For thousands of years metallurgists have been mixing metals to change their characteristics, often with world-altering consequences. And although the materials scientists of today have largely swapped a hammer for some of the most expensive and advanced scientific equipment money can buy, the quest for materials with superior qualities continues.
Take, for example, the nickel-based superalloys used extensively in the hottest parts of the gas turbine engines that power passenger jets. Among manufacturers there is an increasing desire to operate the engines at higher temperatures and under greater stresses, meaning the materials inside them also have to withstand much harsher conditions. Why? Because running a gas turbine engine faster and hotter means it can be more fuel-efficient, keeping down the cost of a plane ticket and making the aircraft more environmentally friendly.
The aims sound simple, but achieving them means metallurgists need to come up with new materials capable of meeting the challenge. One way to do this is with computer modelling, which allows more efficient and cost effective development by employing a mixture of quantitative mathematical techniques over a range of length-scales to optimise a material's performance (see figure 1, below).
At a microscopic scale, a material can be seen to be made up of many tiny crystals, which exhibit different atomic arrangements and different compositions referred to as the microstructure. The physical properties depend, in turn, upon the interactions between and within these crystals, and the nature of these interactions can be correlated to the chemical composition and to the arrangement of atoms.

So, to design an improved superalloy, the composition and microstructure need to be optimised to provide a material with an increased temperature capability. But at this high temperature, the material must also have exceptional mechanical properties and excellent environmental resistance to ensure operational longevity.
To design a new superalloy, a chemical composition must first be chosen. Rather like an artist spoilt for choice by a massive mixture of colours with which to paint, a metallurgist must select the right chemical elements - and in the right proportions - to come up with just the right metaphorical colour combination. For any alloy there are up to 20 potential elements that could be used, meaning that there are literally trillions of potential compositions to pick from. We can consider this array of options as a huge "compositional space", containing every possible combination.
So how is the ideal recipe determined? To do this, we could adopt a manual method, whereby compositions of possible merit are identified using the proceeds of previous research coupled with a metallurgist's experience. But this sort of manual approach is demanding, very time consuming and therefore limited in terms of what it can achieve. Instead, a more powerful alternative is to use a computer to evaluate vast arrays of experimental data which can then be combined with appropriate materials modelling. Computer-based methods like this also mean that the previously un-investigated corners of compositional space can be probed.
To do so, a choice is first made regarding which "phases" are desirable in the material and then elements which favour the formation of any other phase are eliminated. A phase is defined as a structure (an example is shown in the below figure) that is stable for a given composition over a range of temperatures and pressures. This is the job of specialist software that can calculate the phases that will form when different mixtures are made. This enables a metallurgist to identify those compositions that will give a desired combination of phases.
The mechanical properties of each phase, or the whole microstructure, can then be predicted using neural network modelling, which is a statistical technique based partly on experimental data from similar materials tested previously.
However, superalloys usually display very complex behaviours, so it's not possible to model all aspects of their attributes during the initial development, especially when there is limited experimental data. Therefore, during the initial stages, assumptions and simplifications have to be made based on the particular alloy systems being considered. This includes evaluating different phases separately as well as assuming the combination of optimal phases will lead to the production of an optimal alloy. With this in mind, it's usually better to eliminate compositions that clearly do not meet the desired design specification because this narrows the compositional space down to a few regions that are small enough to be evaluated experimentally.
Next, once some experimental results have been gathered, microstructural modelling can be carried out. This is critical because, even for fully-developed alloys, adjusting certain aspects of the manufacturing process can dramatically improve the properties of a material. And, with the advent of advanced materials modelling tools which allow the material behaviour to be accurately predicted, this has become increasingly common.
Also, advances in processing techniques, such as the ability to bespoke-tailor the microstructure within different regions of a material to meet differing chemical or mechanical demands, have been made possible due to computational models.
Nowhere is this more important than in a jet engine, where a turbine blade has to survive in a gas stream approaching 1500°C. At this high temperature, the life expectancy of components is limited by slow, time-dependent plastic deformation of the material. As each blade within the engine rotates, the force felt by the root of the blade is equal to the weight of a family car; and at the high operating temperature, this causes the material to deform plastically. Casting the blade from a single crystal, however, renders it much more resistant to this process.
Nevertheless, a solidification process that allows a single crystal to be grown is not trivial to achieve. The solidification process is "dendritic", resembling a branch-type structure similar to the shapes seen in snowflakes or frost. And once the material is fully solidified, the history of this dendritic solidification remains imprinted within the material, with variations in composition observed in numerous locations. This means that, before this material can go into service, the composition must be made consistent throughout. To achieve this, the material is heated in a furnace for a number of hours at a temperature high enough for the elements to diffuse quickly. This "irons out" the fluctuations in the composition throughout the material, making it much more homogeneous.
Observing and optimising this process using microscopy can be expensive and time consuming. So in recent years it has been aided significantly by a computational tool known as phase field modelling. By understanding how a material solidifies, as well as how rapidly each of the elements in the alloy can move by diffusion, the heat treatment process can be optimised and much better understood.
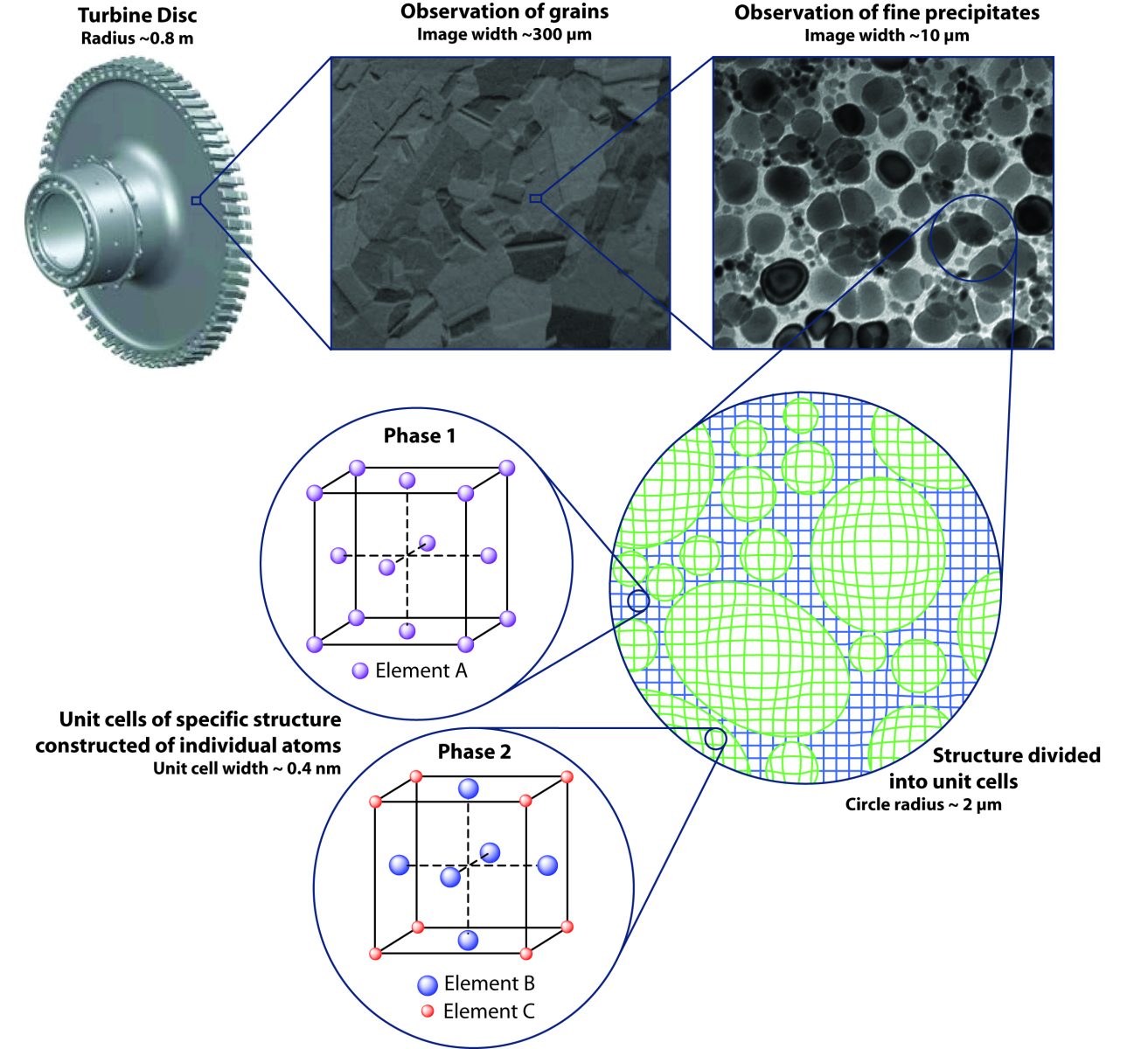
In the case of a turbine disc, which is a large and heavy component often weighing up to 80 kg or more, when this spins inside an engine at rates of up to 10,000 rpm the kinetic energy is huge. This means that the consequences of a "burst disc" could be catastrophic, so they are designed never to fail. But in order to be able to deliver this sort of certainty, it's critical to understand the behaviour and limitations of the material. And as a material is exposed to elevated temperatures whilst in service, its microstructure evolves, giving rise to a gradual change in the material properties.
Therefore, to understand the full behaviour of this component, it is necessary to understand the material over a range of length-scales, as shown in the figure below. By observing a section of turbine disc under an electron microscope it is possible to see individual crystals, or grains. Increasing the magnification further allows you to see fine precipitates. These precipitates are tailored to be a specific shape and size, and are hugely important to the tremendous strength of the material. Zooming into the material even farther would allow us to reveal the individual atoms.
Overall, it's by understanding the relationship between these length-scales, helped by computational tools, the turbine blades and discs of tomorrow will continue to convey people safely, but even more economically and in a more environmentally-friendly fashion...
- Previous Sardines: Fish Food or People Food?
- Next The Superalloys









Comments
Add a comment