Pig liver transplant breakthrough, and weird early galaxies
In this edition of The Naked Scientists: the first transplant of a gene-modified pig liver into a human; also, the James Webb Space Telescope sees one of the first galaxies ever to exist, and it’s very strange indeed; and scientists explain why we can’t recall our early years…
In this episode

01:02 - First gene-modified pig liver transplanted into human
First gene-modified pig liver transplanted into human
Kourosh Saeb-Parsy, University of Cambridge
Chinese scientists have carried out the first known transplantation of a gene-modified pig liver into a human. For ethical reasons, the experimental procedure was performed on a brain dead patient, and showed that the grafted liver remained functional for the 10 day duration of the study. Kourosh Saeb-Parsy is a transplant surgeon at the University of Cambridge, and Addenbrooke's Hospital. He gave us his reaction to the announcement…
Kourosh - What this group were able to do for the first time was to take part of a liver from a pig and transplant it into a patient who was already brainstem dead and show that this liver is able to function after transplantation into a person.
Chris - Why are we going down this route?
Kourosh - This is a really important question. Sadly there are many patients in the UK and around the world that are dying because we don't have enough organs for transplantation. For many conditions where the organs are failing there isn't any other life-saving treatment other than transplantation. Sadly we just do not have enough organs for transplant at the moment.
Chris - Unlike a kidney that we can make a machine to do dialysis, not perfect but it keeps people alive, is there nothing that can do something like that for a liver?
Kourosh - Nothing that is sufficiently effective that can keep somebody alive for a substantial period of time. So really if somebody has very pronounced failure of their liver, liver transplantation is the only life-saving treatment we currently have available.
Chris - This group which have written this paper in Nature, what did they actually do then? What organ did they use, where did it come from, how did they get it into the patient etc?
Kourosh - We've been thinking about xenotransplantation or transplantation from animals into humans for a very long time. In fact the very first organ transplant to patients was done with monkeys. Now those organs all failed because there's so many differences between the cells and the tissue between an animal and a patient. So what this group and others have done is to use current technology to modify the genes in pigs in this case. What this allows them to do is to basically remove some of the molecules on the cells of the pig that would result in very rapid rejection of the pig organ. So this is one of the advances that this group have made and that's allowed the organs to be transplanted at least for a short period of time into humans and for the organs to work. What they did in this case was not replace the complete liver but rather put a part of a liver to basically allow that liver to function in the patient.
Chris - You said they did this in someone that was already dead, they were brain dead. Why do it with that sort of person?
Kourosh - This technology is still in its early stages and therefore there are risks with it. We do not know whether these organs will function for the long term. There is a risk to patients who receive these treatments and here it was decided that actually it was appropriate for the organ to be first transplanted into a patient who was already brainstem dead. That means the patient is legally dead and therefore there is no risk of harm to that patient. So this is a first step to ensure that we can test transplantation from pig organs, pig livers in this case, without causing harm to the patient.
Chris - What did they measure in order to work out whether it was working so we know that were we to go the next step and put this into a person who really did need a liver that we'd have a chance of success?
Kourosh - The liver is a complex organ and performs a variety of functions. So some of these is to for example manufacture or make various proteins and various molecules or clear various toxins from the blood. So they used a combination of these tests to show that the liver that was transplanted was actually performing these functions. So this is a good step forward in this particular case. The patient, if you like, a deceased patient wasn't fully relying on being kept fully supported by the pig organ but they did show that the pig liver was functioning.
Chris - Given that we're not pigs and pigs will make slightly different versions of the chemicals that we have in our bloodstream, it may make some chemicals that we don't have, it may not make some chemicals that we do have. Are those gaps going to be showstoppers or is there a way around that?
Kourosh - That's a great question. There are significant differences between different species and pigs and humans but there are some fundamental biological processes that are preserved through evolution. So for example a removal of toxins or manufacturing some proteins are preserved. Now these pigs are being genetically edited to try and reduce the number of differences between the pigs and the humans. However that technology is not sufficiently advanced to make those organs identical and this is one of the potential side effects of using these organs in the future.
Chris - When they did it. Critically, did it work?
Kourosh - It worked sufficiently for us to want to continue to explore this approach. So for example it would have been a complete failure if they were not able to sustain blood flow into the organ or the organ disintegrated or failed to function. They did not see that. So the results were sufficiently positive for us to continue to do this. And it's also worthwhile saying that other pig organs, namely kidneys and heart, have also been transplanted into patients and have continued to function at least for a period of time.
Chris - So to your mind then, as a transplantation surgeon very much involved in this particular space, what does this paper say to you? What do you think the implications are?
Kourosh - This paper I think provides a hopeful strategy for meeting the demands that we have for the number of organs to be transplanted. I think this will hopefully in the future form part of the solution and help save lives. It is unlikely to be the only strategy. There are other technologies that are being investigated such as printing new organs or bioengineering new organs. So it is likely that this will form part of the repertoire of treatments that we will have for patients in the future.
Chris - There's an old saying, a cynical one, that is xenotransplantation is the future and always will be. They say the same thing about nuclear fusion. Do you think though that before long you and I will be sitting here having a conversation about how this is naturally taking place? Do you think this is literally on the cards now that we're going to start doing this? It's sort of gazing into your crystal ball.
Kourosh - That scepticism I think is well founded. I mean we've been trying to do xenotransplantation for a very long time and we've not been successful. But the number of critical things have happened in recent years, probably in the last five to ten years, and that's our ability to modify more and more genes in pigs. So therefore get them closer and closer to what a human organ would be. And critically these organs are now being transplanted into living patients including hearts and kidneys. So I think we are in the cusp of acceleration where the technology and this approach will become more and more suitable. Now how long that will take remains to be seen. There are still significant challenges and hurdles to be resolved but potentially in the next five or ten years we can see more pig organs being transplanted into patients.
08:18 - James Webb Space Telescope spots mysterious early galaxy
James Webb Space Telescope spots mysterious early galaxy
Joris Witstok, University of Copenhagen
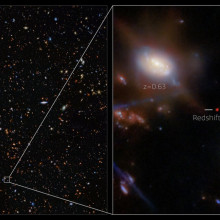
Our Universe formed about 13.8 billion years ago. At some point - we think after just a few hundreds of millions of years - the first galaxies formed, and the first stars began to ignite. And now, believe it or not, we can see some of them. The James Webb Space Telescope can detect the red-shifted light emitted by those first galaxies, giving us our first glimpse of what the very young Universe looked like, and how it went on to form the fabric of space - and the bigger galaxies like our own - that surround us today. This is amazing stuff, and one of the scientists behind these new observations is Joris Witstok, a space scientist at the appropriately-named Cosmic Dawn Centre at the University of Copenhagen…
Joris - One of the really fundamental questions about the early universe is when exactly we had the first stars and the first galaxies. So we start out after the Big Bang with a completely empty universe. All we have is hydrogen and helium. They start to contract by gravity, which starts to form stars. And one of the main questions we're trying to answer is when these first stars start to have an effect on their surroundings.
Chris - Why is that a challenge then, seeing back to that time, what's in our way that prevents us getting a clear picture of that?
Joris - The main challenge here is just the distance. So when you want to look at these very early galaxies, what we're exploiting is that the light takes a finite amount of time to reach us. And so in our case, we're talking about light that's been traveling for over 13 billion years, so really the majority of the current universe's lifetime. And during that time, the universe is continuously expanding. What that means is the light we're seeing from these very, very early galaxies actually gets stretched by a factor of up to 14 in this case.
Chris - And so by picking up the light that's still here today, but very stretched out, we can extrapolate to what that would have looked like over 13 billion years ago. So we can get a glimpse into the early universe, but it's tricky to do because the light must be very dim.
Joris - Yes, the light is very dim and it also shifts across wavelengths. So whereas this light was originally radiated in the ultraviolet, it's actually been stretched out all the way into the near infrared. And that's where we're seeing it with the James Webb Space Telescope.
Chris - What has this revealed to you? Now we've got this in our hands, the ability to really push back the envelope of time in this way. You are able to see back how far do you think and what are you seeing?
Joris - Yes. And in this case, we're looking at a galaxy that we're seeing as it was 330 million years after the Big Bang. What it looks like, if you actually look at the image of the galaxy, it's very, very small. This one in particular measures less than 230 light years across, which sounds fairly big, but for a galaxy that is really tiny.
Chris - The Milky Way equivalent would be about 100,000 years, wouldn't it, for our own galaxy right now. So that is really very small.
Joris - Yes, about 10,000 parsecs or 100,000 light years. But at the same time, it's forming stars at a rate of about one star per year, which is the same as the entire Milky Way together. So it's really compressed all that star formation into this tiny, tiny region. So in that sense, these are really quite extreme galaxies. But what was really unique about this one is that when we looked at the different colors of light, we saw that one very specific color stood out and had an enormous brightness. We know that this color of light corresponds to the emission from hydrogen atoms. And this was telling us some very interesting things about what was going on inside the galaxy.
Chris - And what were they?
Joris - It's telling us two things. So one of them being the source of light inside this galaxy must be extremely powerful, really as powerful as anything we know of. We know that if we take stars, for example, these can't be normal, regular types of stars like we see in the Milky Way. They have to be somehow extremely massive and hot in order to be powerful enough to explain the feature that we're seeing. The other option there is that we're actually looking at a black hole inside the galaxy. And what happens there is that there's a lot of gas that's being consumed by this black hole. And as the gas reaches close to the black hole, it's heated up to incredibly hot temperatures and becomes very, very bright as well.
Chris - Do you think that that massive black hole would have been born out of one of those massive stars exploding, collapsing in on itself, forming the black hole and the black hole then fed on all the other stuff? So you could have actually both models going on very massive, bright, hot stars, but they also produce a massive black hole that then produces brightness. So it could be both things.
Joris - Yeah, definitely. This is one of the possibilities. And this is exactly the kind of question we're trying to answer. And at the moment, we can't say conclusively which of the possibilities it is.
Chris - Do you think the reason that what you're seeing is so small is because the black hole's eaten it all?
Joris - Yeah, that is one of the pieces of evidence that could point towards this being a black hole that does fit in with how compact we see this galaxy to be.
Chris - What's the fate then? Once you've got those initial, very small, early galaxies with potentially massive black holes, supermassive black holes at their centres, what's the next generation then? How do they feed into where the universe evolves to next?
Do they disappear or do they then act as the nucleus to form a bigger next generation galaxy? What do you see happening next?
Joris - The current best understanding we have is exactly that. These tiny, tiny, let's call them baby galaxies grow into present day big galaxies. So what we're seeing at the moment might be the very core of a very, very big, massive galaxy that's seen today, such as the Milky Way.
16:14 - Why we can't remember being a baby
Why we can't remember being a baby
Nick Turk-Browne, Yale University
Have you ever wondered why you can’t remember being a baby? New research suggests it’s not because we didn’t form memories at that time, but because we can’t access them. The study found that babies do indeed appear to be encoding memories in the brain’s hippocampus from very early on. This agrees with other previous studies done using mice, and it leads to the tantalising hypothesis that all of us may potentially have infant memories locked away in our heads that we can’t directly recall. Here’s Nick Turk-Browne, a professor of neuroscience at Yale University…
Nick - We learn an astounding amount as infants. It's in fact, I think, the greatest period of learning in life. And so it's particularly striking in that context that we're lacking a specific kind of memory from that time, which is memory for specific events in space and time. That's the kind of memory we're studying here. It's called episodic memory. That kind of memory is what's missing from early life. In this case, we tried to hone in on when this ability to store specific memories for events in our life comes online.
Chris - So were you actually brain scanning babies to do this?
Nick - Yeah, we have been working for several years to figure out how to do this kind of research in babies. And this is very challenging. They're the worst possible study participants for this kind of a method because you can't move a millimetre. Imagine taking a photograph in low light with your phone. If the subject moves at all, you get a blurry image. It's very similar in the case of functional MRI. If the baby moves, then we can't figure out what's going on in their brain. They're also challenging because they have very short attention spans. And to perform these kinds of experiments, you typically need a lot of data. But in a baby, we're lucky if we get five or 10 minutes of data. So we had to design really engaging tasks that the baby could do to probe their memory.
Chris - Talk us through what the experimental design was then. What did the babies have to do that enabled you to get at this question of, well, are there memory circuits for episodic memories? What I ate for dinner last night, are they working or not?
Nick - Well, most of the babies we're working with were pre-verbal. They can't speak and possibly can't fully understand language yet. And so you can't tell them what to do or ask them to do things. So we had to come up with indirect ways of probing what a baby remembers. And so in this case, we're going to use a measure called looking time. Where a baby looks sort of reveals what they know. And so we can use patterns of looking time to figure out what it is that they remember. The experiment involves showing them photographs of faces and objects and places. This is while they're lying in the fMRI machine and we're recording their brain activity. And then we test their memory by showing them two photographs at a time. And one of those photographs was something that they had seen earlier in the experiment. And the other photograph was another image from the same category. So it might be the same woman's face paired with another face that they hadn't seen before. If they remember having seen that woman's face before, they'll look longer at that photograph than at the new one. And so with this, we can quantify how strong their memory was for that picture. And based on that measure of where they're looking and their memory, we can go back and say what was happening in the brain when they first saw that picture. And we can compare brain activity during that period of storage with what was happening in their brain when they saw a picture that they later didn't remember. And so that's the basic logic of this experiment is to compare brain activity during encoding. That is the initial storage of a memory for things that are later remembered in this case, looked at more versus later forgotten.
Chris - And where do you, based on these experiments, see the bottleneck then? Is it that they don't encode or make the memory in the first place? Or is it that there is some kind of issue with encoding that into long-term memory? Or is there a third possibility? They can do all those things, but they just don't know where they encoded the long-term memory, so they can't get it back.
Nick - Yeah. What we demonstrate in this particular study is that hippocampal activity is greater when babies are looking at something for the first time that they later remember. And so what this study establishes is that beginning around 12 months of age, the infant hippocampus has the capacity to encode memories. What this means is that our inability to remember early life, or what's called infantile amnesia, is not a deficit of storage. Memories are getting into the system, but how long they last and why they're not accessible are the next questions that we're asking.
Chris - And why do you think that might be the case?
Nick - Yeah. Well, there's some really beautiful animal research in mice where you have a baby mouse, they have some experience using molecular techniques. You can tag which neurons help to store that baby memory. And then when the mouse grows up to maturity, which in the case of a mouse is just a few months, then you can stimulate those neurons that were part of the memory that was formed in the baby mouse, and they'll express the memory. So there's evidence in these experiments that the mice are able to form memories as a baby that persist until when they're adults. And so what this suggests is that at the limit, this may be possible in humans that into some period of childhood or perhaps beyond, that we may still have these memories in the hippocampus, but that they're not accessible. So what's going on is a deep mystery. There's one data point in the mouse experiment that's really, or mice experiments that's really critical, which is if you just put the mouse back in the environment where they formed the memory as a baby, now when they're an adult, they don't show memory. So just being back in the environment wasn't enough. Their hippocampal memory had to be stimulated directly. So what this tells us is that in humans, as we grow up, we may have memories in our brain that are inaccessible because the cues from the environment might not be enough to retrieve the memory. And this is the hypothesis that we're developing now. This is just a speculative theory that, whereas people originally thought that infantile amnesia was because the hippocampus is immature, I actually think what might be going on is that the rest of the brains develop and may be responsible.

22:42 - How biological enzymes are fighting the plastic crisis
How biological enzymes are fighting the plastic crisis
Andy Pickford & Brooke Wain, University of Portsmouth
The work was carried out with the support of UK Research and Innovation.
Plastic is an amazing material that has revolutionised our world - but at a staggering cost. From food packaging to medical devices, it’s quite literally everywhere. Yet its durability means it also lingers long term in our environment, to the detriment of - as we heard last week - our health, and the health of animals and even plants. With global plastic production set to double by 2050, the need for sustainable solutions and, particularly, better ways to recycle and reuse the constituents of plastic is a high priority. One area showing considerable promise is the development of enzymes, originally sourced from nature, that can attack certain plastics and break them down into their constituent building blocks, or monomers. Scientists are in the process of evolving these enzymes to optimise their activity. Will Tingle has been along to see one such venture in action…
Will - The world is producing about 400 million tonnes of plastic every single year, and it's not going anywhere by itself. We can burn it, we can bury it, but A, those are not permanent, and B, we just end up needing to produce more plastic. So, how can we close that plastic loop in a way that's sustainable and useful? Well, I've come down to the University of Portsmouth to visit the Preventing Plastic Pollution with Engineering Biology, or P3EB, Mission Hub, to find out more.
Andy - My name's Professor Andy Pickford. I am the lead of the P3EB Mission Hub. The P3EB mission is to advance technology for biological recycling and upcycling of plastic waste, so to support this transition towards what we call a circular plastics economy.
Will - Now, the means of plastic breakdown that P3EB is heavily focused on here, it seems, is using enzymes, biological molecules, to break down the bonds in plastic polymers. This isn't a novel concept, though, is it? We have been doing it for years, so how is that idea being furthered here?
Andy - Yes, we've been breaking down plastic with enzymes, certainly in the laboratories for a decade or so. And for certain plastic, like polyethylene terephthalate, or PET, we are nearing industrial application of the technology, but we really need to continue to advance the technology, not just for PET, but for other types of plastic, and make it more economically viable, so that recycled plastic becomes desirable from an economic point of view.
Will - Why is plastic traditionally so difficult to break down? Is it a case of the bonds in those molecules, in those plastics, just we've designed them to be so tough we now don't know what to do with them?
Andy - Yeah, that's certainly part of it, and certain plastics have different strengths of bonds within them, so as I mentioned, we've really made great advances in the breaking down of PET. PET has ester bonds in it, and these are actually quite weak bonds, and so enzymes can target those bonds and break the polymer chain down into its constituent molecules.
Will - Is it this way? It was time to take a trip down to the lab to get the breakdown on the breaking down.
Brooke - I'm Brooke Wain, and I'm a final year PhD student at the Centre for Enzyme Innovation.
Will - Okay, I appreciate this is one of the most difficult and convoluted questions of our time. If we want to use biological enzymes to break down plastic, how do you even start looking for the right thing to use?
Brooke - Well, to start with, it's the enzymes themselves. So plastics are polymers, similar to natural polymers, they comprise of monomers connected together by bonds which produce the resulting polymer. Now, in nature, you have enzymes which target those bonds to break down the polymer into the monomers, and natural polymers are things such as cutin, which is the waxy cuticle layer on your leaves that you see, that shiny surface, and there's enzymes in the natural environment which break down that cutin layer, called cutinases. So one large part of our research into enzymes which break down plastic focuses on engineering and taking those cutinases to be able to break down the synthetic plastics such as polyester. Natural enzymes are typically not very fast and not very tough, so we can introduce changes to their structure to improve these characteristics. So here at Centre for Enzyme Innovation, we are blessed because on one floor, in one huge lab space, we have the whole process start to finish. We do the discovery of the enzymes, we do the engineering of them, various approaches to work out the optimal conditions for each enzyme. Once we have a really fast, efficient enzyme, we take it to the next lab, which is where we do all the bioprocessing. That's these noises you're hearing now, these are bioreactors. Now, we can use these in two ways. We can grow them up and use them to recombinantly express the protein we need in high yields, which is also important because if you want to break down a lot of plastic, we're going to need a lot of enzyme in the lab to be able to do that. The other aspect is we actually do the plastic digestions in these bioreactors. So because one of the monomers released after PET breakdown is an acid, we can then use a base to be added to the system to neutralise the pH, which keeps our enzymes happy, but also allows us to quantify and track that reaction.
Will - As you said, we've got sort of jars of ground down what was once plastic alongside spinning milky vats of fluid. Are we looking at this breakdown process in action?
Brooke - Yeah, you're seeing exactly what I was describing about how you have that enzymatic digestion in a bioreactor. If you see this bottle on the right, this has the sodium hydroxide, which as the pH changes inside the vessel, it will automatically register, detect that change in pH and start adding in the sodium hydroxide to neutralise the pH so we can quantify how much TPA that acid monomer is being produced. Once we finish an enzymatic digestion, we can filter off anything that's undigested and characterise that.
We can pass it through activated carbon to remove any organic contaminants, anything like that. We filter it and once you get this, what we call a monomer soup, we can drop the pH to crash out the TPA. We can filter that and we get almost quite pure terephthalic acid ready to use and ready to dry for a future reaction. The other part is ethylene glycol, which is a bit more tricky to get out. Now this is not ideal, but there's technologies that are going on at the to improve this monomer recovery aspect.
Will - When this process is finished, what are we going to be left with?
Brooke - Once this bioreactor digestion has finished, what you have inside that vessel is a combination of bits and bobs. So you have undigested PET plastic, you also have buffer, you have leftover monomers, but that's it. The highlight of this technology is that those monomers can be purified and then either up-cycled into alternative resources or repolymerised back together to make the same virgin quality PET along with the catalyst.
Will - We're back now with you Andy and I can't believe I'm saying this, but thank you for a fascinating tour of a chemistry lab. But the obvious question is, once you've broken down these polymers into their constituent monomers, their building blocks, what do you do with them? Do they go back into making plastics? Do they go into making something completely new? Or is it a bit of both?
Andy - We can simply stitch them back together in a very simple chemical process to remake plastic that has identical properties to a virgin plastic, as we call it, that you might make from oil and gas. Or we can take those chemical building blocks and we can turn them into something else. So the terephthalic acid that you get from PET could be converted into fragrances or flavourings such as vanillin, and the ethylene glycol could be fed to microbes to turn into all sorts of other chemicals.
Will - To take nothing away from this fascinating science, 400 million tonnes of plastic year is an almost incomprehensible amount. This does need scaling, doesn't it?
Andy - Yes, scaling of this process is actually ongoing. One of our project partners on the Mission Hub, Carbios, a French biotechnology company, have a pilot plant currently running with this technology and they are building an industrial plant which they aim to open in 2027, and that will handle 50,000 tonnes of PET waste per annum, so equivalent to about five Eiffel Towers. There's clearly a considerable way to go, but we are at the point now where this can be scaled up.

31:32 - Should a 73-year-old exercise on a trampoline?
Should a 73-year-old exercise on a trampoline?
So, can trampolining put a spring back in your step, Francisco? To help us find the answer, we reached out to John Travers, author of 'A Shot of Hope: Stories of Quiet Resilience' and clinical senior lecturer at Trinity College Dublin.
John - My work in reversing frailty and building resilience in older adults has made me a big advocate for resistance exercises like trampolining. This should be combined with consuming sufficient protein to turn that work into stronger muscles and bones. Jumping on a trampoline can improve cardiovascular health, strengthen muscles and bones and improve mental health.
Will - Francisco mentions coordination, specifically. Is this something trampoling can help?
John - Yes, it can stimulate the vestibular system, located in the inner ear, which gives us our spatial awareness and helps maintain balance and coordination. Jumping creates multiple changes in speed and direction which is an excellent way to support the vestibular system and sense of balance.
Despite all this potential upside, as with all exercise, there are risks. Falls and injuries to backs and knees are possible. A key factor in such injuries can be the technique used. People with existing low back pain due to pinched nerves or osteoporosis would be well to avoid these risks. A low-to-the-ground rebounder with a bar for holding and maintaining slightly bent knees when jumping is essential for safety. Enjoy the lift you will feel from bouncing but be aware of the risks and if in doubt, leave it out.
Will - So, Francisco, yes, trampolining, or rebounding, can give you all the benefits of physical activity, including a workout for your vestibular system to help with balance and coordination, but it would be wise to take some precautions before starting the exercise. Thanks to John Travers from Trinity College Dublin for the answer, whose new book ‘A Shot of Hope: Stories of Quiet Resilience’ is out this month.










Comments
Add a comment