Derek - Hello and welcome to Colchester County High School, which is where we've come this evening. A very warm welcome to you. With me is a scientist new to the kitchen science feature and who's going to do a great experiment you can do at home. Please do listen out for the details because it's very easy. Could I get you to introduce yourself?
Sheena - Hello my name's Sheena Elliott and I'm a PhD student at Cambridge University studying physics.
Derek - Ok, and just quickly, what have we got set up? What are we going to be looking at today in kitchen science?
Sheena - We've got a Cartesian diver, and what we're going to do is simulate how a submarine works in a lemonade bottle.
Derek - Fantastic and that's all coming up. Also with us is a student from Colchester County High School, so would you care to tell me your name and what year you're in please?
Amanda - My name's Amanda and I'm in upper sixth.
Derek - Excellent, thanks for coming along. We love to know from all our volunteers whether they like science. So are you doing science at school?
Amanda - I'm doing all three sciences and maths.
Derek - Ok so are you going to carry on and do more of that?
Amanda - Yes.
Derek - So we already have a convert but hopefully we'll be switching you onto science even more. Right then, to do this experiment at home, you will need some very simple things and hopefully you will have them. Firstly, a straw or a pen lid. Some kind of drinking straw or the lid of a biro will work very well. Secondly, you need a lemonade bottle. The bigger the better basically: if it's as big as two litres then that's very good and it's needs to be emptied and filled with water. Thirdly you need some blu-tac or some plasticine, and that's it. Now Sheena's going to tell us what to do with these things and how to set it up.
Sheena - First we need to take the drinking straw which we might cut up into pieces two or three centimetres long, or if not we can use our pen lid, and then we need to block up the ends. If it's pen with two ends, then we need to block it up so there's only an air pocket inside it. It's the same with the drinking straw; you just block up both ends. So what you want is an air pocket enclosed in the drinking straw or the pen lid. It has to be a very good seal. You then need to make sure that it floats, but you have to make it so that it's only just floating. You can practice in a mug of water. Put it into the water. If it floats too high then add a little more plasticine. If it sinks to the bottom then you might have to take some off and play around with it until you get it just right.
Derek - Ok, so the plasticine is weighing it down and you just need to get the right amount.
Sheena - Yes that's right.
Derek - And then what do you do with the lemonade bottle?
Sheena - You want to fill your lemonade bottle right to the very top, right to the brim, so that there's no air at the top. Then you want to put in your little pocket of air which was your straw or pen lid and just it in the top. Then you need to tighten the lid on the bottle quite hard.
Derek - Ok so that's the set up. Then what do people do?
Sheena - All people have to do is squeeze the bottle and see what happens.
Derek - So there it is then. What you have to do is get that air pocket, which could be a pen lid or a piece of straw about one inch long, and plug it at both ends with the right amount of plasticine so it just about floats. Then fill a lemonade bottle right to the top with water and put the little air pocket thing in the top and put the lid on. Screw it tight, squeeze the bottle and see what happens. Of course, you can do this at home, and we're going to be doing later on in the show. Of course, we have a volunteer who's going to be doing it, and I wonder what, Amanda, do you think's going to happen when we do this?
Amanda - I don't really know but I think it might rise.
Derek - Any reason? Why do you think that that might happen?
Amanda - I don't know.
Derek - Ok, that's fair enough because all will be explained later and Sheena will be telling us all about how this relates to real world things that we see around us. Anyway, you at home, we want you to do this too and hope you've got all the details now. If you like, then please call us with the result because there are prizes! So until later in the show, we'll be waiting poised next to this air pocket inside our lemonade bottle to see what happens. Do come back to us later at Colchester County High School. Goodbye until then.
LATER....
Derek - Hello again and welcome back to Colchester County High School where we have been waiting for the last part of the show to do this experiment with a lemonade bottle, which has a kind of floating thing ready inside it. Sheena's here too, and Sheena, could you instruct Amanda about what she has to do now?
Sheena - Ok Amanda. All you need to do is step towards the bottle, put your hands around it and give it a little bit of a squeeze.
Derek - And tell us what you see.
Amanda - It's gone down.
Derek - Ok tell me more. What's gone down and how far has it gone?
Amanda - It's gone all the way to the bottom.
Derek - Ok and when you release your hands, what happens then?
Amanda - It rises up again.
Derek - So what we've seen then is that when Amanda squeezed the bottle, the bit of straw with the plasticine on the ends and the air pocket inside sank all the way to the bottom of the bottle. As soon as she took her hands away and released the pressure from the bottle, it came back up to the top. So Amanda, have you any idea why that's happened?
Amanda - I have no idea.
Derek - Well that's ok because Sheena Elliott is here who set up this experiment. So Sheena, what's happening here firstly?
Sheena - Well before we can understand what's happening we really need to know why it floats. What's the difference between something that's floating and something that's sinking? For this, we're going to turn to Archimedes' law. What Archimedes said was that for something to float, then the amount of water it displaces has to weigh more than the thing you're trying to make float. When we have our straw floating to begin with, it's obviously taking up more space of water and that water weighs more than the straw ensemble with its plasticine. Therefore it's floating.
Derek - Ok, let's just have an example here. I'm imagining something very big and very light like a big piece of polystyrene. I suppose that takes up much less weight of water if you kind of put it on the water and so it doesn't displace any and it doesn't sink.
Sheena - Yes exactly. It's all down to density because you're talking about the amount of volume it takes up and the amount it weighs. What it really comes down to is that if it's less dense than water then it will float and if it's more dense than water then it'll sink.
Derek - So we've made something there, the bit of straw with plasticine on the end, that's just about less dense than water. So what happened then when Amanda was squeezing the bottle?
Sheena - When we squeeze the bottle we're increasing the pressure inside the bottle. That's inside the whole bottle that the pressure is increasing. The thing is that water is very difficult to compress but air is comparatively much easier to compress. So by compressing the air inside the straw, you're making it smaller. So although it weighs the same, it's taking up less space so it's density has increased and has therefore increased in density relative to the water and begins to sink.
Derek - So when you squeeze the bottle you squeeze the water in the bottle, but because it can't be squeezed it has to take it out on something, and that is the straw.
Sheena - Yes exactly because the air is easier to compress than the water.
Derek - So when do we actually see this effect in the practical world?
Sheena - This is actually how fish can control their height in the water. They have little air sacs inside them and muscles to compress those. So the fish is actually changing its density by compressing these air sacs. It's also how submarines work and how their ballast tanks work. They let water in to increase their density and they pump water out to decrease their density.
Derek - So a ballast tank is really like this air pocket. They just compress it however much they want.
Sheena - Yes. That's just how they work.
Derek - Wow. Amanda does that make sense to you?
Amanda - Yes it does.
Derek - Yeah, I've always wondered how submarines and fishes managed to do that. What about you?
Amanda - Me too.
Derek - Well it's been explained. So how did you like our experiment?
Amanda - I really enjoyed it and it was totally amazing.
Derek - Will you be going home and doing the same again with everyone you know?
Amanda - I will.
Derek - Excellent, a convert! We make lots of these on this part of the show. Well thanks very much to Amanda who's at Colchester County High School and also to Sheena Elliott who will be back with us doing some more kitchen science. So that's all from Colchester County High School and we will see you again for more kitchen science somewhere in the East of England. Until then, goodbye.
Ingredients
 | A large lemonade bottle full of water |  | Some plasticine or blue tack |
 | A pen lid |
It also works with a ketchup packet weighted with a paperclip instead of the pen lid
Instructions
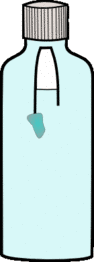
1- If you are using the penlid, use some plasticine to block the hole in the top, then add plasticine to the bottom of the lid until it just floats
2- Fill the bottle up to the top
3- Float the lid or sachet in the bottle.
4- Put the lid on the bottle
5- SQUEEEZE
Result
When you squeeze the bottle you should find that the diver ( the penlid / sachet ) sinks and then when you stop squeezing it should float again. You should be able to repeat this as often as you like.
Explanation
If something is surrounded by a fluid, it gets an upthrust (push upwards) that is as strong as the weight of the liquid it is pushing out of the way. This means that if an object takes up more space than it's weight in water (is less dense than water) it will float, and if an object is smaller than it's weight in water it will sink.
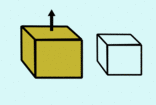 | 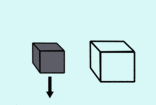 |
| less dense means it floats | more dense so it sinks |
Both the pen lid and the sachet have an air pocket in them - the one in the sachet is better hidden, but you may be able to see it if you hold the sachet up to the light.. This makes them quite a lot bigger and so gives them lots of upthrust, whilst only increasing their weight by a minute amount, so it makes them less dense.
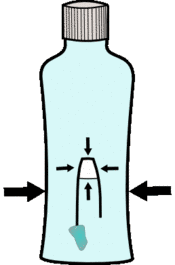
If you squash the bottle, because the water is virtually incompressible this pressure is transferred to the air pocket, squashing it and making it smaller. This makes the penlid smaller so it is more dense and sinks.
If you stop squashing the air will expand again reducing the lid's density so it floats again.
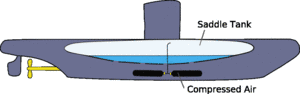
How is this related to a real submarine?
Real submarines can affect whether they float or sink in a similar way. They have large tanks on their sides which they can fill with water to increase the submarine's density and sink, or fill with compressed air from tanks inside the submarine to reduce their density agian so they float again.
- Previous Making music with wine glasses
- Next Why is the sky blue?










Comments
hello i think your experience
hello i think your experience is very interesting so please continue your work
Add a comment