If a doctor offered you live eggs from a parasitic worm to treat your medical complaints, would you accept?
In the last article, we saw that the hygiene hypothesis suggests that a lack of exposure to certain infectious organisms, notably parasitic worms 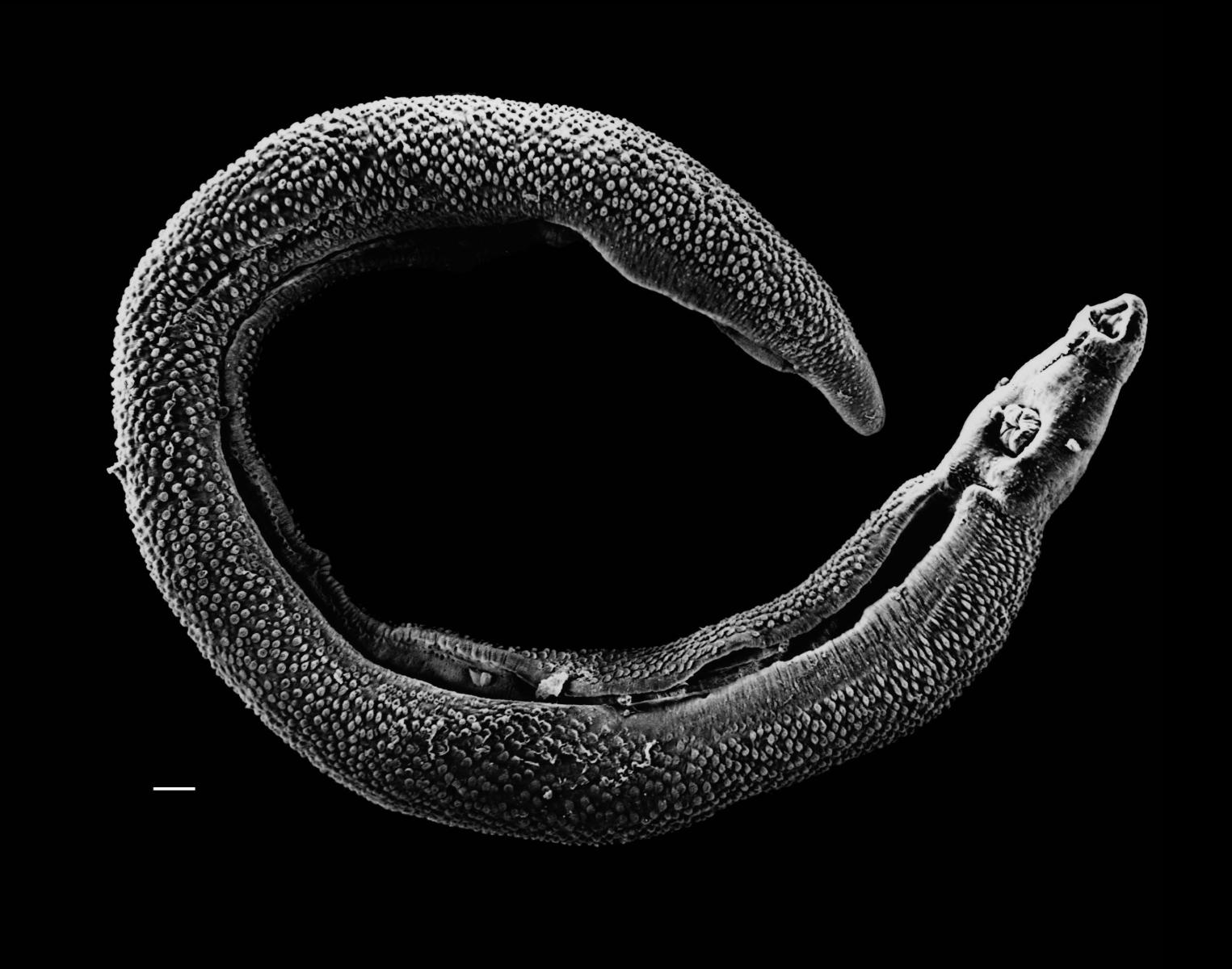 or 'helminths', is causing a rise in immunological diseases in the developed world. These diseases include allergy and autoimmunity, where the immune system initiates excessively aggressive or inappropriate responses and damages our own tissues in the process.
or 'helminths', is causing a rise in immunological diseases in the developed world. These diseases include allergy and autoimmunity, where the immune system initiates excessively aggressive or inappropriate responses and damages our own tissues in the process.
Reintroducing certain parasitic worms may therefore inhibit the development of allergy and autoimmune conditions. But how close are we to helminths becoming a viable, mainstream medical treatment? Professor Joel Weinstock, from Tufts University in the United States, thinks we may not be far off at all...
Weinstock has pioneered the use of Trichuris suis, or pig whipworm, as a treatment for potentially devastating autoimmune conditions known under the umbrella of 'inflammatory bowel disease', or IBD.
In IBD, the immune system damages the gut lining, potentially causi
 |
|---|
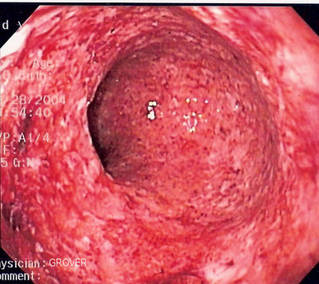
| Figure 1. Severe inflammation and immune-mediated damage to the inner walls of the intestine, visible in this person with Crohn's disease. |
diarrhoea, discomfort, severe pain, bleeding, malabsorption of food and life-threatening complications (Figure 1). There are two main forms of IBD, known as 'Ulcerative Colitis' (UC) and 'Crohn's Disease' (CD). Although the cause of these illnesses is not known, it is thought that the immune system may be reacting to harmless bacteria that live naturally in our guts, with a violently excessive response.
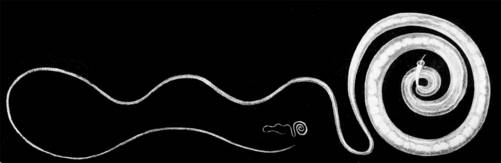
Trichiura suis is a nematode worm related to the human whipworm, Trichuris trichiura (Figure 2), which is the third most common human helminth infection in the world.
In 2003, Weinstock and colleagues gave 2,500 pig whipworm eggs to three patients suffering from ulcerative colitis and four with Crohn's disease. The eggs are microscopic, and are swallowed in a liquid suspension.
"Nobody can see the eggs, you can't taste them. They're invisible," said Professor Weinstock.
Once ingested, they develop in the human gut into adults that are less than a centimeter long and thinner than a human hair. Unlike human whipworm, T. suis dies after a few months and apparently causes no symptoms.
"[They] interact with the host immune system in a totally stealth fashion - you wouldn't know they were there, you wouldn't feel them," said Weinstock.
 |
|---|
| Figure 2. Trichiura trichiuris (human whipworm). |
In this study, six out of the seven patients (86%) entered clinical remission in their disease, suggesting the eggs could be of value. However, seven patients is a tiny sample and with no controls, there is always the risk the results could have been due to chance.
To gain more concrete evidence, Weinstock published two further investigations in 2005. This time, he used the gold standard of clinical investigations: randomized double-blind, placebo-controlled trials.
"We were the first group to test the biological agent in a double-blind study... it's a way of proving whether a drug really works," said Weinstock.
In one study, 54 patients suffering from ulcerative colitis were divided randomly into two groups. One group ingested 2,500 T. suis eggs at 2-week intervals for 12 weeks, while the other group took a placebo. Neither the patients nor the doctors knew whether they were getting the real eggs or the dummy placebo.
Out of 30 patients given the eggs, 13 experienced improved symptoms (43.3%), while only 4 out of the 24 patients receiving the placebo derived any benefits (16.7%).
In the second study, 29 patients suffering from active Crohn's disease were given a dose of 2,500 T. suis eggs every three weeks for 24 weeks. 23 patients (79.3%) experienced some benefit, with 21 (72.4%) going into remission. There was no control group for comparison.
"The results were positive, suggesting that it would be of value, and that's why the agent went into the pharmaceutical industry at that point," said Weinstock.
Importantly, no side effects were reported by patients taking the whipworm eggs.
"In our study it didn't show any significant side effects. Any time you're using an experimental drug there's always a risk until it's been properly tested. But I suspect that the relative risk will be low," said Weinstock
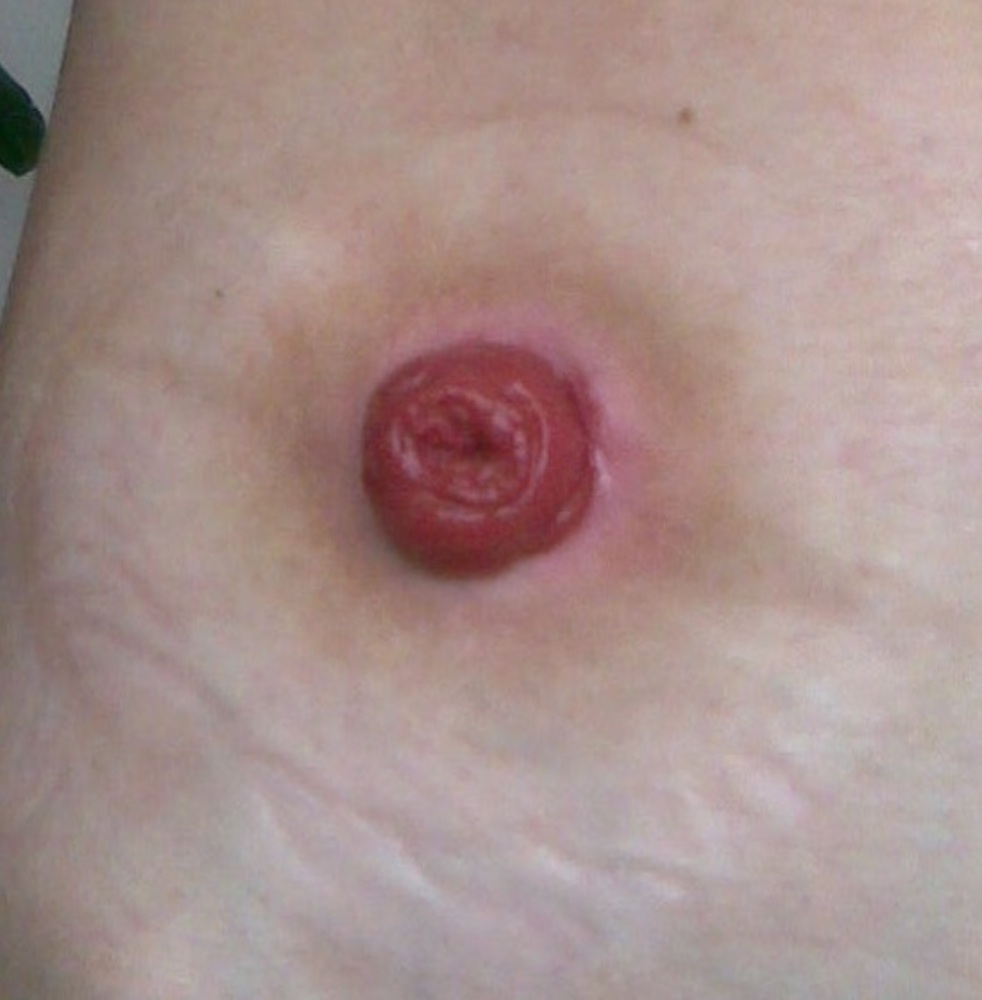
This contrasts with current therapies for inflammatory bowel conditions which often carry severe side effects. For example, long-term use of steroids, a major treatment option in IBD, can cause thinning of the bones and skin, increased risk of infections and psychological disturbances. Radical surgery performed in cases of severe IBD can leave patient's requiring a colostomy bag for life (Figure 3).
 |
|---|
| Figure 3. Radical surgery to treat severe inflammatory bowel disease. A loop of bowel is cut and the free end is brought to the surface for emptying into a stoma bag. |
"If [patients] are offered the option of taking something that they can't see or taste, that has a safety level much, much better than current therapies, and works equally as well if not better, which one do you think they're going to choose?" said Weinstock.
Laboratory work by Weinstock and colleagues suggests that the worms activate special cell types which regulate other immune cells in the body. These include 'T-regulatory cells', or Tregs, which recognize specific molecules from the worm's surface and then dampen down the ensuing immune reaction from other cells. They also produce chemical signaling molecules that dampen down the immune inflammatory response, including IL-10 and TGF-β, mentioned in the previous article.
"The worms seem to work by triggering and modulating the regulatory side of our immune system," said Weinstock.
A similar finding has been reported in a detailed study conducted on one man who suffered from ulcerative colitis and infected himself with human whipworm, T. trichiura. His symptoms improved while he was heavily infected (releasing >15,000 eggs per day in his stools), but worsened as the worms died off and egg production waned (down to <7,000 eggs per day). He then re-infected himself and once again experienced symptomatic relief.
Regions of the man's bowel that were heavily colonized by T. trichiura were less affected by ulcerative colitis, as visualized by colonoscopy. The immune cells in these regions produced a chemical signaling molecule called IL-22, which is in the same family as the anti-inflammatory molecule IL-10, described previously.
The conclusion is that whipworm can be a safe and effective treatment for inflammatory bowel disease, and operates via the induction of immune regulatory mechanisms and production of signaling molecules like IL-22.
Professor Weinstock was optimistic for the future of using pig whipworm eggs as a mainstream therapy.
"Potentially, if studies went very well, in two or three years there could be a product approved by regulatory agencies," he said.
But not everyone agrees that infection with live parasitic worms is the best way forward.
Professor John Croese, from James Cook University in Australia, thinks we may be running before we can walk, by jumping straight in to using parasitic worms as a treatment before we really understand how they work.
"We're starting at the wrong end - and I did this myself - just throwing [helminths] in and hoping to treat a disease. I think we need to start at the other end and understand the relationships between these organisms and our health and then work towards using them to develop treatments," said Croese.
In 2006 Croese published a small study on nine people who suffered fr
 |
|---|
| Figure 4 - Blood-sucking hookworms attached to the intestinal mucosa. (Species: Ancylostoma caninum). |
Crohn's disease. Each person was infected with a type of helminth called a hookworm, which is a blood-sucking nematode that latches on to the gut lining and feeds on human blood (Figures 4-5).
Unlike the pig whipworm studies performed by Weinstock, Croese's research uses a species that naturally infects humans, called Necator americanus, collected from Papua New Guinea. There were initially side-effects to infection, including itching, rash and stomach pain. These dissipated after a few weeks, and the more severe effect of hookworm infection - anaemia, or lack of blood caused by the feeding worms - was not encountered owing to the low number of worms per person.
The results did suggest that hookworm provided moderate symptomatic relief in Crohn's disease, although it only became noticeable from 17 weeks after infection. However, when Croese tried reducing the amount of mainstream pharmaceutical treatment the patients were taking, they relapsed back into disease.
"It was fairly clear that conventional treatment was the more robust in controlling the patient's disease process," said Croese.
| Figure 5. Video of mating hookworms, of the 'Necator americanus' species, in a human host. Video taken via colonoscopy. |
Croese decided to change his research strategy and investigate the more subtle effects that hookworm infection might be having on the host immune system.
"We've changed now in that we're really not trying to find a helminth that'll cure every disease, but rather trying to understand the change that helminths impose on the immune system at a more basic level," he said.
To investigate this, Croese examined how hookworms influence the immune response in coeliac disease. This is another autoimmune condition that affects the gut lining, and in some ways mirrors the problems seen in Crohn's disease and ulcerative colitis. However, with coeliac disease we know exactly what the trigger is that sets off the immune response: gluten, a chemical found in wheat, barley and rye (Figure 6).
Croese conducted a randomized placebo-controlled trial, published earlier this year (2011), testing the capacity for hookworm to suppress gluten sensitivity. Twenty people who suffer from coeliac disease were split into two groups: one group received two installments of Necator americanus, while the other received placebos that contained no parasites. They were put on a gluten-free diet and so did not experience any symptoms.
Twenty weeks after the first dose of hookworm was given, all twenty subjects
 |
|---|
| Figure 6. Gluten is contained in many foods, making coeliac disease difficult to manage. |
were put on a gluten-rich diet and the researchers looked to see whether the hookworms made any difference to the immune reaction induced by gluten in coeliac disease sufferers.
The short answer is that the hookworms made no difference.
"What we found was that, as treatment - as an alternative to a gluten free diet - the hookworm suppression of the immune system was nowhere near enough to allow people with coeliac disease to go back onto a gluten-rich diet," said Croese.
But it's not quite as simple as that.
The researchers collected tissue samples and did blood tests on the study participants and found that hookworms have a more subtle influence on the coeliac disease process. Inflammatory mediators that activate the immune system, such as a chemical called Interferon gamma, or IFNγ, leak out of gut tissues when someone with coeliac disease ingests gluten. But the amount of IFNγ secreted in the hookworm-infected group was significantly reduced compared with controls. So helminths did have an immunological effect, even if it wasn't sufficient to work as an effective treatment.
Croese did use far fewer parasites for his study than the 2,500 T. suis eggs used by Weinstock. But this is because hookworms cause quite severe blood loss in high doses.
Croese conceded that, "there is some exciting evidence to suggest that whipworm might work in inflammatory bowel disease." But his overall message was that we should not automatically assume that live infection with helminths will make effective treatments for immunological diseases, even if a lack of those same helminths is implicated in causing the diseases to start off with.
"It's wrong to assume that the hygiene hypothesis suggests that using helminths, or introducing people to poor hygiene, once they have an established disease, will switch the disease off. The epidemiological evidence suggests that these diseases are more common when a society becomes hygienic, but that's acquisition of a disease, it's not treatment," said Croese.
Instead, Croese suggests that the focus should be on further elucidating the interactions between helminths and the human immune system, and then isolating molecules secreted by parasites that modulate immune processes. Then, rather than a live infection, we could use helminth-derived products or their derivatives to design new drugs that are more effective and safer than current medications.
"The stronger prospect is to understand the processes of the disease and what particular chemical components helminths produce that might be used as a template for future drugs," said Croese.
Whether we go down the road of live infection with helminths like pig whipworm, or design helminth-derived products as drugs, or - my personal preference - do both, parasitic worms will almost certainly play a key role in furthering our understanding of immunological diseases and will guide the development of new treatments for these conditions.
In the next and final article on the hygiene hypothesis, we will look at why parasitic worms might have evolved to apparently help us out in the first place. Are worms friend or foe?
Glossary of terms:
Allergy - Diseases caused by an excessive and unnecessary immune reaction to harmless particles in our environment, like grass pollen, which causes hayfever.
Autoimmunity - Diseases caused by the body's immune system targeting its own tissues 'by mistake', resulting in tissue damage.
Celiac disease - Immune response mounted against gluten, which is found in wheat, barley and rye. When ingested the immune reaction causes diarrhea, discomfort and pain.
Cytokine - A chemical signaling molecule that influences processes concerning the immune system. An 'inflammatory cytokine' promotes immune reactions, activating immune aggression. An 'anti-inflammatory cytokine' has the opposite effect, suppressing and subduing the immune system.
Crohn's disease - see 'Inflammatory Bowel Disease'.
Helminth - A multi-cellular animal that lives in or on humans and which has an 'earthworm-like' shape. These include nematodes, like hookworm and whipworm, flukes, like Schistosoma mansoni, and tapeworms, which can grow to meters in length. These creatures are not actually very closely related to each-other.
Immune system - A complex network of molecules, cells and organs which have evolved to seek out potentially damaging threats, like infectious bacteria and viruses, and destroy them.
Immunological disease - Diseases characterized by a dysfunction of the immune system. See 'Allergy' and 'Autoimmunity'.
Inflammation - This is a strong response mounted by the immune system against potential threats. Causes the inflamed area to become hot, swollen and painful.
Inflammatory bowel disease - Autoimmune disease, where the immune system damages regions of the gut causing pain, diarrhea and blood in the stools. The immune system may be reacting to harmless bacteria that live in our gut. Includes Crohn's and Ulceratice Colitis (UC).
Parasite - An organism that lives in or on another creature, the 'host', from which it derives benefits at the host's expense. Most medical doctors think of parasites as separate from bacteria and viruses, covering 'all the rest'- from single-celled organisms like amoeba to helminths, like tapeworms.
Ulcerative colitis (UC) - See 'Inflammatory Bowel Disease'.
T-regulatory cells, Tregs - A special type of immune cell that is activated by specific molecules and functions to inhibit other cells in the immune system, dampening down immune responses and producing anti-inflammatory chemicals.
References and further reading:
* For a thorough general overview of the hygiene hypothesis, see the 'Review series on helminths, immune modulation and the hygiene hypothesis', in Immunology, January 2009, Volume 126, Issue 1.
* Whipworm as a treatment for inflammatory bowel disease
Summers, R.W., et al. 2003. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol 98(9):2034-41.
Summers, R.W., et al. 2005. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology 128(4):825-32.
Summers, R.W., et al. 2005. Trichuris suis therapy in Crohn's disease. Gut 54(1):87-90.
Broadhurst, M.J., et al. 2010. IL-22+ CD4+ T cells are associated with therapeutic trichuris trichiura infection in an ulcerative colitis patient. Sci Transl Med 2(60):60ra88.
* John Croese's work on hookworms:
Croese, J., et al. 2006. A proof of concept study establishing Necator americanus in Crohn's patients and reservoir donors. Gut 55(1): 136-137
Daveson, A.J., et al. 2011. Effect of hookworm infection on wheat challenge in coeliac disease--a randomised double-blinded placebo controlled trial. PLoS One 6(3):e17366.
- Previous Teaching old dogs new tricks
- Next Making Maths Interesting










Comments
Add a comment