Ingredients
 |  |
| Two balloons | A little water |

| |
| A candle | |
Instructions
- Light a candle
- Blow up a balloon
- Try holding it in the flame - what happens?
- How add a little water to the balloon, and then blow it up.
- Hold the part of the balloon that is covered with water to the flame.
Obviously be careful with the candle, and as the balloon may burst, do the experiment away from anything electrical, or which may be damaged by getting slightly wet. Don't leave the balloon over the candle for more than a few tens of seconds or the water could get very hot.
Result
You should find that the empty balloon pops immediately, but the balloon with the water is stable for ages over the flame.
| Fireproof balloon |
Explanation
The candle is a source of heat and will add heat energy to any object it touches. If this is well thermally insulated like the rubber in the balloon, the object will heat up to many hundreds of degrees Celsius. Rubber can't cope with these temperatures and it fails, causing the balloon to pop.
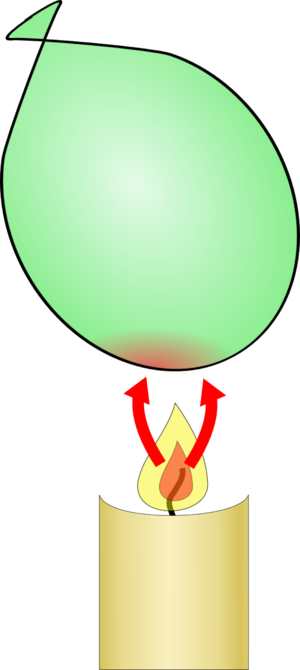 | 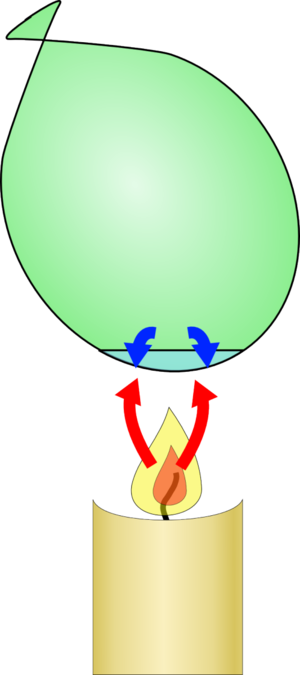 |
| If you put a normal balloon over a candle, the candle adds a lot of heat energy to the rubber until it heats up to the point at which it fails, and the balloon bursts | If the balloon has some water inside, the heat is transferred to the water which takes a long time to heat up, so the rubber never heats up enough to fail. |
If there is water in the balloon the heat can be very efficiently transferred to the water. Water takes a huge amount of energy to heat up, so it stays relatively cool for a long time, cooling the balloon and stopping it heating up.
In fact if there is any liquid water at all, it must be at below 100°C as otherwise the water would boil absorbing even more heat.










Comments
what would be the data
what would be the data analysis?
Thanks for the information.
Thanks for the information.
Add a comment