about yet little action has been taken. There is a risk that we could be faced with a post-apocalyptic like, post-antibiotic world, where minor infections and common injuries which have been easily treatable for generations are now life threatening. Currently in the United States 23,000 people die every year as a result of anti-biotic resistant infections1. In recent years we have made huge advances from HIV medication to transplantation, however none of this would be possible without antibiotics to prevent infections.
What are bacteria?
Bacteria are everywhere and it's not as simple as good and bad, despite what pro-biotic yoghurt companies would lead you to believe (a study published in 2002 showed that 68.4% of the bacteria in probiotic products were antibiotic resistant)2. We live extremely closely with bacteria; our bodies could contain ten times more bacterial cells then our own. Cellular life is characterised by the three-domain system as Prokaryotes, Eukaryotes and Archea. Bacteria are known as prokaryotes where as human cells are eukaryotic. Prokaryotes do not contain membrane-bound nucleus like our cells and are very small, only 1-5 millionth of a meter (1-5µm) in length3.
An antibiotic is a substance that, at low concentrations, inhibits growth of bacteria; they can be produced naturally by moulds and other bacteria, extracted and chemically modified. The first to be discovered was Penicillin in 1928 by Sir Alexander Fleming. Fleming was tucking into a mouldy sandwich whilst working in the lab and a mould spore from the bread contaminated the petri dish containing the bacteria Staphylococci. The mould produced the chemical we now know as Penicillin, which acts by breaking apart the bacterial cell wall by producing a chemical called β-Lactam. This marked the start of the modern age of antibiotics. Deemed as the wonder drug, Penicillin saved thousands of lives during WWII. Flemming was already aware of the risks of antibiotic resistance and emphasised this in many of his speeches, cautioning that Penicillin should not be used unless the disease has been diagnosed correctly4.
How do antibiotics work?
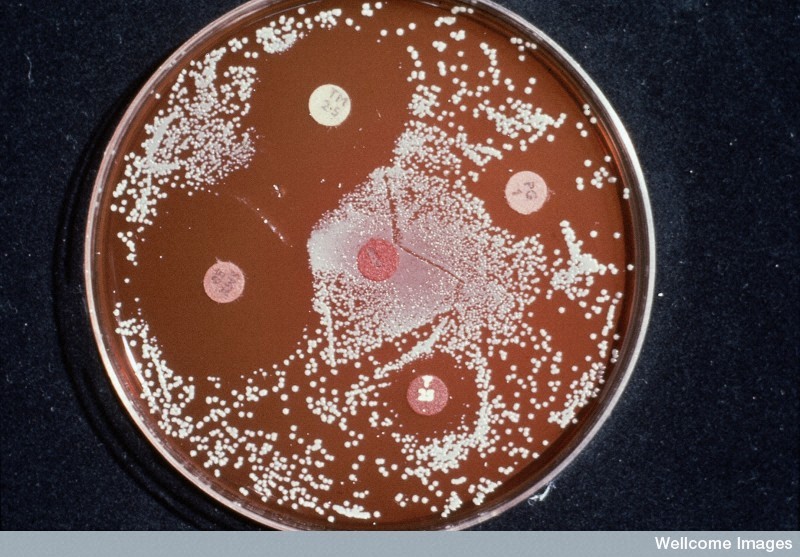 There two main classes in which antibiotics target bacteria, bacterialcidal and bacterialstatic. Bacterialcidal agents actively kill the bacteria (some are classed as bacteriolytic which kill by breaking open the cell). Bacterialstatic antibiotics inhibit the growth and replication of the bacteria but do not kill them, which allows the immune system to destroy the pathogens.
There two main classes in which antibiotics target bacteria, bacterialcidal and bacterialstatic. Bacterialcidal agents actively kill the bacteria (some are classed as bacteriolytic which kill by breaking open the cell). Bacterialstatic antibiotics inhibit the growth and replication of the bacteria but do not kill them, which allows the immune system to destroy the pathogens.
Penicillins are bacterialcidal, acting by inhibiting enzymes involved in maintaining the structure of the cell wall resulting in holes forming.
Another well known group of antiobiotics; Tetracyclines, are Bacterialstatic, binding to the machinery within the cell that reads the genetic code and interfering with protein synthesis.
What causes resistance?
Resistance has been around for as long as we have used antibiotics. It occurs when some or all of a population of the microbe survive after exposure to an antibiotic.
Bacteria develop resistance due to their high replication and DNA mutation rates. Staphylococcus Aureus (a common cause of skin infection, respiratory disease and food poisoning) can replicate every half an hour, in 30 hours it is possible for the bacteria to have mutated on every single piece of DNA code (base pair) 30 times5.
Extensive mutation and selection pressure create the perfect breeding ground to develop resistant genes, which enable to bacteria to find a way of surviving the attack by antibiotics, ensuring any of the bacteria containing the protective gene survive and pass on the gene to future generations. This means only one bacteria needs to stumble onto this recipe for survival, and soon they will all be resistant.
A strain of Staphylococci contains a resistant mutation which allows it to produce an enzyme called β-lactamase. Two types of antibiotic, Penicillin and Cephalosporin, have a structure made up of a ring known as a β-lactam ring. The β-lactamase produced by Staphylococci acts by cutting the ring, rendering the antibiotic unable to destroy the bacteria. The genes that code for the β-lactamase can even be transferred to neighbouring bacterial cells, as the code exists on plasmids, small pieces of DNA molecule that can be exchanged with other bacteria6.
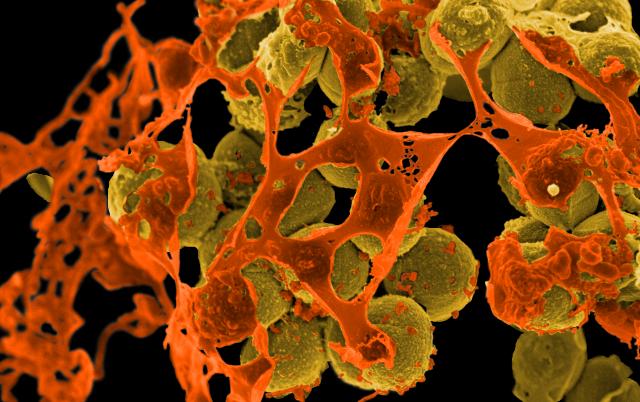 In the past when bacteria developed resistance, there were alternative antibiotics that could be used. However, with the emergence of multi-drug resistant bacteria the situation became a lot more serious. Methicillin-Resistant Staphylococcus Aureus (MRSA) has been found to show resistance to Streptomycin, Aminoglycosides, Chloramphenicol, Trimethoprim, Rifampicin, Fusidic acids and Quinolones. Until recently Vancomycin was seen as the antibiotic of last resort against MRSA, however between 2002 and 2006 seven cases of Vancomycin resistance have been reported in the USA7.
In the past when bacteria developed resistance, there were alternative antibiotics that could be used. However, with the emergence of multi-drug resistant bacteria the situation became a lot more serious. Methicillin-Resistant Staphylococcus Aureus (MRSA) has been found to show resistance to Streptomycin, Aminoglycosides, Chloramphenicol, Trimethoprim, Rifampicin, Fusidic acids and Quinolones. Until recently Vancomycin was seen as the antibiotic of last resort against MRSA, however between 2002 and 2006 seven cases of Vancomycin resistance have been reported in the USA7.
Some strains of Enterococci and Mycobacterium tuberculosis have also developed resistance to most current antibiotics6.
Discovery of new classes of antibiotics has slowed in recent years. The last new antibiotic class to be discovered was Bedaquiline in 1997, becoming available on the market in 2012, with antibiotic resistance to the drug being observed in 2006 before it was even introduced8.
Are there any antibiotics left to discover?
Currently we have only cultured 1% of the bacteria on the planet, leaving an untapped resource that could contain the next generation of antibiotics9. Pharmaceutical companies are not investing in research and development of new antibiotics despite requirements because the companies are unlikely to see a return on their investment. Many companies have discontinued research into new antibiotics leaving only five of the largest companies GlaxoSmithKline, Novartis, AstraZeneca, Merck and Pfizer still conducting research into the area.
It could be deemed as greed on part of the companies; many have argued the pharmaceutical industry has a obligation to ensure the worlds health needs are met and indeed many have philanthropic schemes, such as GSK's HIV medication for non-profit price to developing countries10. Despite pharmaceuticals being some of the richest companies in the world, discovering new drugs is a lengthy and costly process. Discovering and bringing a new drug to market on average costs around £1.2bn and can take up to 12 years11. Despite the huge costs and length of time 1 in 5 never make it to market failing during clinical trials. Companies need to be sure they will re-coop investment, antibiotics are only taken for a short duration and due to the age of antibiotics currently on the market, generic alternatives are available cheaply. The National Health Service is unlikely to pay the high price tag associated with new antibiotics unless it's a last resort situation. Pharmaceutical companies see larger returns on therapies for chronic illnesses, such as Arthritis, Alzheimer's and Obesity, where patients will be dependent on drugs long term.
Why is resistance so prevalent?
Anti-biotic resistance develops from over, and incorrect, use. We are routinely prescribing antibiotics for conditions where they are not completely effective. In developing countries there is poor regulation on the use of antibiotics, leading to the availability of antibiotics without prescription, often containing inadequate instruction to their use. In many cases counterfeit antibiotics are produced, which contain a small dose of the active ingredients12. Another major cause of resistance is people not finishing the course of treatment, often the bacteria are weakened and the symptoms of illness disappear, at this point people regularly stop taking the medication, allowing a proportion of the bacteria to survive, enabling them to grow in numbers this time armed with a resistance to the drug.
Another major problem is the use of antibiotics in the agricultural industry; this  practice is banned in the EU but still widespread throughout the world. Livestock are fed a constant, low level of antibiotics to prevent any infections that would reduce productivity. 90% of antibiotics are used in agricultural in this manor rather then to treat disease13. Low concentration use of antibiotics in this manor can lead to a reservoir of anti-bacterial resistance that can then spread to humans. It has been found that MRSA originated in humans as Methicillin-Susceptible S. Aureus (MSSA), before transferring to livestock where it acquired resistance to two types of antibiotic Tetracycline and Methicillin before transferring back to humans as MRSA14.
practice is banned in the EU but still widespread throughout the world. Livestock are fed a constant, low level of antibiotics to prevent any infections that would reduce productivity. 90% of antibiotics are used in agricultural in this manor rather then to treat disease13. Low concentration use of antibiotics in this manor can lead to a reservoir of anti-bacterial resistance that can then spread to humans. It has been found that MRSA originated in humans as Methicillin-Susceptible S. Aureus (MSSA), before transferring to livestock where it acquired resistance to two types of antibiotic Tetracycline and Methicillin before transferring back to humans as MRSA14.
It is estimated that every year a million people suffer from foodborne illnesses, costing the NHS around 1.5billion a year15. In serious cases antibiotics maybe required making any antibiotic resistance in foodborne bacteria a real concern.
Antibiotic resistance has a profound effect on children; diseases such as malaria, pneumonia and dysentery are common in children from developing countries. In many cases these diseases can no longer be treated with older antibiotics, accessibility to effect antibiotics in these areas are critical for saving children. In high-economic countries routine surgery and cancer chemotherapy will become unavailable if resistance increases12.
In order to prevent a post-antibiotic era we need to use the antibiotics we do have correctly and focus on finding new classes of antibiotics. On the 1st of October 2014 the WHO published a draft report suggesting actions which need to be taken in order to combat antimicrobial resistance. Proposals included improving awareness, increasing research and surveillance, reducing infection rates by effective hygiene and ensuring all current antimicrobials are used correctly.
One novel form of preventing bacterial resistance has emerged in the form of 'Crispr'. The molecule can be programmed to specifically recognise the resistant gene in a bacteria and cut at the plasmid deleting resistant gene and preventing the resistance spreading to neighbouring bacterial cells16,17.
Only a combination of innovative treatments such as this one and prevention of the common misuse of antibiotics will solve this ever-looming threat to the world's health.










Comments
Add a comment