eLife episode 17: Stress, fertility and remote-controlled sperm
In this episode of the eLife podcast we hear about controlling sperm by optogenetics, hibernation, body clocks, enzyme structure, and a way to overcome stress-induced infertility.
In this episode
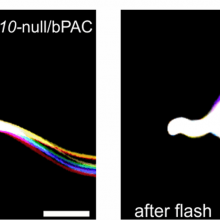
00:36 - Remote controlled sperm
Remote controlled sperm
with Dagmar Wachten & Vera Jansen, The Center of Advanced European Studies and Research.
How sperm gear themselves up to fertilise an egg is very difficult to study. For a start, standardising the conditions and then being able to reproducibly study each sperm cell without unintentionally altering its biochemistry is extremely tricky. And this is what inspired a German team to come up with a way to make remote controlled sperm that you can turn on with a flash of light. Chris Smith spoke to Dagmar Wachten and Vera Jansen from The Center of Advanced European Studies and Research.
Dagmar - We wanted to control sperm function by light. And the reason was because light has no side-effects, at least in the way we are using it. So pharmacology, for instance, when you use pharmacological substances they have loads of side-effects and we wanted to get away from that by using optogenetics.
Chris - You can shine a light on sperm and adjust its level of activity?
Vera - Yes, that's true. So, basically what we have done is we have genetically engineered mice. Introducing an enzyme that is sensitive to light and what this enzyme does is by stimulating it using light, it produces cyclic AMP. This is an intracellular messenger that is really important for sperm function. And what cyclic AMP does in sperm is that it controls sperm motility. And now, when we activate this enzyme in sperm by shining light on them, they produce cyclic AMP and thereby we can control sperm motility.
Chris - And when you shine the light one the sperm, Vera, what's the response? They make this second messenger but how do they respond? Is it just a change in motility or do other effects kick in as well?
Vera - So, various functions in sperm are controlled by cyclic AMP. One immediate effect of the increase in cyclic AMP is the change in motility which is an acceleration of the flagella beat frequency. But there are also long-term effects of elevated cyclic AMP and this the so called sperm capacitation which is a maturation process which is needed for sperm to fertilize an egg successfully. By illumination, we could actually trigger this long-term maturation process which is called capacitation.
Chris - Does this mean, Vera, that you could potentially follow the signals through the sperm cells. So, if you were to illuminate one small part of the sperm could you then unpick how the signal is propagated or cascades through the sperm turning on these different effector functions which are all involved in activating a sperm so it can fertilize an egg?
Vera - We really hope to solve this issue in the future. So, we want to expand actually the optogenetic tool box in sperm by not only introducing a photoactivated adenylyl cyclase but also photoactivated phosphodiesterase. And we want to combine these tools together with tools to actually follow the dynamics of cyclic AMP on the single cell level. And with this expanded tool box, we really hope to decipher the processes in the sperm which actually make sperm fertile.
Chris - How reliable do you think your method is from a physiological standpoint? Because if one takes a sample of sperm and shines light on them, the amount of response or the amount of light hitting an individual sperm is not going to be the same from one sperm to the next. And therefore, the degree of activation to make a certain amount of cyclic AMP may differ from one sperm to the next. So, how sure are you that this is a faithful and reproducible way to study how these sperm work?
Vera - We're really trying to use the very same light dose that we shine on individual sperm. And thereby, we can more or less get the same response in individual sperm. When we do that in sperm populations, the response is also similar in the whole population. So, we're quite sure that independent of the light dose that we're using, we get a very similar response in the whole population of sperm.
Chris - And there's no danger, Vera, that by making this particular construct, adjusting or altering the sperm in this way to make them light-sensitive, that you have in some way changed them in a way you don't know and therefore some of the responses you're seeing to light might be ones that you didn't expect or can't account for?
Vera - The side-effects, of course, we don't really know if we changed something by introducing these enzymes but the processes that we know which are regulated by cyclic AMP, these processes we checked by control experiments to show that actually the normal cyclic AMP dependent processes which take place in the sperm are not altered in the transgenic sperm.

05:30 - Heating up in a hurry
Heating up in a hurry
with Sandy Martin, The University of Colorado, Denver
When winter comes there are several ways to survive; one is to wear a thick coat to stay warm; another is to surrender to the cold and enter a state of hibernation. But with a body temperature sitting - in some cases - just above freezing - how do you bring yourself back to life? Working with hibernating squirrels, Sandy Martin has found that the animals store up not just nuts for winter but a population of messenger RNA molecules that can rapidly reboot their heat-producing brown fat tissue every two weeks...
Sandy - One of the big mysteries of hibernation is how these animals are able to rewarm their body temperatures from near-freezing back to temperatures like ours in just a matter of hours and one of the really key organs is a tissue called brown fat. It has lots of little energy power houses called mitochondria but it also has lipids that will be used as fuel to run metabolism at very high rates, not making the energy currency that normal mitochondria make called ATP but all the energy that comes from burning the fat is turned into heat. And they do this periodically, approximately every 2 weeks. So, we were interested in how this organ was able to function immediately at the time the animal decided it needed to rewarm in a few hours.
Chris - How quickly does it do that? So when the squirrel says, "Right. I'm gonna wake up. It's been two weeks, I'm now gonna come back to life for a little while." How quickly does their body temperature recover?
Sandy - Not even two hours.
Chris - Wow!
Sandy - Somewhere between an hour and a half and two hours.
Chris - That's very quick, isn't it?
Sandy - It is. They do the first part completely with the metabolic activity of brown fat and then as they get a little warmer, they start to shiver as well. And so shivering also contributes to the rewarming process.
Chris - And this is somewhat paradoxical because at these very low - sustained low temperatures - with very low metabolic activity, it's hard to imagine how the cells would very quickly put themselves into a state where they could generate so much metabolic activity literally at the flick of a switch like that.
Sandy - Yes, that's right. And although we know the signal comes from the brain, at the molecular level in the cells there has to be machinery that does the work to make the heat. That machinery's been shut off during the long period of torpor and yet suddenly, it has to work. Originally, we were looking for changes in gene expression that cycled with the activity of brown adipose tissue.
Chris - So by comparing metabolically what's going on in the two states, you can say, "Well, what's different between the tissue in those two conditions?"
Sandy - Yes, except we're looking more on it now at a molecular genetic level. So, we're going one step back in this study. We were looking at the messenger RNAs at given times across the torpor-arousal cycle. So, if you can imagine, we have animals entering torpor. Two weeks torpid and then rewarming very rapidly; and ask, "What happens to the mRNA population in brown fat?" And the big surprise to us was that there was a culprit of messenger RNAs that actually increased in abundance when the animals were very cold. And we knew from previous work, transcription didn't work in ground squirrels whose body temperature was near-freezing.
Chris - So, it's also paradoxical, isn't it? You've got these very, very cold cells and environment where they shouldn't be able to make very efficiently at all any more of these messenger RNA transcripts, yet their numbers are rising?
Sandy - Exactly, and that was the big surprise. And so, if you think about it, there are two ways to change the abundance of a single RNA given a pool of a lot of other RNAs. You can either make more, i.e. transcribe it, but we've already said we don't think there's any new transcription in the cold or you could protect some transcripts from being degraded while allowing the bulk population to be degraded.
Chris - And which is it? Is it the protecting, or is it the making more mechanism which is at play here?
Sandy - Right! At the end of the 2 weeks of torpor, the change in that of RNA seems to be strictly due to protection.
Chris - And when the animal wants to reawaken itself, how does it then deploy these transcripts? And then when it wants to go to sleep again, how does it get rid of them so that it doesn't stay too hot?
Sandy - Yeah. There's a whole lot we don't know for sure but our data suggests there's a mechanism for stabilizing transcripts that involves a particular class of proteins, called poly (c) binding proteins. And they're shown to have effects on stabilization of mRNAs and affect the ability of those mRNAs to be taken the next step further in the gene expression program, and that means to make the actual proteins that do the work. So our thought is that as animals enter torpor they can sequester the group of RNAs that are most important for metabolic heat production - i.e. that process of rewarming - at the end of the torpor bout they just hold those in a safe place; don't allow them to be used but don't allow them to be degraded. Meanwhile, the bulk of messenger RNAs in the cell are being slowly degraded. By the end of the torpor bout, these are the dominant RNAs in the cell and maybe by virtue of their interaction with these specialized proteins, are the first to go back on to ribosomes for translation and therefore the first to be replenished and activated during the arousal process. So, that's our model of how it works.
11:03 - A second body clock
A second body clock
with Florian Storch, McGill University
At the heart of the brain is a neurological clock, called the suprachiasmatic nucleus, which ticks "genetically" to keep a roughly 24 hour schedule. The signal from this "master clock" is relayed around the body where it helps to set the metabolic tone throughout the day. Disturb the clock and you suffer from jetlag; disable the clock and scientists had thought that animals would lost track of time. But if you look carefully enough another rhythm - indicating the existence of a second body clock - this one running with a 4 hour rather than 24 hour rhythm - appears. Florian Storch explained to Chris Smith...
Florian - When you eliminate the circadic clock for mice and study them in constant darkness where they are not exposed to any timing cue, these mice will not show complete arrhythmia but often times still show some rhythmicity which indicates there is another timer which drives rhythm in the range of 2 to 6 hours.
Chris - So, if it isn't the suprachiasmatic nucleus, the master clock in the brain, which is driving this 4-hour cycle; what is?
Florian - Yeah. So, when we looked at these animals under constant condition, constant darkness which are just like in the circadian clock, and we observe these 4-hour rhythms. We were thinking this rhythmicity locomotor activity can also be considered as a rhythmicity and arousal. So, when the animal jumps into the running wheel, the animal is aroused. And there is quite some literature about arousal regulation and what has been prominently associated with arousal promotion is dopamine because mice which lack dopamine are actually very lethargic and this is very different from the other monoamines. And so we were thinking maybe dopamine might play a role in these 4-hour activity cycles.
Chris - And how would you know that the dopamine was driving the activity, and that the escalation in dopamine wasn't just a consequence of the act of the activity?
Florian - When we draw our attention to mice which were lacking the dopamine transporter, and the mice will show elevated dopamine tone and show great length in the activity cycle at 12 hours about. This kind of pointed to us that the dopamine somehow is dictating the period of this oscillation.
Chirs - Why would the brain need this? What purpose could it possibly serve?
Florian - There's evidence that it actually can provide a kind of selective advantage. This has been demonstrated in the common vole which shows 3 to 4 hour oscillations in its foraging behaviour. And this is very synchronous amongst the vole population. And it's believed because they are in the synchronous fashion, coming out of the burrows and exposing them self to a predator, that it actually reduces predatory risk. So, we think that really this other oscillator which we now describe in this publication is really conferring social synchrony and it might be the reason why we have, for instance, three meals a day and not five, not one, might be due to the function of this oscillator. So, generally it confers social alignment and social synchrony which is, especially with species which are social species, might be a real selective advantage.
Chris - Now moving from an animal and being a bit speculative for a minute. There are a number of human conditions which are associated with an elevation in dopamine tone in the brain - schizophrenia and some of the psychotic illnesses is one example. Bipolar disorder overlaps with that. Do you see any association in what happens to humans afflicted by these conditions and what you found in your experimental animals?
Florian - We were really struck when we looked at sleep-wake recordings from bipolar and schizophrenic patients, how similar in some cases the sleep-wake abnormalities are to our animal models. So, there are some bipolar patients which suffer from rapid cycling. They observe a manic episode every 48 hours. And we have animals which have been treated long-term with methamphetamine which also increase this dopamine tone; and these animals will show 48-hour cycling suggesting that in bipolar and also in schizophrenics, the sleep-wake cycle operation associated with these illnesses may be based on dysregulation of this oscillator we describe here.
15:26 - Modelling molecules
Modelling molecules
with Michele Vendruscolo, University of Cambridge
Enzymes hold the key to biochemistry. Without them, life-critical chemical reactions couldn't happen fast enough to keep anyone alive. And now we know much more about how they work, we'd like to engineer these catalysts to do other important jobs. But to do that we need to know how they grab hold of their chemical substrates, and what changes in shape they temporarily need to adopt to bring about a reaction. Cambridge Chemist Michele Vendruscolo has been using nuclear magnetic resonance images of the enzyme lysozyme to model the process...
Michele - The problem is to understand how the molecule grabs another molecule and then releases it. This is a problem of molecular movement. So, we need techniques that are able to characterize the movements but a molecular level, typically nanometres. The particular technique that have used is called nuclear magnetic resonance spectroscopy, and is based on the properties of the interaction of electro-magnetic radiation with atomic nuclei. In simple terms, this is a technique that enables one to detect how molecules move. And this is the ideal technique for describing the set of movements that are needed to capture and release molecules.
Chris - Are the proteins, the enzymes, not continuously moving around all the time. So, why do you not get a sort of blurry image when you try to see those movements? How do you get them down to a resolution where you can watch them almost like clockwork to see those movements?
Michele - You actually get a blurred picture. And so the point is how to disentangle from this average information all the individual components of it. In case that we have studied, there is a fundamental stage which is called ground state, and then there is another interesting state which is called intermediate state and the intermediate state has a much lower probability of being present but is essential because it's the state that the molecule that is acted upon by enzymes as to go through in order to be released.
Chris - So how do you do it? Do you somehow freeze time with the enzyme in one form and look at it, and then freeze time in the other form and look at it? Or do you look at all the forms and then work out what shape it must be in order to produce the mixture of images you see?
Michele - It is model building in the end. So, because we have an average information about the population of different structures, but we know there are different structures. So, we use theoretical modelling and computer simulations to build realistic models of these structures and then we require that the signal that we see is coming out as an average from the models that we generate.
Chris - And when you do this, what has this revealed about the behaviour of this enzyme system that we didn't have an insight into before?
Michele - Well, despite the fact that it's fairly obvious that the molecule, that the enzyme has to capture and release its substrate. We simply didn't know their atomic structures. We knew that they ought to be there but we didn't have a model for that. So, what we contributed is, in the particular case of an enzyme - it's called lysozyme - we know now the atomic structure of this intermediate state for release. And this is helpful because in the long term this may lead to a better ability to design enzymes.
Chris - Because we're obviously in a position now where we can begin to manipulate proteins. Now if you understand how they work, then we can make proteins actually do certain chemical reactions that we want them to do that they currently don't exist in nature with the capacity to achieve.
Michele - Yes. This is actually a very small component of a much broader area of research, which as you said, we are starting to become capable of manipulating molecules and this is an astonishing ability because the scale at which these processes take place are typically a billion times smaller than our everyday life experience. So, the ability of working over these landscapes I think is continuous source of amazement.

20:19 - How stress causes infertility
How stress causes infertility
with Daniela Kaufer & Anna Geraghty, UC Berkeley
It's well known that stress can interfere with fertility. But what's less well understood is how. It turns out that there's a stress-sensitive signal in the brain's hypothalamus that can block the release of the hormones that normally coordinate reproduction. UC Berkeley scientist Anna Geraghty found that temporarily deactivating this signal can prevent the effects of stress on reproduction in rats. Co-author on the study Daniela Kaufer spoke to Chris Smith...
Daniela - The first thing that you see and that you would expect to see is that there's a rise in the level of circulating stress hormones, what would be cortisol in people. You also have a rise in a peptide called gonadotropin-inhibitory peptide or in mammals it's called RFRP3. It's a top-down switch, if you want, of the reproductive system - it sort of shuts down the system.
Chris - Where does this come from, this inhibitory hormone, and what sorts of cells is it targeting? What's seeing it and what effect is it having?
Daniela - The RFP3 hormone is produced in an area of the brain called the hypothalamus. It is known to inhibit cells that produce GnRH, the gonadotropin-releasing hormone. So, controlling from the top, the hypothalamus pituitary-gonadal axis.
Chris - And the fact that it comes on in response to stress, it's downstream of the effects of stress. How is that achieved then?
Daniela - Several years ago, we looked at the cells that produced GnH and we found that they have the right receptors for the stress hormone, for the cortical steroid - cortisol. So, that's called a glucocorticoid receptor and that's expressed in the cells that produce GnH. And in response to this elevated stress signal, they start producing GnH and that stays around.
Chris - And then when a person recovers from stress or an animal recovers from stress, you would expect their cortisol levels to drop right down? Do you see, eventually, the inhibitory peptide falling off, or does it stay elevated?
Daniela - The furthest that we looked was one full oestrous cycle after distress. At this point we see the stress hormone that's going down so don't see any more glucocorticoids that are elevated, but the GnH is still elevated. We haven't looked further out at the expression but we did look at functional relevance of that. And when we look at what happens functionally to the animals, they still have difficulty in mating. Less of them that mated get pregnant and more of the ones that got pregnant have problems carrying the pregnancy throughout, and we see embryo survival that is decreased.
Chris - So, Anna. How did you explore what the relationship was between RFRP and the fertility effects?
Anna - So what we used in our study in our study was actually an inducible knockdown virus, and what that means is that the virus itself turns off the RFRP gene in the hypothalamus. And so we're putting it directly into the brain to shut it down. But what the inducible aspect of it is, is that we could actually control when that virus is on and off. And so what we were able to do is control the virus to such a degree that it was only turned off during that stressful period. So, the same period we did earlier where we found the fertility problems. We repeated those 18 days but during this time we blocked RFRP from being released - actually look at the effects RFRP increases might be causing in these. However, because it's inducible, we can actually turn it back on once the stress is over and what we found was that by turning off RFRP just during those 18 days, you completely prevented all those reproductive problems we saw in the stressed animals. And they actually had the same levels of stress hormones between the two groups. So, not having RFRP didn't change how they experienced the stress, just the downstream effects of it.
Chris - What do you think the implications are for what you found?
Anna - Oh. I think the possibilities are really great for what we could do, although, a lot of research needs to be done. The ability to start thinking about maybe it's not just the stress hormone we should be looking at when humans or animals are having fertility issues. That maybe it's something downstream of stress that might be persistent, even though the stress levels are normal. So, in this case RFRP, might give us a much wider range of problems to look for in fertility so that we could actually better identify problems humans are having.










Comments
Add a comment