In the first part of this mini-series we looked at the earliest stages of mammalian development, from the egg and sperm to the ball-like blastocyst. In this second part we turn to the story of cloning, and the technical problems and limitations of the process. Although cloning burst onto the global media scene with the arrival of Dolly the sheep in 1997, the technology has been around for decades. At its most basic the process involves taking an egg cell and replacing its chromosomes (DNA) with the DNA from another cell, and kick-starting the 'developmental programme' with chemicals, or an electric pulse (figure 1). This process is known as Somatic Cell Nuclear Transfer, or SCNT for short. Hans Spemann first proposed such a "fantastical experiment" in 1938, and in 1952 tadpoles were cloned from embryonic frog cells. John Gurdon first successfully cloned frogs from differentiated (developed) cells in 1962 and since then the full list of successfully cloned animals has expanded to include also mice, cows, rabbits, pigs, sheep, cats, horses, zebra fish and monkeys.
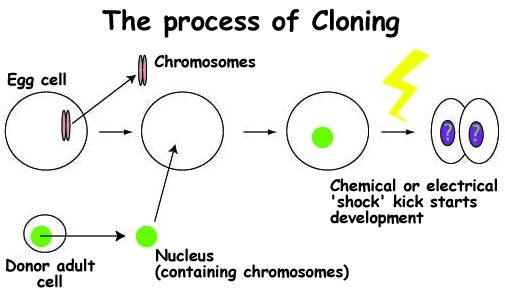 | |||||||
1. Cloning requires an egg cell, and an adult donor cell. The (unwanted) chromosomes are removed from the egg cell and discarded. The nucleus, containing the DNA to be cloned, is removed from the donor cell. | 2. The donor nucleus is inserted into the empty egg cell, a process called somatic cell nuclear transfer (SCNT). Afterwards the egg contains a full (adult) set of chromosomes as if it had been fertilised normally. | 3. A pulse of electricity, or a chemical 'shock', kick-starts the development process, and the embryo begins to grow. | 4. Cell division begins. The subsequent development of the embryo depends upon how successfully the donor nucleus has 're-programmed' the egg. | ||||
Figure 1 - The process of embryonic cloning | |||||||
However, not all clones are created with equal ease. Cloning success is much more likely if embryonic cells are used as nuclear donors. These could be embryonic cells taken from an early fetus, or cells from the inner cell mass of the blastocyst (see Cloning Part 1). What made Dolly so special was the fact she was the first mammal to be cloned using a cell taken from an adult - and even then she was the only live animal to be born out of nearly three hundred attempts. Since then, other animals have been cloned from adult cells, but the efficiency of achieving live births is still extremely low. So what's the big difference between embryonic and adult cells? The key lies in the concept of epigenetics, which was introduced in the first part of this series (see also an earlier article about genetic imprinting).
Cloning requires the reprogramming of an adult or embryonic cell right back to the very start of development. Adult cells are more differentiated (specialised) than embryonic cells - they have made decisions about what sort of cell to be and these choices are said to be imprinted within the cell. For example, an embryonic cell has the potential to become all sorts of cell types, whereas an adult liver cell can only be a liver cell. So embryonic cells only have to "forget" a few decisions when they are cloned, whereas an adult cell has been through many more choices so, essentially, the clock has to be wound back further.
Cells remember what type they are due to epigenetic modifications of their DNA: molecular tags which mark certain genes as being switched on or off. These tags are very similar to those on the sperm which are removed shortly after fertilisation by reprogramming factors in the egg. In order for an adult or embryonic cell to be reprogrammed by cloning (by exposing it to the reprogramming environment of the egg), the epigenetic marks within the DNA must be removed. Adult cells are believed to have more of these tags than embryonic cells, so are harder for the egg to reprogramme.
Whether the donor cell is adult or embryonic, a successful clone must negotiate all the stages of development from one cell to a blastocyst and then on to being a fully-grown baby. But embryos can only be grown outside the womb in a test-tube until the blastocyst stage. After this, the embryo needs to form a placenta and take resources from the mother. Researchers must transfer the little balls of cells into the womb of an appropriate surrogate mother, where it can grow further. In fact, embryos created by mixing eggs and sperm in IVF clinics are usually transferred before the blastocyst stage- often when they only consist of two to eight cells.
Experiments in animals such have mice have shown that there is a high proportion of success in growing cloned embryos up to the blastocyst stage. Even embryos containing twice the normal number of chromosomes can grow quite happily to this point, even though a baby with this many chromosomes could never be born. Yet if these embryos are transferred into surrogate mothers, very few of them produce babies. This is because the egg contains many of the ingredients required for the early stages of development, and will direct the formation of a blastocyst even if there are severe problems with the DNA and chromosomes within the embryo. But to grow from a blastocyst to a baby requires complex patterns of genes being switched on and off. This can only happen if the DNA of the donor nucleus has been fully and correctly reprogrammed by the egg during the first stages of the cloning procedure. So it appears that even if you can create a clone, and even if that clone makes a blastocyst, you are still highly unlikely to end up with a baby.
But all is not lost. Blastocysts are extremely useful even if they don't go on to make a fetus. They are the source of the infamous embryonic stem cells, or ES cells for short. ES cells are made by removing the inner cell mass from the blastocyst and growing it in a dish (figure 2). After a time, a colony of special cells with almost magical properties appears. These cells have the capacity to form any tissue of the body, and can be treated with various chemicals to achieve this. They can also replicate themselves many times, to create large populations of useful cells. Genetic engineering techniques work very well in ES cells, allowing mutant genes to be repaired, and new genes to be added.
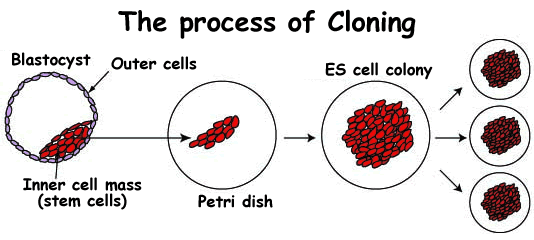 | |||||
| 1. To make embryonic stem cells (ES Cells), the inner cell mass is removed from a blastocyst. | 2. The inner cell mass is transferred to a petri-dish, covered with a nutrient solution, and allowed to grow. | 3. The inner cell mass soon develops into a large cell colony which can then be divided into smaller clusters of cells that will themselves continue to grow and multiply. | |||
Figure 2 - How to make Embryonic Stem Cells (ES Cells) | |||||
Much weight has been placed on human ES cells as a viable therapy for many diseases such as Alzheimer's and Parkinson's. Although we are far from fully understanding the biology of these mysterious cells, they are certainly brimming with promise. In part three of this series we'll look at the latest developments from South Korea, combining ES cell technology and cloning. Are human clones just around the corner? Or are they already here?










Comments
Add a comment