Ingredients
 | Some PVA glue also known as white, school or Elmer's glue. |  | Borax, which is used as a laundry aid. |
 | Various containers, including some cups. |  | Some teaspoons to measure and mix with. |
Instructions
Dissolve about 1 level teaspoon of borax in 2-300ml of warm water. - Borax powder is a strong cleaning agent and quite caustic, so get an adult to do this.
If you are using standard PVA glue pour about half a cup full into a container, and then dilute it with another half cupfull of water (if you want to end up with more goo, then use more glue!)
Add 1-2 teaspoons of borax solution and stir vigorously.
You may want to repeat the experiment with different amounts of borax.
After it starts to solidify, take it out of the container and knead it in your hands for a few minutes. - If you have sensitive skin you may want to wear gloves.
Have a play with it, how does it behave?
Wash your hands thoroughly after playing with it. Don't eat it, and don't put your fingers in your eyes when you are playing with it as any left over Borax is an irritant.
Result
You should find that the dilute PVA is very runny, but when you add the borax it will solidify and form a rubbery mass.
 |  |
| Gloopy stage immediately after adding the borax. | More solid stage but still needs kneading |
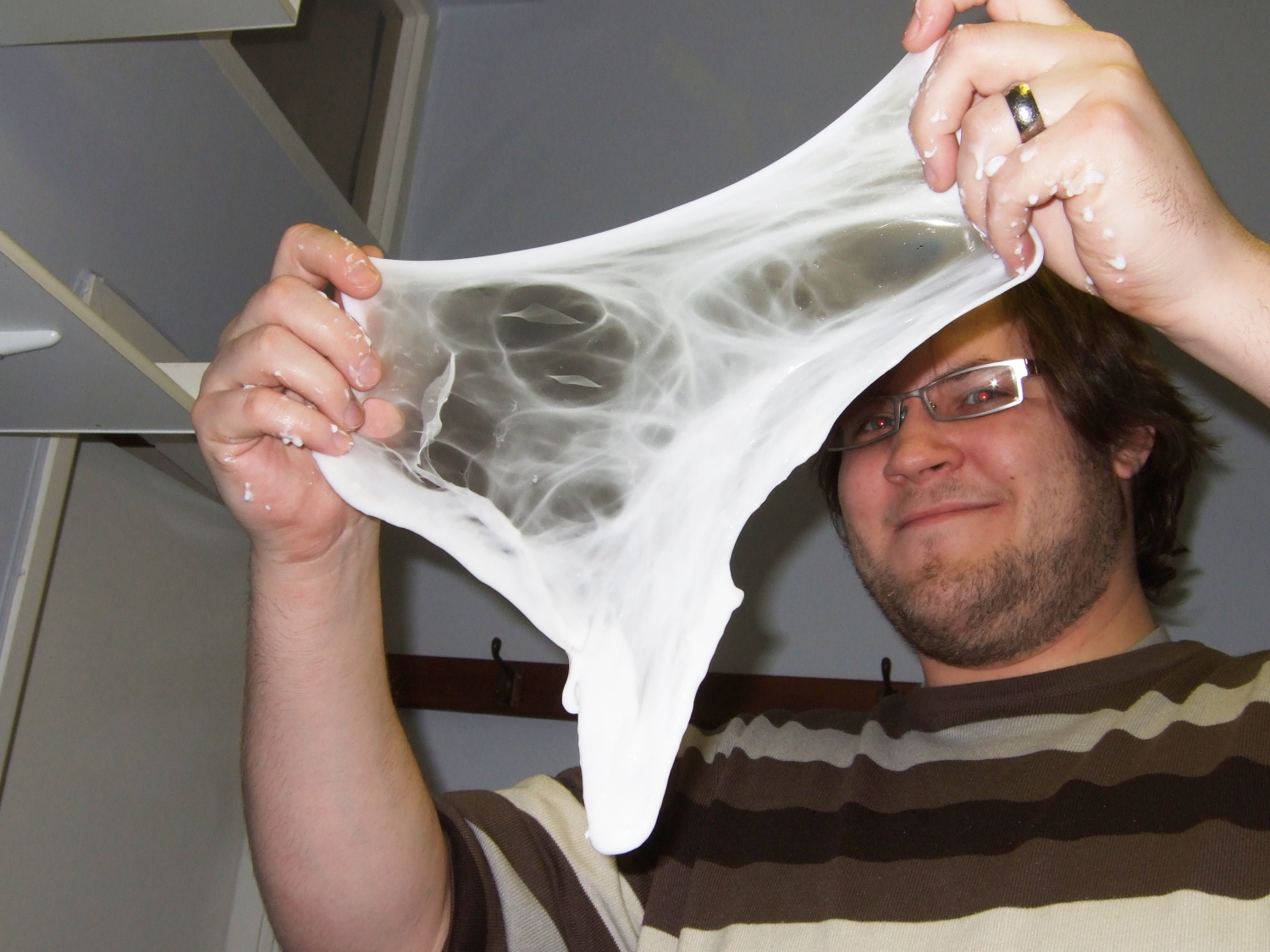 | |
| Evenually it forms a relatively dry bouncy solid which is great fun to play with. | If you move slowly the flubber will stretch |
| | |
| The flubber bounces if you throw it at the table | If you leave it the flubber spreads out - The film is speeded up by a factor of 15 |
Explanation
PVA glue is made up of PVA - poly vinyl alcohol molecules, in water. These are very long molecules which will tangle up with each other a bit when you stir the glue, making it quite thick and viscous, but there is nothing to stop it from flowing slowly.
 |  |
| PVA glue is made up of long molecules | If you stretch the PVA glue the molecules can move past one another slowly so it flows. |
The borax will form bonds to PVA molecules, and as each borax molecule can bond with more than one PVA molecule, it acts to stick them together. If there is just a little borax this will effectively make the PVA molecules longer, and it will go much more slimey. But if you add enough Borax it will form a great big interconnected network which will distort if you pull on it but will then wriggle back to its original shape, Molecular vibrations, a result of it being warm, cause each molecule it to become less straight. This means that it is stretchy like rubber and will bounce.
 |  |
| The borax crosslinks different PVA molecules together. | This means that when you stretch the tangled network parts of the PVA molecules straighten out. Then when your let go they wriggle to become shorter again. |
Why does the flubber flow very slowly?
The bonds between the borax and PVA are not very strong so occasionally they break and reform somewhere else. Each time this happens while the flubber is stretched it will flow slightly, and so it flows very slowly.
- Previous Silver from soot
- Next Science of fruit jellies










Comments
Add a comment