In a memorable scene from the 2004 revenge flick Kill Bill 2, deadly assassin Elle Driver has just outwitted the unsympathetic villain, Budd, by hiding a Black Mamba snake in a suitcase of money she's given him. As Budd counts the cash, he encounters the Mamba and receives multiple strikes to the face. As he lay moaning on the floor of his rotten trailer, Elle reads from her notepad. "'The venom of a Black Mamba can kill a human in four hours, if, say, bitten on the ankle or the thumb" she reads. "However, a bite to the face or torso can bring death from paralysis within 20 minutes.' Now, you should listen to this, 'cause this concerns you" she says to Budd, glancing down at him with her one cold, unpatched eye.

This morbidly comical exchange illustrates the most vile and basic use of toxins: to immobilize, and ultimately, to kill. In this classic Tarantino scene, the toxins are wielded as a weapon, coming directly from the source. This technique may in fact be an ancient one, as the renowned Roman military commander, Hannibal of Carthage, reportedly threw cauldrons of angry, venomous snakes into enemy ships in one of the earliest examples of biological warfare - sometime around 200 B.C.. 1
In contemporary science, however, toxins have seen a redemption of sorts and are being used to serve, rather than to harm, humans. Toxin researchers have investigated exactly what makes venom, and the toxins within it, so deadly and in the process, a virtual smorgasbord of useful properties has emerged. Areas as diverse as cell biology and cosmetics have benefitted from toxin research. In essence, the tables have been turned on toxins - they were finely tuned through evolution to kill, but are now used to improve the lives of people through neurological research and medical treatments.
What are toxins?
Toxins are poisonous substances produced by living organisms that are harmful in very small doses. They are typically used for prey capture, as seen in snakes and scorpions, or for defense, as seen in snails and jellyfish. One species may have over one hundred different toxins contained within its venom.
Toxins can be divided up based on their targets or their effects on cells: hemotoxins attack red blood cells; necrotoxins cause general cell death to occur, usually in skin and muscle tissue; and neurotoxins affect the nervous system. It is this last group that has proved most useful in a research setting.
 Venom Neurotoxins
Venom Neurotoxins
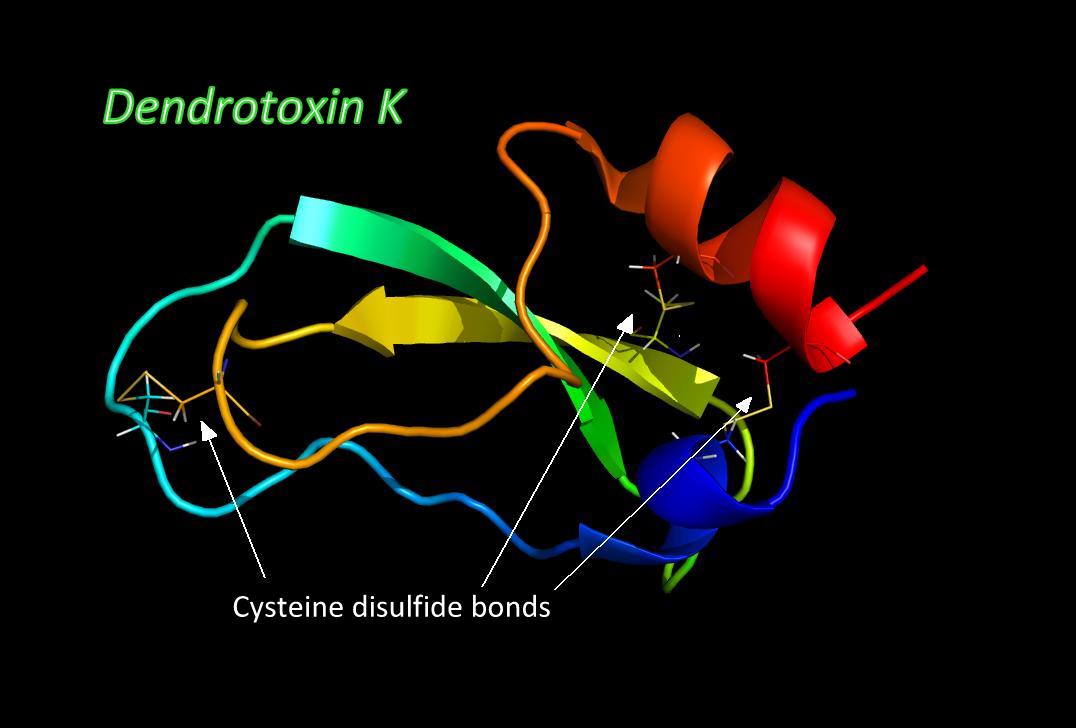
Venom neurotoxins share certain properties that define their mode of action. Most of them are peptides, meaning they are relatively short protein chains, about 12 to 80 amino acids, or residues, in length. These short chains become folded into a tight ball and are held that way by special disulfide bonds between cysteine residues. Once folded, this tight ball has active faces - special binding regions - that help it bind to its target within the cell membrane and in some cases, to the membrane itself. Toxic peptides can bind to their targets for long periods, wreaking havoc on the nervous system and the muscles they innervate by interfering with the normal functioning of the neuron cell.
Neurotoxins in Research
How can these lethal peptides help unravel the intricate mystery of the nervous system? The answer lies in the fact that venom neurotoxins interfere with bioelectricity, the ability of cells to pass an electrical current and the basis of nervous transmission.
Bioelectrical current is not carried by electrons, as it would be in mechanical circuits. The charged particles are ions, or atoms that have lost or gained electrons; sodium, potassium, calcium, and chloride ions predominate. The cell membrane does not allow these ions to pass through, except through special pore-forming proteins called ion channels that act like ultra-fast switches. Most venom neurotoxins interfere with theseion channels and alter ionic current in the cell.
Depending on the type of channel affected, this can be like holding the nerve switch "on" - causing convulsions - or holding it "off" - causing paralysis. This is quite advantageous to the toxin-bearer, and, as it turns out, quite advantageous to scientists as well - and not just the mad variety.
In early research, the very nature of the nervous signal was revealed with the help of neurotoxins. Blocking currents with different toxins allowed the signal, called an action potential, to be separated into its component currents of sodium and potassiumions. This led researchers to speculate on the existence of the pores selective to specific ions we now know as ion channels.2
A more modern use of toxins is in the pharmacological classification of unknown ion channels. New channels can be divided into toxin-sensitive and toxin-insensitive groups, allowing for quick characterization. The specific and strong interaction of the toxin with the channel also allows the channels to be more easily purified, during affinity chromatography, for example. This creates opportunity for more experiments, and therefore more knowledge to be gained about ion channels and the nervous system in general.
There are more direct applications as well. The size and shape of the toxin peptide can be revealed by various methods, and this may then be used to map out the region of the channel to which it binds. The scorpion toxin charybdotoxin has been used in this way to map the pore region of potassium channels.3
Antivenom
Humans have long been plagued with the ill effects of neurotoxins so the production of antivenom (or antivenin) was an important advance in toxin research. The first was created by Albert Calmette, a French scientist of the Pasteur Institute, in 1895 against the Indian Cobra (Naja naja). He injected a small amount of the venom into a lab animal which then produced antibodies against the venom peptides. These antibodies were harvested and given to patients to counteract the poisoning. 
Many different bites and stings can now be treated this way, provided there is access to medical care and the effort has been made to produce the antivenom. Sadly, most pharmaceutical companies do not invest many research dollars into antivenoms as they largely affect third-world countries where profits are prohibitively low. There has, however, been a recent advance that created a safer, more useful snake antivenom.4 It is active against more types of snake venom than just one species, as in traditional antivenom, and causes fewer allergic reactions making it more amenable to mass production.
The Future of Toxin Research
More and more toxins are being discovered each year, each one with a specific target, and each one carrying great possibility. They come from unlikely sources and have unexpected benefits: some commercial, like the use of Botulinum toxin (Botox) to reduce wrinkles, and some medical, like the use of the same toxin to treat debilitating muscle spasms. While toxins can benefit the population directly in such ways, new discoveries contribute even more to basic neuroscience research. Learning about the building blocks is essential to understanding the system. Neurotoxins are an increasingly important tool in picking apart the building blocks: neurons, ion channels, and cell membranes.
So, while deadly assassins and ancient warriors wield snakes, neuroscientists in clinics and laboratories throughout the world now wield the power of millions of years of evolution in the perpetual combat against human suffering.
Rheanna M. Sand
References Cited:
1. History-world.org. Hannibal. Retrieved 26 May, 2008. http://history-world.org/hannibal.htm
2. Hille B. 2001. Ion Channels of Excitable Membranes (3rd ed.). Sinauer. Sunderland, MA
3. Garcia, M.L., Ying-Duo Gao, O. B. McManus and G. J. Kaczorowski. 2001. Potassium channels: from scorpion venoms to high-resolution structure Toxicon 39: 739-748
4. MacKenzie, D. 2008. New antivenom could save more snakebite victims. NewScientist.com news service. 06 June 2006. Retrieved 25 May, 2008. http://www.newscientist.com/article.ns?id=dn9277&feedId=online-news_rss20
- Previous The Science of the Supernova
- Next Donated to Science










Comments
Add a comment