We were asked why councils don't use seawater to melt ice on the roads, so why not find out the answer with an experiment?
Ingredients
 | Ice |  | Salt and optionally screenwash (be very careful, this should definitely not be drunk) |
 | Small containers |  | A thermometer |
Instructions
Mix up some seawater by adding about 9g of salt to 250ml of water (or if it is easier, by dipping some out of the sea).
Fill several containers with ice.
Add some seawater to one container, salt in another, and some screenwash in a third.
Measure the temperature change.
Result
You should find that all the ice that you have added salt or antifreeze to will reduce in temperature, but the stronger the solution, the greater the reduction in temperature.
| If seawater is added to ice the temperature falls to just below 0 celcius. | |
| Adding salt to ice, the temperature drops very significantly | |
| It would appear that this screenwash is not very good, and would explain why it froze in my car. |
Explanation
Salt, and other soluble substances will reduce the melting point of water, so if you put salt on ice, it will now be above its melting point, and start to melt. The stronger the solution, the more it reduces the melting point, so strong solutions will melt ice to lower temperatures than weak ones.
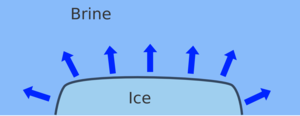 | 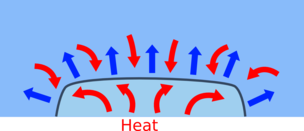 |
| When you add brine (salt solution) to ice the melting point of the ice will be reduced and the ice melts | This takes a huge amount of heat energy, which comes from the surroundings, cooling them down, until the ice melts or reaches the freezing point of the brine. |
Melting ice involves breaking bonds between the water molecules, so it requires a huge amount of heat energy: This means that it will cool down as it melts, until ether it all melts or it reaches the melting point of the salt solution you have applied. The temperature the ice gets to gives you a good idea of how cold your de-icer will work on the roads.
Why does ice melt when you add salt?
The surface where ice and water meet is a very dynamic; molecules are always melting off and others freezing onto the surface. When the ice is stable (i.e. neither freezing nor melting) these two processes are still happening, but they are in balance. If you reduce the temperature the probability of a molecule melting off will reduce, so the melting slows down, and the freezing speeds up, which means that the ice grows. If you heat it up then the opposite will happen, and melting will dominate.
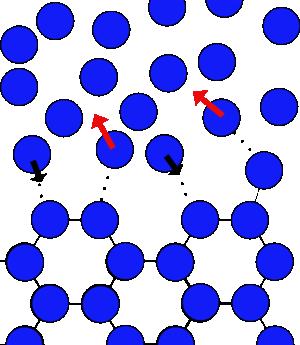 | 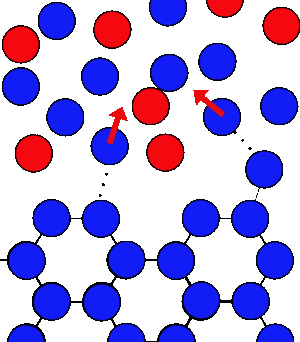 |
| At 0°C there can be the same number of water molecules joining and leaving the ice. | When salt is added there is a lower concentration of water molecules in the liquid to come back and form ice, so the freezing slows, whilst the melting rate is unchanged. So overall the ice melts |
If you add salt, or any dissolved substance, the water molecules will effectively get lost amongst the salt, and take longer to make it back to the ice, so the rate of freezing will slow down, but the rate of melting will be unchanged, so overall the ice will melt at 0°C.
At lower temperatures the rate of freezing is higher, so there is a new freezing point below 0°C. In fact, the lowest temperature it is possible to get water using salt is around -18°C, and this was roughly how 0°F was defined (although using Ammonium rather than Sodium Chloride).
- Previous Exploding Film Canisters
- Next Swapping Sounds










Comments
Thank you for your prompt
Thank you for your prompt reply. But I did not get the reply for my query regarding gain in enthalpy by freezing water which rises when temperature goes down. How can there be gain in enthalpy by freezing water instead of loss.My presumption is that the gain enthalpy referred here is by environment. Please confirm.
The sentence in the above
The sentence in the above explanation "so if you put salt on ice it will now be above its melting point" is confusing .Can you elaborate on it
yeah but what are the sources
yeah but what are the sources of error becasue whe ni looked it up i found this and after reading everythign i couldnt find the sources of error and any improvements whats so ever
Maybe you could suggest some
Maybe you could suggest some improvements?
Add a comment