Ingredients
| Grated Carrot | Felt pen or food colouring | ||
| Oil | Small jars |
Instructions
Put the food colouring or ink from a pen into some water. Now try adding some oil.
Does anything happen to the colour?
Now try extracting some colour from some grated carrot. Try this in two jars one with a little water and another with a little oil.
Add some grated carrot to each jar and shake them vigorously.
Separate off the oil and the water and look at them.
Now try adding some oil to the water and water to the oil and shaking.
You can try the same thing with other colours like the red in tomato skins.
Result
 | When oil is added to inky water the colours stay in the water. |
 | When water is shaken with carrot it goes a cloudy orange colour. |
 | When this is shaken with oil the colour is transferred into the oil |
 | When oil is shaken with grated carrot it can go a deep orange colour. |
 | This is not transferred to water if they are shaken together. |
Explanation
The felt pen is very soluble in water but not in oil.
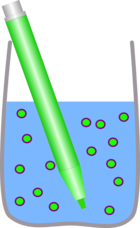 | 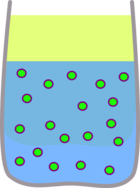 |
| The ink dissolves easily in the water | But none dissolves in the oil |
The orange pigment in carrot (beta carotene) appears to be soluble in both oil and water, but if you look closely at the water or leave it to stand for a long time the orange tends to settle to the bottom as it is mostly in small lumps of carrot.
 |  |  |
| Carrot contains a bright orange pigment called beta carotene | When the grated carrot is shaken with water the water looks orange, but this is mostly tiny lumps of carrot being suspended in the water. | However if the carrot is shaken in oil the beta carotene dissolves very well staining the oil orange. |
This means that if you shake the carrotey water with oil, the beta carotene can still dissolve in the oil, but if you shake the carrotey oil with water the beta carotene can't dissolve so the water stays clear.
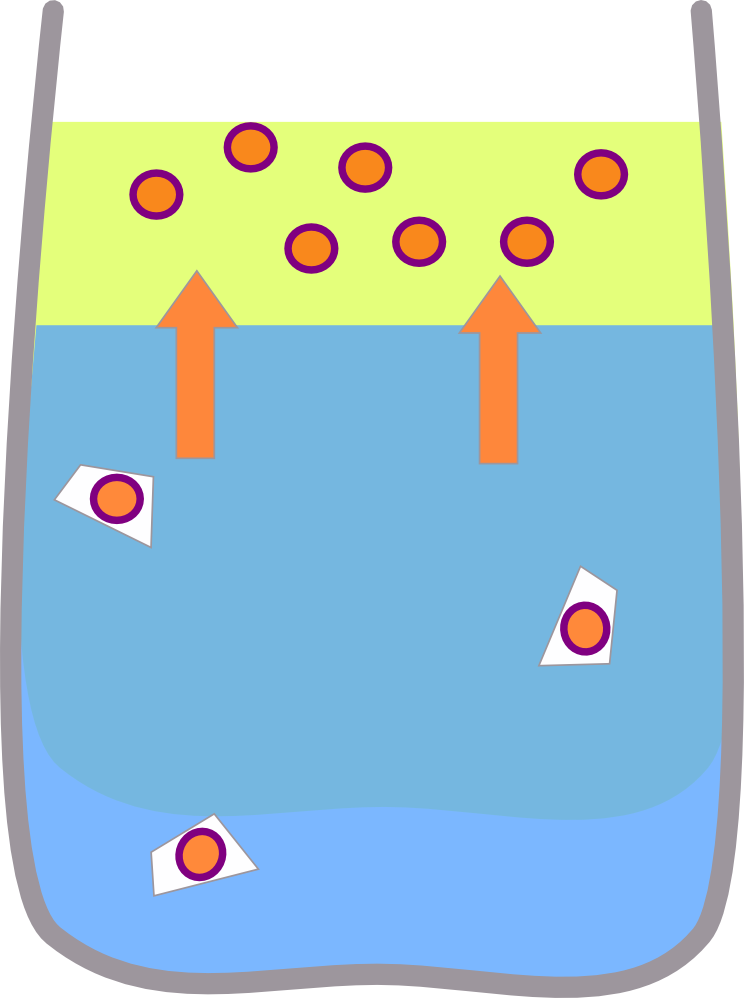 | 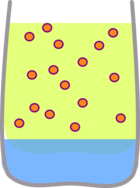 |
| Shaking the carrotey water with oil causes the colour to dissolve in the oil | Shaking the carrotey oil with water has virtually no effect. |
This is a really important process in chemistry. Many reactions produce lots of byproducts and waste products, and your starting materials may not have been pure anyway, and you want to produce a pure product. One of the most straight forward ways to do this is to find a solvent which will dissolve your product but not the other chemicals. You then shake the two solvents together, wait for them to separate and extract the solvent you want. It is then just a matter of removing the solvent from your product.
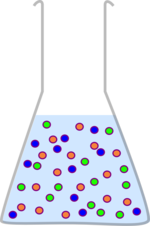 | 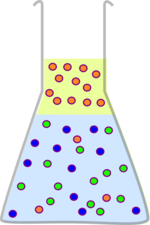 |
| Many chemical reactions produce waste and byproducts which need removing. | If you can find a solvent which will dissolve your product but not everything else, this process is quite straight forward. |
What has this got to to with cells?
A cell is made up of lots of important pieces, the cell membrane, the DNA in the nucleus, fats, proteins etc. But under a microscope they all look very similar, you need an agent to make them look different, these are called stains.
Stains with similar properties to the beta carotene will stain oily cell membranes, other stains will stick to DNA (and are therefore quite good at causing cancer) or particular proteins. So with the right stains it is much easier to tell the parts of a cell, or even see damaged cells which may become cancerous.
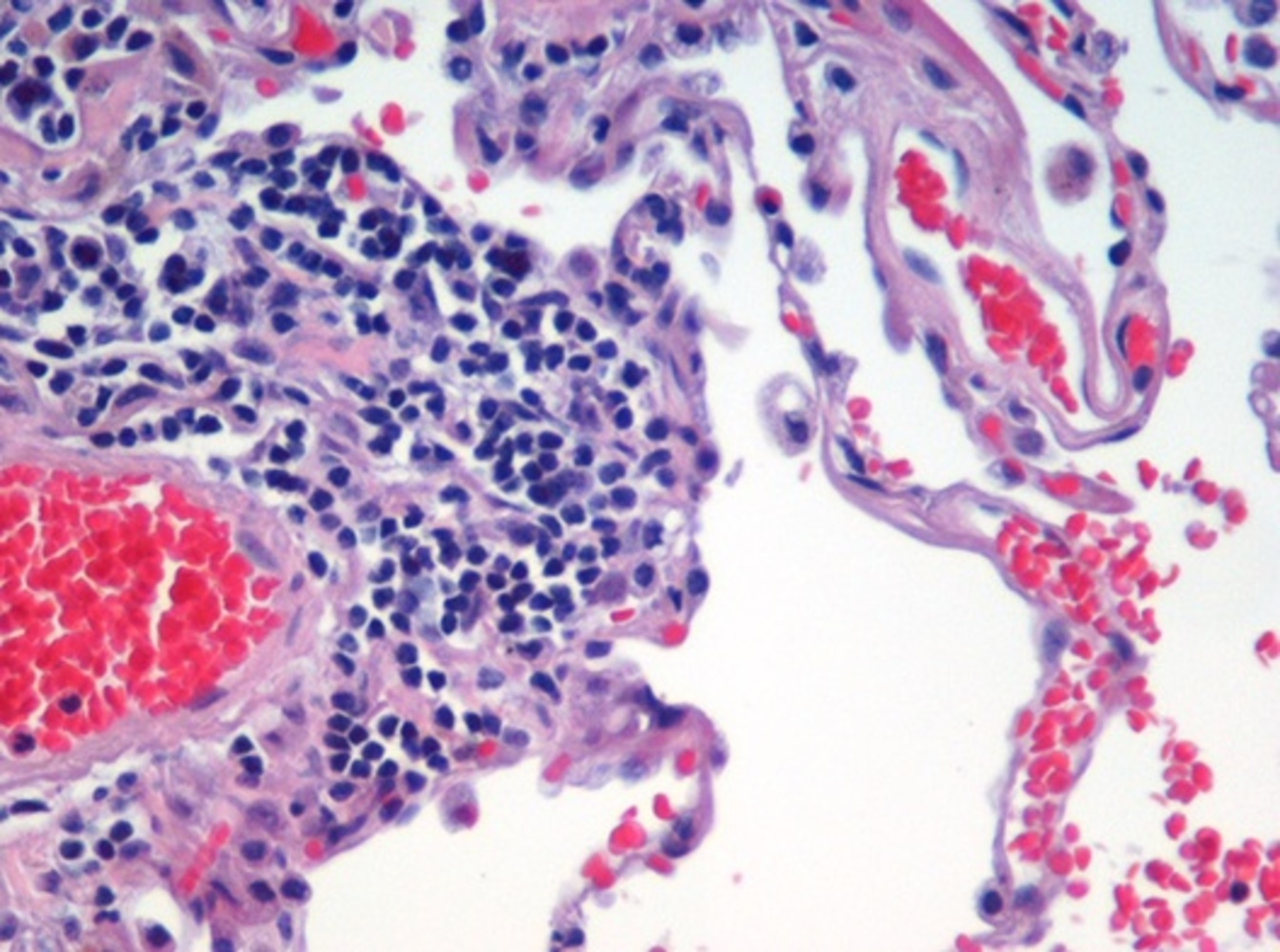 |
| Image is of haematoxylin-eosin stained lung tissue sample taken from an end-stage emphysema patient. Cell nuclei are blue-purple (the eosin), red blood cells are red (stained by the haematoxylin) , other cell bodies and extracellular material are pink, and air spaces are white. © "T Cells Cause Lung Damage in Emphysema PLoS Med 1(1): e25" |
- Previous Blowing out a candle
- Next Dissolving eggs - the power of enzymes










Comments
Add a comment