Eggs capable of being fertilised and turning into healthy mice that are themselves fertile have been produced from stem cells by 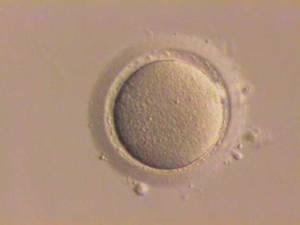 scientists in Japan.
scientists in Japan.
Writing in Science, Mitinori Saitou and his team at Kyoto University took embryonic stem cells and expressed two genes - prdm-1 and Dppa3 - which are normally active in cells destined to become the future ovarian tissue in a developing mouse. These were then mixed with non-egg cells that play a supporting role in the normal ovary.
The resulting "reconstituted ovary" was then implanted into recipient mice to allow the donor ovarian tissue to grow.
By six days, the implanted tissue contained cells with the same genetic, epigenetic and biochemical characteristics that would be expected in an ovary developing in a normal mid-term mouse foetus.
To test their function, the team then harvested the nascent eggs from the reconstituted ovaries one month later and fertilised them with sperm in the dish.
A number of embryos began to develop which were then implanted into surrogate mouse mothers where a small number of them yielded healthy pups that were themselves fertile, proving that the technique can work.
But the success rate - at 4% - was very low, probably because, the team found, many of the resulting embryos produced when the reconstituted eggs were fertilised ended up with an extra set of chromosomes, rendering them inviable. Why, the researchers still need to find out.
But this result, and the fact that they were also able to produce new eggs using another source of cells called iPSCs (induced pluripotent stem cells), which can be made from mature adult cells, is a major step forward, both therapeutically and scientifically. Apart from revealing an approach for making replacement eggs, it also provides a new way to study how the process of egg cell development takes place, and the genetic and epigenetic changes occur around fertilisation.










Comments
Add a comment