eLife Episode 40: Glowing Squid, and Electric Anxiety
In this month's eLife Podcast, the sea urchin immune system, symbiotic bacteria in squid, the brain's anxiety circuitry, and a training course to promote collaboration between scientists...
In this episode
00:34 - A new animal model of immunity
A new animal model of immunity
with Kate Buckley, George Washington University
The mammalian immune system is notoriously complicated and difficult to unpick. But, speaking with Chris Smith, Kate Buckley from George Washington University has found a much simpler creature with just a hundred or so immune cells that - despite being separated from us by half a billion years of evolution - uses many of the same signals our own human immune system does...
Kate - Sea urchins have what we call a biphasic lifestyle or a two-step lifestyle, sort of like a butterfly and a caterpillar. They go through a larval stage before they turn into an adult. As larvae, these animals which are about twice the width of a human hair, they live for about two months in the ocean and they swim and they feed and they're fully competent organisms but they live in seawater which is really a microbe-rich environment. There’s a million bacteria per millilitre of seawater. And so the question is how are these tiny animals able to deal with this onslaught of microbes?
Chris - Because when a human baby is born, it gets preloaded with the cross-section of its mother’s immune system in the form of antibodies fed to it across the placenta. So, what does a sea urchin do?
Kate - It doesn’t have any real pre-programming developmentally. It just has to rely on what’s in its genome basically. And so, it has a small set of about a hundred immune cells that are able to recognize pathogens and respond. It makes anti-microbial peptides but that’s really all that’s known about it at this point.
Chris - So how did you approach it? What did you do?
Kate - We exposed these larvae to many different species of bacteria and just looked at them under a microscope to see if there were any observable changes in how their cells behaved or how the animals looked. And we found this one species of bacteria that caused what looked a lot like an inflammatory response. So, cells came from the periphery of the organism and travelled to the gut and that’s sort of a classic inflammation response.
Chris - What is the bug? And what’s the signal that is then radiating out through the organism’s body to tell these immune cells, “Come here. We’ve got a problem”?
Kate - The bacterium is called Vibrio diazotrophicus. It’s commensal. It forms long lasting relationships with adult sea urchins. And so, what this means is that the animal probably has developed an evolutionary strategy to say, “Okay, a little bit of this bug is okay but too much of it and we have to call in an immune response.” And the signal is this cytokine called Interleukin 17 or IL17.
Chris - That’s interesting, isn’t it, because Interleukin 17 is a lynchpin of my immune system and yours.
Kate - Right. That’s absolutely right. And so, what we found basically is that these larvae which are just a few thousand cells, the way that they control their gut-associated immune response is very similar to the way the human gut immune response is mediated. So, that it was surprising.
Chris - Are you saying then that because we all originated in a similar way that this interleukin signalling is very, very ancient and that’s why sea urchins have it and why we still have it today, it’s how we tell our immune response what to react to and what to ignore?
Kate - Right. Exactly. So, sea urchins are echinoderms which diverged from the vertebrates about 550 million years ago so this is really a deeply conserved element of the gut response.
Chris - And although the signals may be the same, the effects they have on cells and the way they're interpreted may not. So were the same receptors used then in the sea urchins so their immune cells see this Interleukin 17 family of signals in the same way that my immune cells do or not?
Kate - They do actually. Yeah. And so that’s one of the advantages of this model is that it’s basically a simplified version of the immediate response in the vertebrate gut. And so, the IL17 receptors are expressed in the gut and more widely. And some of the genes that are turned on when the receptors interact with IL17 are known to be associated with IL17 response in humans. And so, what this tells us is that there’s some conservation and the signalling pathways between sea urchins and vertebrates. And this gives us hope that we will be able to find additional things that are also conserved first in the sea urchins and then apply them back to humans.
Chris - Because this whole system is notoriously difficult to understand and unpick because it’s so complicated in big higher organisms and getting to the root of it is absolutely critical if we want to understand how things like the microbiome operate and how it influences our immune maturation, so do you think what you’ve got here then is a much simpler, much more tractable model for how that part of our immune system a. evolved and b. actually works?
Kate - That’s correct. So, the frontline of defence in your gut immune system is a genomically-encoded or what’s called innate immune response. And that’s something that sea urchins and humans have shared. And what we’re able to do with this larva is, since it’s transparent, we can actually just look at the gut. We can watch what the cells are doing and it’s a lot easier than getting at a mouse gut.

06:03 - Squid symbiotic bacteria: friend or foe?
Squid symbiotic bacteria: friend or foe?
with Cheryl Whistler, University of New Hampshire
Some squid team up with symbiotic bacteria, which supply light and help the squid to disguise itself. Speaking to Chris Smith, Cheryl Whistler from the University of New Hampshire explains how quickly the animals establish the right relationship with the right bacteria, including cherry-picking the mutants that make the most light...
Cheryl - We work with a squid species called Euprymna scolopes, more popularly called the Hawaiian Bobtail squid and it lives on near-shore areas at the Hawaiian Islands and it associates with a single species of bacterium called Vibrio fischeri. And Vibrio fischeri is a bioluminescent bacterium. So, it produces bioluminescence or light and when it associates with the squid, it lives in a particular special organ that the squid has on the bottom of its body. And when it’s swimming at night, and it’s out looking for mates or food, predators will actually sit on the bottom of the reef and look up for shadows. And when a squid passes over the top of a predator, if it is casting light from the bottom of its body, it just looks like a beam of moonlight and it’s completely camouflaged from the predators.
Chris - So, how does the squid team up with the bacterium to tell it, “Right. I want the light now”?
Cheryl - Good question. So when the squid first hatch, they actually don’t have any of the bioluminescent symbionts. They have to recruit them from the sea water. And they do this by providing attractins that the bacteria can sense and then they swim into the light organ down through ducts and enter the light organ and then start growing there. The problem is if the squid were not selective about who it let into its light organ, it could very easily let in a pathogen or a squatter that doesn’t belong there and that doesn’t produce bioluminescence. So the squid has actually developed really exquisite specific mechanisms to recognize who its symbiont is and to only let its symbiont into its light organ. Once the bacterium is there, the squid feeds it and when the bacterium reaches a nice robust population, it automatically glows and it does so by a method called quorum sensing where the bacteria actually communicate with each other and tell each other to turn on light together.
Chris - So getting the right population of bacteria and getting the right numbers of bacteria that want to cooperate is absolutely critical for the survival of the squid and for the survival of that bacterial strain as a whole.
Cheryl - Absolutely. So the bacteria can live outside the squid but they don’t live a good life. And the squid certainly, the theory is without the camouflage, they would be much more susceptible to predation.
Chris - So, what was the question you were seeking to ask here?
Cheryl - We were interested in understanding how the squid recognized their specific symbiont and how does their symbiont communicate to them that they are the beneficial partner that should be allowed to live in that light organ? And we did it by looking at natural diversity that exists in non-symbiotic populations. So can bacteria that have never seen the squid before through very subtle changes and their DNA, basically evolving through mutation, can they find solutions to the problem that the squid provides in terms of barriers to prevent the wrong symbiont from getting in?
Chris - So how did you do it? Take some squid, take some bacteria, mix them up, and start a family growing and then take some of those bacteria and keep repeating that to slowly select for bacteria and see how they change.
Cheryl - Well, we thought we might have to do it slowly but in fact, it happened very rapidly. So, what we did as we took bacterial strains that were naive to squid. We took strains from sea water that never encounter squid. We took the strains from other hosts like fish and we grew them up in culture and then dropped in a cohort of juvenile squid who had never seen a symbiont yet. And then we let the squid basically select from the natural genetic diversity that arose in culture and then we passage them from that squid to the next squid by allowing the squid to vent out the bacteria. This is one of the things that does is it actually grows up a population and then every morning, it vents out or dumps out 95 per cent of the culture so that they can grow a nice fresh culture and be glowing very brightly at night. So when they go back into the water, they're potentially now genetically adapted to that light organ environment.
Chris - You say potentially but is that actually what happens? Do you see a refinement of the population driven by this selection from a squid?
Cheryl - Absolutely. And this is one of the remarkable things about the experiment is that the mutations likely arose in culture when the populations where very large. But the first squid didn’t have only the beneficial mutations in their light organ population. It was a mixed population. By the second squid, we were able to detect the beneficial mutation that was literally a needle in a haystack in the initial inoculum.
Chris - And what did change?
Cheryl - It was really kind of interesting because it wasn’t a different mutation in every line of squid. So, we did squid in parallel and we used several different strains. And what we found was that five lineages acquired beneficial mutations and the mutations mapped to the same gene. And they were not remarkably dramatic mutations. They were literally tiny little point mutations – so nucleotide changes. And just that change was enough to transform a non-symbiont into a really “kick ass” symbiont.
Chris - Love the phrase! So, there must be something specific about that gene. What does it do that gene, do you know?
Cheryl - Yes. It’s a protein whose job it is to turn off behaviours. And these strains that were not natural symbionts have this repressor blocking the expression of traits that were necessary to be a good symbiont. And what the mutations arose did is that they didn’t completely obliterate the activity of this repressor. They just modified it ever so slightly. So by modifying how this repressor interacted with its targets, the bacteria were able to modify not just one behaviour but a suite of behaviours that were linked to each other and that were necessary to be a symbiont. And these included their metabolism and included their ability to produce light at the right level and a really key trait was that it changed the way they produced their sugary coats that they coat their selves with. They are called polysaccharides. And these polysaccharides allow the bacteria to form aggregates or biofilms that protect them from things like oxidative stress, and chemicals and it also protected them from the squid’s immune cells. So when immune cells monitor who is coming into the light organ, they literally will glob on and kill any bacterium that isn’t the right bacterium. But this change in the sugary coat of these cells allowed them to slip pass the immune cells and get in to the light organ and do their job as symbionts.
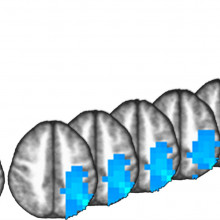
13:04 - The anxious brain
The anxious brain
with Nicholas Balderston, NIH
Anxiety is very common - in fact about one person in 5 at any one time reports feeling anxious. And although we know about some of the brain's "fear centres", what lies upstream of them and actually triggers sensations of anxiety in the first place, is less clear. But, by making volunteers fearful they were about to receive electric shocks, Nicholas Balderston, from the NIH in Bethesda, Maryland, has identified a brain region that may be responsible. Chris Smith hears how...
Nicholas - What we wanted to do is study the experience of being in an elevated state of anxiety and study what happens in the brain during that experience to identify what was the most prominent patterns of activity or connectivity. And it’s a very simple paradigm. You bring people in and you tell them that they're going to get shocked and periodically, you actually do give them shocks. And you can use periods when they're at risk for shock and compare them to periods where they're not at risk for shock and can very reliably evoke increases in physiological responses and then also changes in brain activity.
Chris - While that’s going on, what measurements are you making from the brain and how?
Nicholas - We used two different techniques to measure brain activity. The first technique was functional Magnetic Resonance Imaging. So we bring people in, we put them in an MRI scanner. We have different short blocks of time where they're at risk for receiving a shock and they know that they're going to receive a shock at some point. And then we also have periods of time where they know that they're not at risk for receiving shocks. We extract the data from those individual blocks and compare the safe blocks to the threat blocks. So what we did is we looked at how the pattern of communication between different brain regions changed over those periods of time. We also used a technique known as magnetoencephalography. This technique measures the changes in the magnetic field around the person’s scalp and you can figure out where in the brain is generating those magnetic fields. And, during this session, we were also able to present bursts of white noise so that we could measure their acoustic startle reflex. So, when you’re anxious, you're going to startle more than when you’re not anxious. So, we were able to show that our threat of shock manipulation is actually making them anxious and they were startling more.
Chris - And when you look at the outcome from both the MRI, the imaging study, but also the MEG, the magnetoencephalography recordings, were they the same, and which brain areas appear to be showing a change in their activity consistent with them - in some way - interpreting the threat and the heightened state of anxiety that that person finds themselves in?
Nicholas - Yes. So, with both measures, what we found is that there was one particular part of the brain - the intraparietal sulcus - that showed both increases in connectivity with the rest of the brain and also a pattern of neural oscillations consistent with increased excitability. What I think is happening is threat of shock is increasing the excitability in this region, in a manner that’s consistent with, say, an increase in stimulus-driven attention or bottom-up attention. So it’s potentially making people more aware of their surroundings.
Chris - Do you have any insights into how this region – the intraparietal sulcus – is doing this, how it’s changing the way that the brain processes and attends to stimuli?
Nicholas - One possibility is that this increase in excitability and connectivity may be lowering the threshold for detection of external events. It may make it easier to pick out stimuli that pop up in the environment – something that you otherwise wouldn’t be able to detect. This increases the salience of new stimuli through like bottom-up attentional mechanisms.
Chris - Now, obviously, if this region of the brain – the intraparietal sulcus – lies upstream of a lot of the changes and effects - manifestations - of anxiety, if you turned it off, that should abolish or reduce a person’s anxiety response. Have you tried doing that, because you could do that, for example, with transcranial magnetic stimulation? You could deactivate that brain area...
Nicholas - We haven’t tried that but that’s something that we’re very interested in doing. We’re very interested in trying to develop new techniques for treating anxiety. One potential way as you mention is to use transcranial magnetic stimulation to reduce activity in this region perhaps that would reduce some of the aspects of anxiety in these individuals.

17:39 - Training scientists to collaborate
Training scientists to collaborate
with Natalia Landázuri, Karolinska Institute
Historically, early career science training was dominated by learning which end of a pipette to hold, and doing lots of experiments badly until you learned to do them better. But the era of the sole scientist who beetles away in solitary confinement in front of a microscope for decades is well and truly over. Science has become much more collaborative and much more multidisciplinary. And that means teaching and training need to keep up. Which is why Natalia Landázuri, at Stockholm's Karolinska Institute, has been piloting a way to teach some of these skills by linking her institution to one on the other side of the Atlantic. She tells Chris Smith why the course was created...
Natalia - The way the world is changing, the way it is so easy to interact with people across the world with the help of technologies, the easiness to access information online and gather information has changed the extent to which collaboration can happen. And also, the way knowledge is being built, we have so much more knowledge from so many different areas that gathering that increases the need for collaboration across the world really and across disciplines.
Chris - So you’re saying, it’s important that a student, an early career scientist isn’t just taught how to fiddle with test tubes and do experiments but also how to use this network and how to develop this network for themselves internationally.
Natalia - Absolutely. Well, it is true that a traditional training for a doctoral student would be to have the student in the lab and tell them how to do technical things, how to use a pipette. Those really are just the beginning and even if a student is not willing to embark in an academic research career, skills of internationalization, innovation, creative thinking, those are skills that a person should also be trained for for their future.
Chris - The thing is that a lot of people who are training these students, these early career researchers, have themselves had to develop those skills organically as they’ve grown their career. They haven’t actually ever been taught how to teach them. So, is that really what you were seeking to do here?
Natalia - Exactly. That’s exactly what we were thinking about. I totally agree. It has been a challenge for a person to all of a sudden finish a PhD and be thrown in the middle of an academic setting being told, “You need your own line of research, your own ideas and find your own funding, and your own network” pretty much. It is definitely something that was lacking and that is still lacking in most instances during doctoral education. So that is the gap that we were willing to fill through the development of this course.
Chris - Tell us about the course.
Natalia - We implemented this joint doctoral course in regenerative medicine. It was a collaboration between Karolinska Institute that’s in Stockholm, Sweden and Mayo Clinic in Rochester, Minnesota in the United States. And it was a joint course that we shared between the two institutions through video conferencing and through other online tools. What we did is agreed on the common grounds for the learning outcomes and on the teaching-learning activities for the students. And the way we designed the course is that we would have a synchronous in-class occasions through video conferencing and then we would have the asynchronous off-class occasions where students would work on their own, reflect on different scientific topics but they would also interact with each other.
Chris - What did the students say about it? What do they think they got out of the experience?
Natalia - I think it was highly rewarding. We sent out an evaluation at the end of the course and we got pretty good feedback saying, “This is amazing! Thank you so much. I haven’t ever been in a course like this.” But we first wanted to know if the online and video conference systems we were using were okay for the students if they were actually getting what they were hoping for. And the answer to that was, yes, the students didn’t really see a difference in their interactions with speakers. They didn’t think that the quality of the talks were different. They highly enjoyed the fact that they could collaborate and communicate with the students on the other side of the screen. So, I mean it was really a highly appreciated course.
Chris - Do you think this would translate to other institutions or was this a very special relationship between the Karolinska and the Mayo or do you think it would be something that could be generically offered? And also, to what extent do you think it’s a reflection, its success, on the topic regenerative medicine? Would it work for people doing particle physics or people doing palaeoanthropology?
Natalia - Our thought was this was a proof of concept for a course that could really be expanded to any other area of science of research, of just any other top educational activity or even outside of education. Just the possibility to form collaborative networks using technology so that we can brainstorm together and take ideas further is something that can definitely be implemented in other settings and we would certainly encourage others to test this type of setting.
Related Content
- Previous Do Plants Hibernate?
- Next Marine Month: All at Sea










Comments
Add a comment