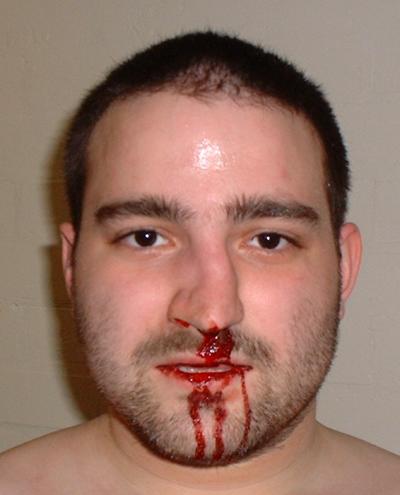
 We all know about pain, or at least we think we do. Things that damage our bodies cause pain. We learn to avoid pain because the experience is nasty, and in the process we learn to avoid stimuli which might damage us. The more the damage, the greater the pain, and the more we avoid it. Put simply like that, pain is obviously beneficial, in that it teaches us to avoid damage, even though the experience of it is unpleasant. But closer examination reveals many puzzles and contradictions. Sometimes we fail to notice even quite severely damaging stimuli. In a rugby match the pain of a broken bone may only be felt after the player has left the field. Soldiers in battle similarly do not feel severe injuries until after the battle is over. I read recently a letter written home by a sailor at the battle of Trafalgar, where he comments that he had been unaware at the time of the battle that a cannon ball had carried away three of his fingers. So pain is not so simple after all. Damaging stimuli do not necessarily hurt.
We all know about pain, or at least we think we do. Things that damage our bodies cause pain. We learn to avoid pain because the experience is nasty, and in the process we learn to avoid stimuli which might damage us. The more the damage, the greater the pain, and the more we avoid it. Put simply like that, pain is obviously beneficial, in that it teaches us to avoid damage, even though the experience of it is unpleasant. But closer examination reveals many puzzles and contradictions. Sometimes we fail to notice even quite severely damaging stimuli. In a rugby match the pain of a broken bone may only be felt after the player has left the field. Soldiers in battle similarly do not feel severe injuries until after the battle is over. I read recently a letter written home by a sailor at the battle of Trafalgar, where he comments that he had been unaware at the time of the battle that a cannon ball had carried away three of his fingers. So pain is not so simple after all. Damaging stimuli do not necessarily hurt.
The opposite can also happen - pain can felt even when there is no stimulus, or it can be caused by a stimulus which is clearly not damaging. One example is neuropathic pain, which persists indefinitely, long after the injury which inititated it has healed. Neuropathic pain is a terrible (and currently untreatable) problem for those who suffer from it. Another example is phantom limb pain. Patients with amputated limbs often complain of a feeling of pain in the ghost of the missing limb. A more day to day example that we are all familiar with occurs if the skin is burned, for instance in getting a hot dish out of the oven. The burned area remains painful for days, and even a quite innocuous stimulus, such as gentle touch or the warmth of contact with a hand, causes pain. So pain can be caused by a non-damaging stimulus, or even by no stimulus. There is clearly no direct or simple relationship between the strength of the stimulus and the magnitude of the pain. What can be going on here?
In fact pain is really very poorly understood, in spite of years of study. We do not even know very much about how nerve fibres detect pain. Do the nerve endings react directly to pain, or is it that damage releases something from other cells, which then stimulates the nerve fibres? And how can pain be so variable? Is this something happening "in the mind" or are there processes happening out at the periphery, at the level of the nerve terminals, which might explain the variability of pain?
These are the questions that my lab in Cambridge is trying to answer. We abandoned the idea of studying pain in intact animals and went instead for the simpler situation of isolated nerve cells cultured in a dish. We have found that they respond in many ways like pain-sensitive nerves in a real animal. For instance, we found that the temperature at which these isolated pain-sensitive nerves respond to heat is very similar to the temperature at which a human subject reports that the sensation from a warmed object changes from a pleasant feeling of warmth to a sensation of painful heat. We found out something about the way in which heat actually stimulates the nerve endings - it is a direct process, caused by sodium ions flowing in through the membrane of the cell. So the actual response to heat is intrinsic to the nerve fibre itself, and does need any factors which might be released from nearby cells damaged by the hot stimulus.
But the isolated nerve fibres did differ in an interesting way from their counterparts in an intact animal. If a hot stimulus is repeatedly applied to skin the sensation of pain gets worse and worse, as we all know, while our isolated nerves in culture always responded with a signal of the same size. This told us that the process of sensitization, by which the feeling of pain increases with time, is not intrinsic to the nerve fibres, but instead depends on factors released from other nearby cells which in an intact animal are damaged by the painful stimulus. Nearby cells are, of course, not present in a culture of isolated nerve cells. We identified one of these factors as a protein fragment called bradykinin, which was already known to be present in elevated concentrations in damaged or inflamed tissue.
So, in summary, transduction, or the process by which a nerve cell detects pain, is quite separate from sensitization, the process by which pain gets worse with time. The first is present in a nerve fibre by itself, and does not need any other partners in order to produce a normal response to heat. The second needs bradykinin, and probably other factors as well, to be released from damaged cells nearby in order to occur. We have now found out quite a lot about what is happening at a molecular level inside the nerve during both of these processes. All this atttracted considerable media attention, and the work was featured in several national newspapers, in the popular science magazine "New Scientist" and on BBC television.
We have recently opened up a new front which is also proving very interesting. We knew all along that sensitization was not going to be caused by just a single process, and that the work I described in the paragraphs above was always going to be just a start on the problem. Pain gets worse for many reasons, some of them happening rapidly (such as the effect of bradykinin described above), some on a longer time scale. Some happen in the nerve endings themselves, others are indeed "in the mind" (or perhaps in the spinal cord). We wanted to make more progress in unravelling these important and interesting processes. We have focussed mainly on processes happening in the nerve endings, out where the pain is first detected. That's not to say that more central processes are not important, but you have to start somewhere.
One possible explanation for the neuropathic pain that I mentioned above is that the properties of the nerve endings might be somehow changed by injury. We looked into the question of whether receptors for bradykinin, which we already knew was important in short-term sensitization, might be increased in number ("upregulated") by injury, in a long-term process. We were very excited recently when we discovered just such a process. It occurs over a long time scale, because factors released by injury have to be transported all the way from the nerve terminal in the damaged area, back up to the nerve cell body located just outside the spinal cord. There the factor (or factors - we are still not sure how many are involved) upregulate production of the receptors for bradykinin, which then have to be transported back down to the terminals. All this takes several days, and it is just this sort of long-term process that might help to explain the slowly-developing agony that sufferers from neuropathic pain feel. We have already identified one of the important factors involved in this long-term process - it is a growth factor, called nerve growth factor (NGF for short). If we could control the production of NGF, or if we could modulate the pathways by which it acts, then we may be able to take the first steps to controlling neuropathic pain. We are just making a start in this area, but it is an interesting beginning.
Last of all, I hope I haven't given the impression that all this work was done by me. In fact, not much of the actual work at all was done by me - I am more the person that starts the ideas running and gets the money necessary to run a large lab (it is, of course, pretty expensive). I am very lucky to have a group of outstanding young scientists working with me. They come from many different countries of the world, from Italy, Spain, Sweden, Taiwan, Korea, Canada - and we even have some from England! It is one of the great pleasures of modern science to work with such a diverse and interesting group of people. Many of our ideas also, of course, depend on progress in other labs. As Isaac Newton once said, you can only see any distance in science if you can stand on the shoulders of giants.
- Previous What's On The Menu ?
- Next The Placenta - a Mathematical Model










Comments
Add a comment